Abstract
Incubation of barley primary dormant grains at 30°C, a temperature at which they cannot germinate results in a reinforcement of their sensitivity to temperature, and in particular in a loss of their ability to germinate at 15–20°C.1 Incubation of the grains at 30°C in the presence of GA3 (1 mM) or of isolated embryos prevents this induction of secondary dormancy. In such a condition, embryo ABA content was lower than that measured in embryos of seeds incubated at 30°C on water. Expression of genes involved in ABA metabolism (HvABA8′OH1, HvNCED1 and HvNCED2) was studied in isolated embryos incubated on water and in the presence of GA3 (1 mM). Expression of HvABA8′OH1, HvNCED1 and HvNCED2 was discussed in relation with ABA content and germination ability at 30°C.
Key words: secondary seed dormancy, abscisic acid, gibberellin, ABA 8′hydroxylase, 9-cis-epoxycarotenoid dioxygenase, barley grain
Introduction
Primary dormancy in cereal grains corresponds to an inability to germinate at temperatures generally higher than 15°C.2,3 It sets in during seed development on the ear4,5 and is under abscisic acid control.6–9 Maintenance of high level of ABA content and sensitivity of the embryos to ABA play a critical role in the expression of dormancy in barley grains.10–12 In addition the balance of ABA and gibberellins (GAs) is involved in the regulation of germination and dormancy.13 When seeds are placed in unfavorable conditions they can enter in secondary dormancy which is highly relevant to seed behavior in the soil where the dormancy is cycling in relation to seasonal variations of the environmental conditions.14 We have demonstrated that in barley, incubation of grains at 30°C, a temperature of which primary dormant seeds cannot germinate, reinforces their sensitivity to temperature.1 This phenomenon which can be considered as an induction of a secondary dormancy is associated with a high level of ABA and an increase in embryo sensitivity to ABA.1 A specific role for HvNCED1 and HvNCED2 in regulation of ABA synthesis during secondary dormancy has been suggested,1 while expression of ABA 8′ hydroxylase (HvABA8′OH1) can be considered as a key gene regulating primary dormancy.11,12 The data presented here allows to compare the changes in ABA content and expression of HvABA8′OH1, HvNCED1 and HvNCED2 genes during incubation at 30°C of grains on water and in the presence of gibberellic acid (GA3), and of isolated embryos placed on water.
Imposition of Thermodormancy Required Seed Envelops and was Suppressed in the Presence of GA3
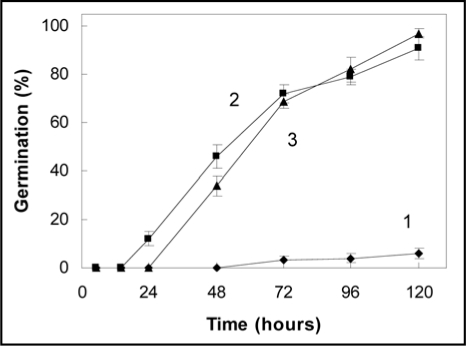
Primary dormant grains did not germinate easily above 20°C.1 Only 6% of the seed population, germinated within 5 days at 30°C (Fig. 1). This sensitivity to high temperature results from an inhibition exerted by the coverings of the grains (seed coat + pericarp) and may be by the endosperm since isolated embryos germinated perfectly at 30°C, the time to obtain 50% germination being about 48 h (Fig. 1). GA3 strongly stimulated the germination of whole grains at 30°C. It largely overcame the inhibitory effect of the covering structures, whole grains germinating as well as isolated embryos (Fig. 1). However this stimulatory effect required high concentration of GA3, particularly as O2 percentage decreased.15
Figure 1.
Time course of germination at 30°C of whole grains placed on water (1), isolated embryos incubated on water (2) and whole grains placed on GA3 1 mM (3). Means of 4 replicates ± SD.
Involvement of ABA in Thermodormancy
Dormancy expression during seed imbibition at high temperature is associated with the maintenance of ABA at high levels in the embryo.10–12 After 24 h of imbibition of whole seeds at 30°C, embryo ABA content remained 1.7 as high as that measured in isolated embryos incubated for the same duration on water (Table 1). Incubation of whole grains in the presence of GA3 (1 mM) resulted in a decrease in ABA content by about 20% as compared with the control grains placed on water as early as 5 h of imbibition (Table 1). Such an effect of GA3 on ABA metabolism was also observed in Nicotiana plumbaginifolia16 and lettuce17 seeds. At 24 h, ABA content remained higher than that in isolated embryos. Fluridone (0.1 mM) an inhibitor of ABA synthesis also resulted in a decrease in ABA content by around 25%1 and allowed the germination at 30°C of 45% of the primary dormant seeds within 5 days (data not shown) against 6% for seeds placed on water (Fig. 1).
Table 1.
ABA content in the embryo from dormant seeds incubated at 30°C on water or GA3 (1 mM) or in isolated embryos placed on water at 30°C
| Incubation condition at 30°C | Embryo ABA content (pg mg DW−1) | ||
| Medium | Duration (h) | Whole grains | Isolated embryos |
| - | 0 | 429.8 ± 5.8 | 429.8 ± 5.8 |
| Water | 5 | 472.4 ± 22.9 | 355.1 ± 3.2 |
| 14 | - | 345.1 ± 29.8 | |
| 24 | 417.0 ± 19.0 | 245.3 ± 24.2* | |
| GA3 | 5 | 420.2 ± 53.9 | |
| 14 | 352.5 ± 22.0 | ||
| 24 | 332.3 ± 20.4 | ||
Germination occurs. Means of 3 measurements ±SD.
Expression of Genes Involved in ABA Metabolism in Relation to Thermodormancy
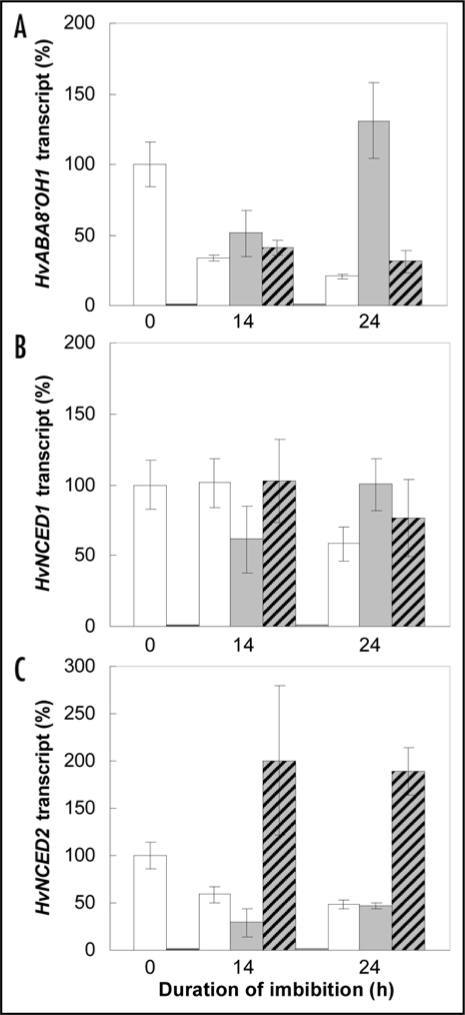
Figure 2 shows the changes in expression of genes implicated in ABA metabolism, HvABA8′OH1 which encodes an ABA hydroxylase involved in ABA degradation and HvNCED1 and HvNCED2 which encode 9-cis-epoxycarotenoid dioxygenase involved in ABA synthesis,11 during 24 h incubation at 30°C. Imbibition of whole seeds or isolated embryos on water resulted in a significative reduction of HvABA8′OH1 and HvNCED2 expression at 14 h (Fig. 2A and C) when HvNCED1 expression did not significantly change (Fig. 2B). Curiously, HvABA8′OH1 expression increased at 24 h for the isolated embryos while it remained low (20.7% of its initial value) for the whole grain (Fig. 2A). These data suggest that the covering structures had no crucial role in ABA metabolism, but were mostly involved in responsiveness of the embryo to ABA probably through the regulation of oxygen availability.2,15 Application of GA3 has almost no effect on HvABA8′OH1 and HvNCED1 expression (Fig. 2A and B) but strongly induced HvNCED2 expression (Fig. 2C). Decrease in ABA content during imbibition in the presence of GA (Table 1) might be then related to ABA degradation rather than ABA synthesis.
Figure 2.
Changes in expression of HvABA8′OH1 (A), HvNCED1 (B) and HvNCED2 (C) in embryos of whole grains (white bars) or of isolated embryos (grey bars) incubated on water and whole grains placed on GA3 1 mM (hatched bars) at 30°C. Data from real-time PCR (mean of 4 replicates ± SD) were normalised with actin transcript abundance and were expressed as percentage of the values obtained in embryos isolated from dry seeds. Absolute transcript amounts were respectively 942.1 ± 143.8, 81.8 ± 13.9 and 42.1 ± 5.7 copies per microliter of cDNA solution after 50 times dilution for HvABA8′OH1, HvNCED1 and HvNCED2 before imbibition.
Conclusion
The primary thermodormancy of barley is under ABA control, being associated with maintenance of high ABA level and strong responsiveness of the embryo to this hormone.10–12 It was prevented by GA3 application and removing the covering structures which both resulted in a reduction in embryo ABA content. However expression of genes involved in ABA metabolism in relation with dormancy depth and/or germination remained unclear. HvABA8′OH1 was considered as a key gene controlling ABA content and primary dormancy in barley11 while it did not seem to play a pivotal role in induction of secondary dormancy1 and ABA content in the presence of GA3 (Fig. 2A). In addition, germination of isolated embryos at 24 h was associated with an induction of this gene in relation with a strong decrease in ABA content (Table 1). Expression of HvNCED2 was strongly improved in the presence of GA3 and not in isolated embryos when the germination ability was similar (Fig. 1). Recent data18,19 underline the interplay between ABA and GA metabolism and signaling pathways. Further studies are then required to determine the role of ABA on GAs metabolism, and reciprocally, at molecular level. Other mechanisms, in particular at the level of ABA and GA signaling pathways should also be investigated in order to obtain a more comprehensive view of the role of the balance between ABA and GA in thermodormancy.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7797
References
- 1.Leymarie J, Robayo-Romero ME, Gendreau E, Benech-Arnold RL, Corbineau F. Involvement of ABA in induction of secondary dormancy in barley (Hordeum vulgare L.) seeds. Plant Cell Physiol. 2008;49:1830–1838. doi: 10.1093/pcp/pcn164. [DOI] [PubMed] [Google Scholar]
- 2.Lenoir C, Corbineau F, Côme D. Barley (Hordeum vulgare) seed dormancy as related to glumella characteristics. Physiol Plant. 1986;68:301–307.. [Google Scholar]
- 3.Corbineau F, Côme D. Barley seed dormancy. Bios. 1996;261:113–119. [Google Scholar]
- 4.Simpson GM. Seed dormancy in grasses. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 5.Corbineau F, Benamar A, Côme D. Changes in sensitivity to abscisic acid of the developing and maturing embryo of two wheat cultivars with different sprouting susceptibility. Israel J Plant Sci. 2000;48:189–197. [Google Scholar]
- 6.Steinbach HS, Benech-Arnold RL, Sanchez RA. Hormonal regulation of dormancy in developing Sorghum seeds. Plant Physiol. 1997;113:149–154. doi: 10.1104/pp.113.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benech-Arnold RL, Giallorenzi MC, Frank J, Rodriguez V. Termination of hull-imposed dormancy in developing barley grains is correlated with changes in embryonic ABA levels and sensitivity. Seed Sci Res. 1999;9:39–47. [Google Scholar]
- 8.Garello G, Le Page-Degivry MT. Evidence for the role of abscisic acid in the genetic and environmental control of dormancy in wheat (Triticum aestivum L.) Seed Sci Res. 1999;9:219–226. [Google Scholar]
- 9.Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant. 2002;115:428–441. doi: 10.1034/j.1399-3054.2002.1150313.x. [DOI] [PubMed] [Google Scholar]
- 10.Benech-Arnold RL, Gualano N, Leymarie J, Côme D, Corbineau F. Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. J Exp Bot. 2006;57:1423–1430. doi: 10.1093/jxb/erj122. [DOI] [PubMed] [Google Scholar]
- 11.Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 12.Gubler F, Hughes T, Waterhouse P, Jacobsen J. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008;147:886–896. doi: 10.1104/pp.107.115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 14.Hilhorst HWM. Definitions and hypotheses of seed dormancy. In: Bradford K, Nonogaki H, editors. Seed development, dormancy and germination. Oxford: Blackwell Publishing; 2007. pp. 50–71. [Google Scholar]
- 15.Bradford KJ, Benech-Arnold RL, C≈me D, Corbineau F. Quantifying the sensitivity of barley seed germination to oxygen, abscisic acid and gibberellin using a population-based threshold model. J Exp Bot. 2008;59:335–347. doi: 10.1093/jxb/erm315. [DOI] [PubMed] [Google Scholar]
- 16.Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M. Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta. 2000;210:279–285. doi: 10.1007/PL00008135. [DOI] [PubMed] [Google Scholar]
- 17.Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, et al. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot. 2004;55:111–118. doi: 10.1093/jxb/erh023. [DOI] [PubMed] [Google Scholar]
- 18.Zentella R, Zhang Z-L, Mehea P, Thomas SG, Endo A, Murase K, et al. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]