Abstract
Protein isoprenylation refers to the attachment of a C15 farnesyl or C20 geranylgeranyl moiety to a carboxyl terminal cysteine residue. Because protein isoprenyltransferases are cytosolic enzymes, it has long been assumed that the isoprenyl diphosphates used for protein isoprenylation are synthesized in the cytosol. However, in the present work, we established that the plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway is predominantly responsible for providing the geranylgeranyl diphosphate for protein geranylgeranylation in tobacco BY-2 cells.
Key words: plastidial MEP pathway, protein geranylgeranylation, protein isoprenylation
Isoprenylated proteins are characterized by the thioether linkage of a C15 farnesyl or C20 geranylgeranyl moiety to a cysteine residue at or near the carboxyl terminus. These posttranslationally modified proteins, the majority of which are further modified by proteolysis and carboxyl methylation of the isoprenylcysteine residue, are involved in a multitude of processes, including regulation of meristem maintenance, abscisic acid and auxin signaling, abiotic and biotic stress responses, and cell division.1 Until recently, the isoprenoid donor molecules used by plant cells for protein isoprenylation (i.e., farnesyl diphosphate [FPP] and geranylgeranyl diphosphate [GGPP]) were widely believed to be cytosolic in origin, products of the well-established mevalonate (MVA) pathway. This belief was, in part, due to early observations that incubation of tobacco BY-2 cells or spinach seedlings with radioactive MVA resulted in labeling of isoprenylated proteins.2,3 However, the quasi-dogma that C5, C10, C20 and C40 isoprenoids are primarily derived from the plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway, whereas C15 and C30 isoprenoids are primarily derived from the MVA pathway, in addition to the observation that [14C]1-deoxy-D-xylulose labeled low molecular weight proteins in vivo,4 cast doubt on the presumed cytosolic origin of the GGPP used for geranylgeranylation of plant proteins. We have shown that the plastidial MEP pathway is predominantly responsible for providing the GGPP for protein geranylgeranylation in tobacco BY-2 cells. This conclusion is based on experiments with BY-2 cells expressing a GFP protein fused to the carboxyl terminal polybasic domain and CVIL geranylgeranylation motif of the rice calmodulin CaM61 (GFP-BD-CVIL). MEP and MVA pathway inhibitors were used to demonstrate that inhibition of the MEP pathway in the presence of oxoclomazone (OC) or fosmidomycin (Fos), but not inhibition of the MVA pathway in the presence of mevinolin, shifted the GFP-BD-CVIL protein from the plasma membrane to the nucleus of transformed BY-2 cells (Fig. 1). Further experiments showed that the plasma membrane-associated form (i.e., in the absence of inhibitors) was strictly geranylgeranylated and carboxyl methylated in vivo and the nuclear form (i.e., in the presence of Fos) was not isoprenylated. In addition, chemical complementation experiments confirmed the predictions that: (1) geranylgeraniol prevents the nuclear mislocalization of GFP-BD-CVIL caused by OC or Fos, but not that caused by an inhibitor of protein geranylgeranyltransferase type I (PGGT1), and (2) 1-deoxy-D-xylulose prevents the nuclear mislocalization of GFP-BD-CVIL caused by OC (OC blocks 1-deoxy-D-xylulose 5-phosphate synthase), but not that caused by Fos (Fos blocks the conversion of 1-deoxy-D-xylulose 5-phosphate to 2-C-methyl-D-erythritol 4-phosphate).
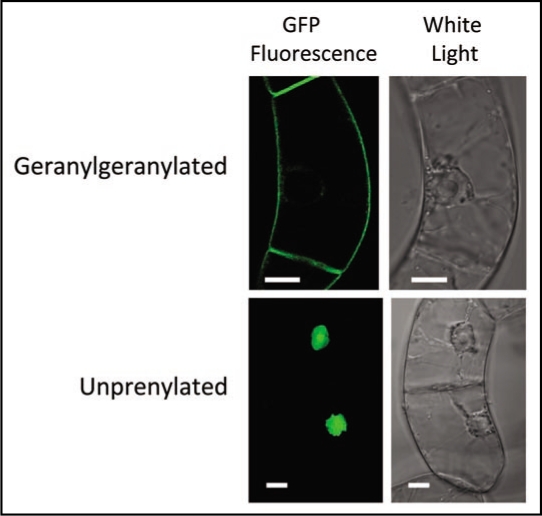
Figure 1.
Geranylgeranylated GFP-BD-CVIL is localized to the plasma membrane in tobacco BY-2 cells, whereas unprenylated GFP-BD-CVIL (i.e., in the presence of Fos or OC) or GFP-BD-SVIL, which cannot be isoprenylated, is localized to the nucleus and nucleolus. Bars = 10 µm.
The results described above established the plastidial MEP pathway as the primary source of the GGPP used for protein geranylgeranylation in tobacco BY-2 cells. However, in plant cells with differentiated plastids, where the metabolic flux through the MEP pathway is directed toward the biosynthesis of chlorophylls, carotenoids, plastoquinones and other isoprenoids, the situation may be quite different. Perhaps in these cells, the MVA pathway plays a greater role in the biosynthesis of the GGPP used for protein geranylgeranylation. Future studies will address this possibility. It is also not clear whether geranyl diphosphate (GPP) or GGPP is exported from BY-2 plastids and used for protein isoprenylation. If the former were exported from BY-2 plastids, a cytosolic GGPP synthase would be necessary to convert the GPP to GGPP. However, if the latter were exported, a plastidial GGPP synthase would be necessary. Future studies will also provide answers to this question.
In addition to establishing the MEP pathway as the predominant source of GGPP used for protein geranylgeranylation in tobacco BY-2 cells, the results described above established a rapid and convenient assay system for screening combinatorial chemical libraries for inhibitors of the MEP pathway, which are potentially useful as herbicides, antibiotics and antimalarial drugs. To screen for these inhibitors, BY-2 cells can be seeded into 96 well plates and induced to express GFP-BD-CVIL following a 3-hour pretreatment with chemicals from a combinatorial chemical library. Nuclear mislocalization of the GFP-BD-CVIL protein can then be observed after 15 hours by fluorescence microscopy (confocal microscopy is not required). To definitively identify inhibitors of the MEP pathway, chemical complementation of the nuclear mislocalization caused by a candidate inhibitor (i.e., restoration of plasma membrane localization in the presence of geranylgeraniol) can be analyzed. As the use of existing herbicides and antibiotics promotes the evolution of resistance mechanisms and renders them less effective over time, screening procedures such as this will be essential to the future of sustainable agriculture and development of new drugs.
Addendum to: Gerber E, Hemmerlin A, Hartmann M, Heintz D, Hartmann M-A, Mutterer J, Rodríguez-Concepción M, Boronat A, van Dorsselaer A, Rohmer M, Crowell DN, Bach TJ. The plastidial 2C-methyl-D-erythritol 4-phosphate pathway provides the isoprenyl moiety for protein geranylgeranylation in tobacco BY-2 cells. Plant Cell. 2009 doi: 10.1105/tpc.108.063248. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7842
References
- 1.Crowell DN. Functional implications of protein isoprenylation in plants. Prog Lipid Res. 2000;39:393–408. doi: 10.1016/s0163-7827(00)00010-2. [DOI] [PubMed] [Google Scholar]
- 2.Randall SK, Marshall MS, Crowell DN. Protein isoprenylation in suspension-cultured tobacco cells. Plant Cell. 1993;5:433–442. doi: 10.1105/tpc.5.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shipton CA, Parmryd I, Swiezewska E, Andersson B, Dallner G. Isoprenylation of plant proteins in vivo. Isoprenylated proteins are abundant in the mitochondria and nuclei of spinach. J Biol Chem. 1995;270:566–572. doi: 10.1074/jbc.270.2.566. [DOI] [PubMed] [Google Scholar]
- 4.Hemmerlin A, Hoeffler J-F, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, et al. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco Bright Yellow 2 cells. J Biol Chem. 2003;278:26666–26676. doi: 10.1074/jbc.M302526200. [DOI] [PubMed] [Google Scholar]