Abstract
Microtubules (MTs) are important cytoskeletal superstructures implicated in neuronal morphology and function, which are involved in vesicle trafficking, neurite formation and differentiation and other morphological changes. The structural and functional properties of MTs depend on their high intrinsic charge density and functional regulation by the MT depolymerising properties of changes in Ca2 + concentration. Recently, we reported on remarkable properties of isolated MTs, which behave as biomolecular transistors capable of amplifying electrical signals (Priel et al., Biophys J 90:4639–4643, 2006). Here, we demonstrate that MT-bathing (cytoplasmic) Ca2 + concentrations modulate the electrodynamic properties of MTs. Electrical amplification by MTs was exponentially dependent on the Ca2 + concentration between 10 − 7 and 10 − 2 M. However, the electrical connectivity (coupling) of MTs was optimal at a narrower window of Ca2 + concentrations. We observed that while raising bathing Ca2 + concentration increased electrical amplification by MTs, energy transfer was highest in the presence of ethylene glycol tetraacetic acid (lowest Ca2 + concentration). Our data indicate that Ca2 + is an important modulator of electrical amplification by MTs, supporting the hypothesis that this divalent cation, which adsorbs onto the polymer’s surface, plays an important role as a regulator of the electrical properties of MTs. The Ca2 + -dependent ability of MTs to modulate and amplify electrical signals may provide a novel means of cell signaling, likely contributing to neuronal function.
Keywords: Cytoskeleton, Biomolecular transistors, Electrical connectivity, Microtubule dynamics, Electrical amplification
Introduction
MTs are important cytoskeletal polymers, composed of hollow cylindrical arrangements of α–β-tubulin dimers [1–3]. Microtubules are essential for, among other functions, cell transport and cell division in all eukaryotes. The regulation of microtubular systems includes transcription of different tubulin isotypes, folding of α–β-tubulin heterodimers, posttranslational modifications of tubulin and nucleotide-based microtubule dynamics [4] as well as interactions with numerous microtubule-associated proteins (MAPs) and motors that are, themselves, regulated (reviewed in [5]). Microtubules are highly dynamic and can switch stochastically between growing and shrinking phases. This dynamic instability [4] is based on the binding and hydrolysis of guanosine triphosphate (GTP) by tubulin subunits. Each tubulin monomer binds one molecule of GTP. The binding to α-tubulin at the N site is nonexchangeable, whereas the binding to β-tubulin at the E site is exchangeable [5]. Only GTP-bound dimers are able to polymerise. After polymerisation, however, the nucleotide is hydrolysed and becomes nonexchangeable.
MTs also show remarkable biophysical properties, likely due to the high density of uncompensated negative electrostatic charges on the MT’s surface. MTs, for example, can be strongly aligned by electromagnetic fields [6–8], which is a property linked to their interaction with external ions [9]. Little is known, however, about the intrinsic electrical properties of MTs and, in particular, as to how these electrical properties are modulated. We recently reported novel features of MTs in solution [10]. Namely, MTs behave as bio-/break molecular transistors capable of amplifying electrical signals. In that report, we used taxol-stabilised, polymerised MTs, which were electrically manipulated with a modified dual “patch-clamp” setup [10]. Our data provided the first direct experimental proof that MTs sustain novel biomolecular transistor-like capabilities, which likely play a yet unknown role in cell function. Due to the relevant role of MT bundles and arrays in neurons and their contribution to neuronal function, it is likely that MTs carry electrical information as a novel means of cell signaling. To gain further insight into the nature of this phenomenon, we explored the effect of changes in the Ca2 + concentration on the electrical amplification and coupling properties of electrically stimulated MTs.
Experimental
Preparation of Isolated Microtubules
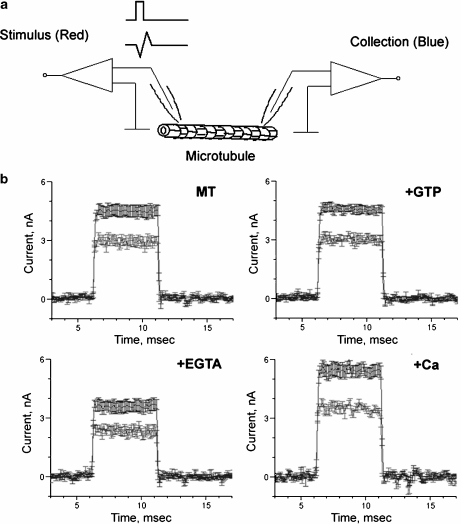
Tubulin was polymerised, as recently reported [10]. Briefly, an aliquot of tubulin in solution (Catalog #T238, Cytoskeleton, Denver, CO. USA) was mixed with 1 mM GTP and incubated for 5 min in a solution containing 80 mM PIPES, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA) and titrated to pH 6.8 with KOH. Paclitaxel (Taxol, Sigma, 10 μM final concentration) was also added to stabilise the MTs. MTs were visualised under phase contrast and electrically manipulated with a modified dual “patch-clamp” setup [10] (Fig. 1, top).
Fig. 1.
Electrical energy transfer by microtubules. Voltage pulses. a Experimental setup for electrical stimulation of MTs as originally described in [10]. Electrical signals are applied to the stimulus end, and received at the other (collection) end of a connected MT. b Voltage pulses applied to the “stimulus” side (blue) and are received on the other, “collection” side (red). Data are shown before (MT ) and after sequential addition of GTP (1.4 mM), EGTA (1.4 mM) and further addition of Ca2 + (14 mM). Data are the mean ± SEM of six to nine experiments
Electrical Recordings and Effect of Ca2 +
Experiments were conducted by placing an aliquot of the prepolymerised MTs in a bathing solution containing in mM: 135 KCl, 15 NaCl, 1.2 CaCl2, 0.8 MgSO4 and 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4. Electrical connections and coupling to the pipettes were assessed with an identical solution filling the pipettes to minimise junction and streaming potentials. To further stabilise the MTs in the bathing solution, GTP (1.4 mM) was added, which had no effect on either the stimulus pipette or the coupling ratio of the connected MTs (see Section 3, Fig. 1). Electrical amplification by MTs was calculated by assessing the ratio of electrical current observed between the “stimulus” and “collection” pipettes. MTs were electrically stimulated at one end (stimulus site) by applying square pulses (Fig. 1) or triangular ramps (Fig. 4). The resulting electrical signal was obtained at the opposite end of the MT (collection site), attached to another pipette, which was kept “floating” at 0 mV.
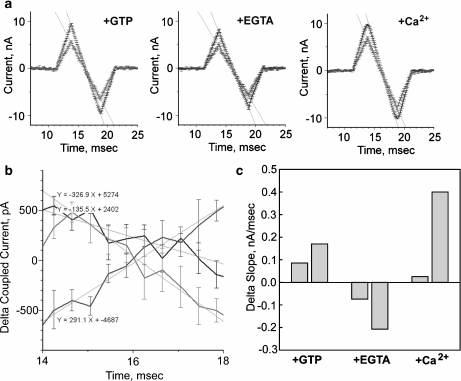
Fig. 4.
Electrical energy transfer by microtubules. Conductance slopes. a Average electrical currents from triangular ramps (±200 mV) for stimulus (blue) and collection (red) sites. Values are the mean ± SEM of 6–8 MTs in the presence of GTP (1.4 mM, +GTP), low Ca2 + (+EGTA) and high Ca2 + conditions (+Ca2 + ). b Subtracted slope currents for MTs after addition of GTP (blue), EGTA (green) and high free Ca2 + (red). Linear fits (solid lines) indicate the change in conductance in low Ca2 + , which is reversed after subsequent increase in bathing Ca2 + . c Change in conductance at the collection site after addition of GTP (+GTP), EGTA (+EGTA) and high Ca2 + (+Ca2 + ) for triangular ramps between ±100 mV (left bar) and ±200 mV (right bar) for each condition, respectively. Data indicate a nonlinear voltage dependent effect of Ca2 + on electrical stimulation
Changes in Ca2 + Concentration
To change the free Ca2 + concentration in the MT bathing solution but minimise the manipulations, which may affect the mechanical stability of the preparation, experiments were initiated in a bathing solution containing in mM: 135 KCl, 15 NaCl, 1.2 CaCl2, 0.8 MgSO4 and 10 HEPES, pH 7.4. Addition of EGTA (1.4 mM) brought the free Ca2 + concentration to approximately 2.4 × 10 − 7 M [11]. A third measurement of free Ca2 + concentration was achieved by further addition of Ca2 + from stock solution (500 mM CaCl2) to reach a final free Ca2 + concentration of 14.5 mM, as calculated in the presence of 1.4 mM EGTA at pH 7.4 and room temperature. Experimental conditions were compared by Student’s t test analysis of paired samples. Whenever indicated (i.e., Fig. 3), the dispersion around the mean from relative changes among groups was indicated by the expanded standard error, which was calculated by addition of averaged standard errors for each condition.
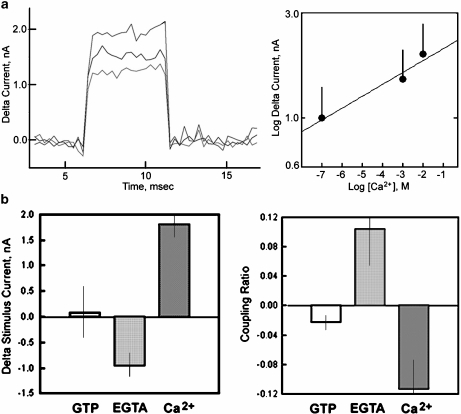
Fig. 3.
Coupling ratio and effect of Ca2 + . aleft Subtracted “stimulus” currents are dependent on the Ca2 + concentration as shown for EGTA (green), GTP (blue) and high Ca2 + (red). Right Electrical amplification is exponentially dependent on bathing Ca2 + concentration. Vertical lines represent the expanded standard error. bleft Delta currents are not dependent on the presence of GTP. EGTA (low Ca2 + ) decreased, while high Ca2 + concentration increased electrical stimulation. Vertical lines represent the expanded standard error. Right In contrast, coupling ratios are highest in low Ca2 + (EGTA), while GTP and high Ca2 + are much less efficient. Vertical lines represent the expanded standard error
Measurement of MT Dimensions
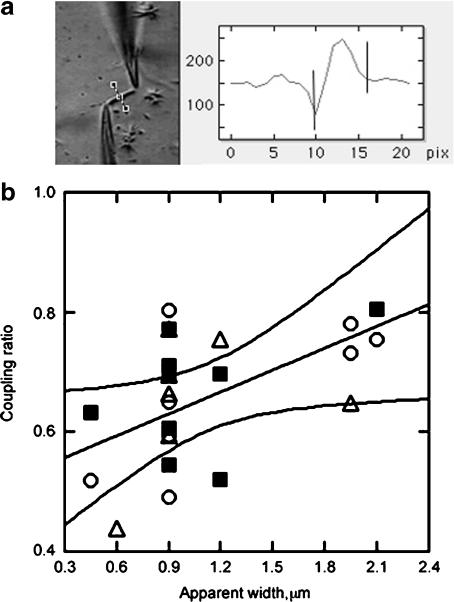
The preparation contained a variable number of stabilised MTs, usually in identifiable bundles (Fig. 2a). We also sought to obtain a correlation between both the apparent length and width of the MT bundles and their electrical properties. The distance between the pipette tips was measured to obtain an apparent length of the connected MTs. The MT apparent width was instead calculated by constructing cross section histograms with a subroutine of Image SXM v1.62 (S.D.Barrett@liv.ac.uk; Fig. 2a, right).
Fig. 2.
Coupling ratio and MT width. aleft MT bundles of various lengths and widths were connected to the electrical system. Right Apparent width was measured from cross section histograms obtained from profiles such as shown in dashed line in brackets, with Image SXM software. b Coupling ratio as a function of apparent width as calculated in a is shown. The linear correlation (straight line) indicates a role of MT width in determining the coupling ratio. The 95% boundaries are also shown. Data indicate control and GTP-treated (open circles), EGTA-treated (filled squares) and high Ca2 + treated (open triangles) MTs, which were not statistically different from each other
Results
To test the effect of Ca2 + on MT electrical amplification, experiments were conducted in three sequential phases. First, MTs were electrically connected as shown in the diagram of Fig. 1 and detailed in [10]. Voltage steps were applied to the connected MTs such that one end would be considered the stimulus and the other end the “collection” site. Thus, two responses were attained, namely, the electrical effect of the attached MT to the stimulus pipette and, second, the electrical response received at the collection site. From these, two distinct phenomena were originally observed in [10], namely that the attached stimulus end amplifies the electrical signal after the MT is connected and also that this signal can be transferred and received at the collection site. Addition of GTP (1.4 mM) to the MT bathing solution (containing in mM: 135 KCl, 15 NaCl, 1.2 CaCl2, 0.8 MgSO4 and 10 HEPES, pH 7.4) had no effect on either the electrical signal at the stimulus pipette or the coupling ratio of the connected MT (Fig. 1). In a second sequential step, further addition of EGTA (1.4 mM), which brought the free Ca2 + concentration to approximately 2.4 × 10 − 7 M [11], decreased both the electrical signals at the stimulus and the collection pipettes. In a third sequential step, further addition of Ca2 + from a stock solution raised the free Ca2 + concentration to 14.5 mM. This manoeuvre reversed the inhibitory effect observed in low Ca2 + and made both signals even higher than those observed for the same MT under control conditions in the presence or absence of GTP. It is important to note that neither addition of GTP, EGTA, nor concentrated Ca2 + modified the electrical properties of the pipette tips in the absence of a connected MT (data not shown). As mentioned above, the MT preparation contained bundles of stabilised MTs (Fig. 2a). The variable number of connected MTs likely affected the electrical coupling and stimulation. The apparent distance between pipette tips was used as the average length of the connected MTs. MT apparent width was instead calculated by constructing cross section histograms as described above and shown in the right panel of Fig. 2a. A positive linear correlation (r = 0.5495, n = 24, p < 0.02) was observed between MT bundle width and the coupling ratio of the connected MTs (Fig. 2b). No correlation was apparent, however, for average length (data not shown). No statistical differences were observed among the various experimental groups (i.e., GTP, EGTA and high Ca2 + ), suggesting that MT bundle width affected but did not significantly support the differences observed between high and low Ca2 + concentrations. The results from the voltage pulse stimulation at the stimulus pipette indicate that, while addition of GTP had no effect on energy transfer, changes in bathing Ca2 + did (Fig. 3a, b). A reduction of external Ca2 + decreased while an increase in the Ca2 + concentration enhanced the electrical stimulation (Fig. 3a, left). However, the coupling ability of the MT to transfer electrical energy (i.e., magnitude of the electrical signal) from one end to the other was highest in the presence of EGTA, i.e., at the lowest Ca2 + concentration (Fig. 3b, right).
The effect of Ca2 + on the electrical coupling by connected MTs was further investigated by obtaining a relationship between electrical amplification and the bathing Ca2 + concentration (Fig. 3a, right). As a result, electrical amplification at the stimulus site was found to be exponentially dependent on the free Ca2 + concentration. To further explore the effect of Ca2 + on electrical amplification and coupling in electrically stimulated MTs, triangular ramp stimuli were also imposed on control MTs (+GTP) before and after addition of both EGTA and high Ca2 + (Fig. 4a). The resulting data were in agreement with those obtained for the voltage pulses. The currents obtained after subtraction of the previous condition indicate a decrease in amplification in the presence of EGTA and its reversal after addition of high bathing Ca2 + (Fig. 4b). Interestingly, the triangular ramps between ±100 mV rendered much lower differences as compared to those applied between ±200 mV (Fig. 4). This difference in electrical response suggests a non-linear voltage effect compared with the observed changes by establishing a constant voltage in the voltage pulses (Fig. 1). The high voltage dependence of the Ca2 + response is evidenced by the differences in coupling slopes at ±100 vs. ±200 mV in the presence of EGTA and high Ca2 + conditions (Fig. 4c).
Discussion
Our findings demonstrate that the electrical amplification supported by MTs is strongly modulated by Ca2 + , thus modifying the polymer’s ability to act as a biomolecular transistor. The experiments herein extend our original observations that MTs support both amplification and conduction of electrical signals [10]. Interestingly, our new findings indicate that this phenomenon is modulated by surrounding Ca2 + within a wide concentration range spanning five orders of magnitude. This is a rather remarkable phenomenon considering the destabilising properties of Ca2 + on MTs [12]. Interestingly, electrical amplification by MTs was supported at approximately 200 nM Ca2 + , a concentration normally found in dendrites of CA1 hippocampal pyramidal cells [13]. This supports the contention that MTs support electrical amplification at physiological Ca2 + concentrations, ranging from 80 to 400 nM in various cell systems. This is particularly evident under stimulated conditions or active transport impairment [14, 15] and most clearly in physiological oscillations of intracellular Ca2 + [14]. Thus, it is tempting to postulate that yet unknown previously unattended physiological consequences of MT electrical amplification may play a significant role in a number of biological cell signalling events, such as cytoskeletal modulation of electrical activity in excitable tissues, e.g., the electrical and mechanical activity of cardiac myocytes [16].
The transistor-like properties of electrically stimulated MTs rely on a permanent electrical polarisation, which follows localised Nernst potentials arising from asymmetries in the ionic distributions between the intra- and extra-MT environments. Our data indicate that while low Ca2 + lowers electrical amplification at the stimulus site, the coupling ratio is highest under this condition, while increasing bathing Ca2 + produces the opposite effect. Based on the present results, the electrical polarisation of the connected MT seems modulated by external Ca2 + , which modifies the forward–reverse-biased junctions in the transistor model (Fig. 5). In this model, an MT’s surrounding Ca2 + controls both proper MT electrical amplification and the coupling of axially transferred electrical signals. Ca2 + is a highly MT-destabilising cation, however [12, 17]. Both our previous experimental data [10] and those presented here support the transistor model. In this model, the small current in the centre or base region of the transistor should control and, thus, modulate a larger current flowing between the end regions (emitter and collector) of the connected MT (Fig. 5). Electrically speaking, the larger collector current (ic) should present a certain proportionality to the base current (ib) according to the relationship ic = βib, or more precisely it should be proportional to the base-emitter voltage (vbe). This is indeed suggested by the exponential amplification as a function of bathing Ca2 + (Fig. 3a, right). Thus, the trans-MT molecular properties allow the length of the MT to behave as a current amplifier, having many implications for amplification and switching in biological settings. A “signal” in the form of changes in availability and binding of local Ca2 + or other ions, would generate a change in the base current, eliciting larger variations in the collector-to-emitter currents, with the consequent amplification of that signal. The model suggests other straightforward implications. In order for the MT transistor to amplify, there should be a structural asymmetry between the larger “collector” and the smaller emitter regions to promote conduction. This structural asymmetry, surprisingly, is supported not by the addition of GTP but rather by bathing Ca2 + . This would suggest that the very thin base region is a Ca2 + -dependent gate, such that the base-collector diode is reversed-biased (see original model in [10]). Conversely, the base-emitter diode, in contrast, is forward-biased. Thus, the base current is strongly dependent on the base-emitter voltage vbe. It is early to ascribe topological features to this gate. However, it is obvious that amplification requires that the polarity of the junction derive from strong differences in charge distribution between the collector-base and the emitter-base regions. The model, thus, implies that each “PNP-like” junctional connection should have an orientation, as is expected in the plus–minus orientation of MTs both in vivo and in vitro. It further follows from this model that the collector may be more positive than the emitter. This, in turn, suggests that a perm-selective conductance, which is either driven by K + or Ca2 + ions, to implicate two of the most relevant cations in the cytoplasm, compete to bias the vbe. Therefore, at a given Ca2 + concentration, the vbe is small (high Ca2 + ) or large (low Ca2 + ), thus, rendering different amplification gains. Namely, the electrical properties of the MT transistor largely depend on the availability of Ca2 + surrounding the MT. Because our results were obtained with in vitro polymerised tubulin, no apparent role of MAPs or other microtubule Ca2 + -dependent proteins may be invoked. Thus, the findings herein provide an electrodynamic picture of the intrinsic properties of the MT. This is particularly relevant in the potential uses of MTs as nanotechnological devices [18], where predictable changes in amino acid residues may have an important effect on the electrodynamic properties of the polymer and structures thereof [19]. It is, however, expected that MAPs may also be affected and indeed effect changes in MT behaviour, which can be translated into further modulation of electrical signals as well. Optimal electrical amplification requires specific Ca2 + concentrations, and any departure from this Ca2 + -MT interaction decreases the efficiency of both electrical amplification and the coupling mechanism by the MT. The data are consistent with the possibility that Ca2 + binding to a finite number of sites, which act as transistor gates, is critical to the most efficient electrical amplification by MTs.
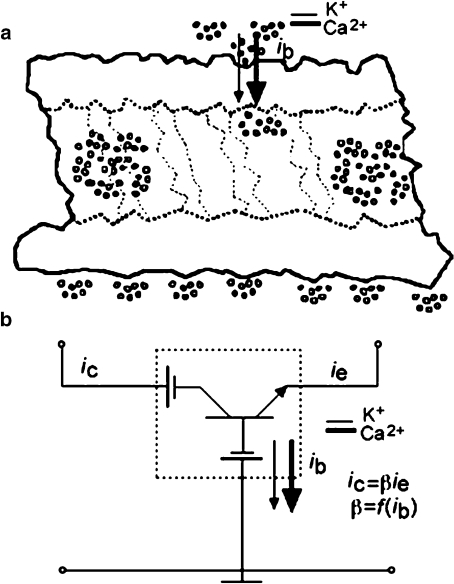
Fig. 5.
Electrical model of the Ca2 + -mediated regulation of MT transistor properties. a Cross-section of MT’s length, showing the surface of the electronegative MT and adjacent cations, including K + ( filled symbols) and Ca2 + ions (open symbols). The selective competition of ions for specific sites, “shunts” the wall’s electrostatic potentials, creating currents, which modify the electrical coupling of unitary sections of the MT, which, from left to right, may represent regions of higher and lower potential, respectively. b Following the diagram in (a) and the model in [10], the experimental data are consistent with intrinsic currents across the wall of the MT, which bias the potentials established across the MT. Thus these currents effectively behave as Ca2 + -dependent base-collector and base-emitter diodes, which control the trans-wall potential and, thus, the amplification properties of an axial length of the MT, as indicated by the β ratio
There are a number of potential biological implications for a MT-based Ca2 + -dependent electrical device, which may provide a number of novel features to neuronal conduction and regulation. It is tempting to postulate, for example, that electrodynamic properties of MTs may provide a suitable explanation for other, better known microtubular-supported phenomena such as fast axonal transport [20]. Most cells, particularly neurons, where this active transport is absolutely necessary for materials to travel from the cell body to the synapse, support MT-dependent organelle movement. Interestingly, while axonal MTs are highly polarised, the MTs in dendrites have both plus and minus ends pointing outward [21, 22]. This poses an interesting question for the conventional understanding of directional MT-dependent transport [20]. It is entirely possible that MT-supported electrical amplification may provide a novel means for directionality in MTs. It has been postulated, for example, that electrical sorting may be a suitable mechanism of vesicle trafficking. There is neither direct experimental evidence nor a clear understanding of the role Ca2 + plays in MT-dependent organelle movement. Fast axonal transport continues in the presence of EGTA, indicating that a high level of free Ca2 + is not required. Increases in free Ca2 + to as high as 100 μM have little or no effect on the transport of small vesicles [23] (see also [20]). There is evidence, however, that retrograde transport in MTs requires higher Ca2 + concentrations than anterograde transport in both chromatophores [24] and axons [25]. This is particularly significant in view of recent findings on the presence of strong transient electric fields inside cells [26], which could influence MTs and, thus, subsequently transmit electrical signals as proposed in the present study. This could be achieved by the effect of Ca2 + , which may be implicated in the process of MT directionality switching itself. Thus, electrical amplification and modulation by Ca2 + of MTs can provide novel means for explaining relevant cell biological features in neuronal function and, it is likely, in other aspects of cell biology in general.
Acknowledgements
Funding from NSERC (Canada), MITACS and Technology Innovations, LLC of Rochester, NY, USA supported this research (AP & JT). HC was partially funded by the PKD Foundation. AJR is the recipient of a PKD Foundation postdoctoral fellowship.
References
- 1.Dustin, P.: Microtubules. Springer, Berlin (1984)
- 2.Inclán, Y., Nogales, E.: Potential for self-assembly and microtubule interaction of α-, β- and γ-tubulin. J. Cell Sci. 114, 413–422 (2000) [DOI] [PubMed]
- 3.Li, H., DeRosier, D.J., Nicholson, W.V., Nogales, E., Downing, K.H.: Microtubule structure at 8 Angstrom resolution. Structure 10, 1317–1328 (2002). doi:10.1016/S0969-2126(02)00827-4 [DOI] [PubMed]
- 4.Desai, A., Mitchison, T.J.: Microtubule polymerization dynamics. Annu. Rev. Dev. Biol. 13, 83–117 (1997). doi:10.1146/annurev.cellbio.13.1.83 [DOI] [PubMed]
- 5.Nogales, E.: Structural insights into microtubule function. Annu. Rev. Biochem. 69, 277–302 (2000). doi:10.1146/annurev.biochem.69.1.277 [DOI] [PubMed]
- 6.Minoura, I., Muto, E.: Dielectric measurement of individual microtubules using the eletroorientation method. Biophys. J. 90, 3739–3748 (2006). doi:10.1529/biophysj.105.071324 [DOI] [PMC free article] [PubMed]
- 7.Van den Heuvel, M.G., de Graaf, M.P., Dekker, C.: Molecular sorting by electrical steering of microtubules in kinesin-coated channels. Science 312, 910–914 (2006). doi:10.1126/science.1124258 [DOI] [PubMed]
- 8.Vassilev, P., Kanazirska, M., Tien, H.T.: Intermembrane linkage mediated by tubulin. Biochem. Biophys. Res. Commun. 126, 559–565 (1985). doi:10.1016/0006-291X(85)90642-4 [DOI] [PubMed]
- 9.Stracke, R., Bohm, K.J., Wollweber, L., Tuszynski, J.A., Unger, E.: Analysis of the migration behaviour of single microtubules in electric fields. Biochem. Biophys. Res. Commun. 293, 602–609 (2002). doi:10.1016/S0006-291X(02)00251-6 [DOI] [PubMed]
- 10.Priel, A., Ramos, A.J., Tuszynski, J.A., Cantiello, H.F.: A biopolymer transistor: electrical amplification by microtubules. Biophys. J. 90, 4639–4643 (2006). doi:10.1529/biophysj.105.078915 [DOI] [PMC free article] [PubMed]
- 11.Patton, C., Thompson, S., Epel, D.: Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium 35, 427–431 (2004). doi:10.1016/j.ceca.2003.10.006 [DOI] [PubMed]
- 12.O’Brien, E.T., Salmon, E.D., Erickson, H.P.: How calcium causes microtubule depolymerization. Cell Motil. Cytos. 36, 125–135 (1997). doi:10.1002/(SICI)1097-0169(1997)36:2<125::AID-CM3>3.0.CO;2–8 [DOI] [PubMed]
- 13.Regehr, W.G., Tank, D.W.: Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J. Neurosci. 12(11), 4202–4223 (1992) [DOI] [PMC free article] [PubMed]
- 14.Zarkovic, M., Henquin, J.C.: Synchronization and entrainment of cytoplasmic Ca2 + oscillations in cell clusters prepared from single or multiple mouse pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 287, E340–E347 (2004). doi:10.1152/ajpendo.00069.2004 [DOI] [PubMed]
- 15.Hallaq, H.A., Haupert Jr., G.T.: Positive inotropic effects of the endogenous Na + /K + -transporting ATPase inhibitor from the hypothalamus. Proc. Natl. Acad. Sci. USA 86, 10080–10084 (1989). doi:10.1073/pnas.86.24.10080 [DOI] [PMC free article] [PubMed]
- 16.Calaghan, S.C., Le Guennec, J.Y., White, E.: Cytoskeletal modulation of electrical and mechanical activity in cardiac myocytes. Prog. Biophys. Mol. Biol. 84, 29–59 (2004). doi:10.1016/S0079-6107(03)00057-9 [DOI] [PubMed]
- 17.Karr, T.L., Kristofferson, D., Purich, D.L.: Calcium ion induces endwise depolymerization of bovine brain microtubules. J. Biol. Chem. 255, 11853–11856 (1980) [PubMed]
- 18.Astier, Y., Bayley, H., Howorka, S.: Protein components for nanodevices. Curr. Opin. Chem. Biol. 9, 576–584 (2005) [DOI] [PubMed]
- 19.Vizcarra, C.L., Mayo, S.L.: Electrostatics in computational protein design. Curr. Opin. Chem. Biol. 9, 622–626 (2005) [DOI] [PubMed]
- 20.Sheetz, M.P., Steuer, E.R., Schroer, T.A.: The mechanism and regulation of fast axonal transport. Trends Pharmacol. Sci. 12, 474–478 (1989) [DOI] [PubMed]
- 21.Baas, P.W., Deitch, J.S., Black, M.M., Banker, G.A.: Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA 85, 8335–8339 (1988). doi:10.1073/pnas.85.21.8335 [DOI] [PMC free article] [PubMed]
- 22.Burton, P.R.: Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res. 473, 107–115 (1988). doi:10.1016/0006-8993(88)90321-6 [DOI] [PubMed]
- 23.Brady, S.T., Lasek, R.J., Allen, R.D.: Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 5, 81–101 (1985). doi:10.1002/cm.970050203 [DOI] [PubMed]
- 24.McNiven, M.A., Ward, J.B.: Calcium regulation of pigment transport in vitro. J. Cell Biol. 106, 111–125 (1988). doi:10.1083/jcb.106.1.111 [DOI] [PMC free article] [PubMed]
- 25.Smith, R.S., Bisby, M.A. (eds.): Axonal Transport. In: Neurology and Neurobiology, vol. 25, pp. 311–26. Alan R. Liss, New York (1987)
- 26.Tyner, K.M., Kopelman, R., Philbert, M.A.: “Nanosized voltmeter” enables cellular-wide electric field mapping. Biophys. J. 93, 1163–1174 (2007). doi:10.1529/biophysj.106.092452 [DOI] [PMC free article] [PubMed]