Abstract
Abnormal DNA content in tumor cells represents large scale chromosomal alterations and reflects later changes of genetic instability. Her-2/neu oncogene is amplified in 20% to 30% of breast and ovarian cancer patients and is associated with poor prognosis. Therefore, we evaluated prognostic value of Her-2/neu expression and DNA content measurements in 252 clinically localized PCa patients with long-term follow-up after radical prostatectomy for progression, metastasis and PCa-specific death. Her-2/neu expression was determined by immunohistochemistry and DNA content measurements employed Feulgen-stained cancer nuclei captured using static image cytometry system. Cox proportional hazard regression & Kaplan Meir plots were used to identify significant prognostic factors for progression, metastasis and PCa-specific death. The proportions of Her-2/neu positive and high %DNA index tumors significantly increased from non-progressor to progressors without metastasis to progressors with metastasis (p<0.0001; <0.0001). Further, the proportions of Her-2/neu positive and high %DNA index tumors significantly increased from patients who died from another cause without progression to those who died from another cause with progression to those died with PCa-specific death (p=0.027; <0.0001). Her-2/neu expression and %DNA index were significant prognosticators for progression (p≤0.001), metastasis (p≤0.01) PCa-specific death (p≤0.04) in univariate analyses. Multivariately, Her-2/neu expression and %DNA index were also significant for progression (p=0.001), metastasis (p=0.001), and PCa-specific death (p=0.02). When all other clinicopathologic information is available, the increment in concordance index by addition of either Her-2/neu or DNA index was ∼2% and of both biomarkers was ∼3% for progression, metastasis and PCa-specific death free survival models. Therefore, patients with Her-2/neu positive and high %DNA index are at a higher risk for disease progression, metastasis and PCa-specific death. Further, Her-2/neu expression and %DNA index may be used with clinicopathologic parameters for prediction of long-term prognosis in PCa.
INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer death among men in the United States, with an anticipated with an anticipated 186,320 newly diagnosed cases and 28,660 deaths in 2008 1. The majority of men with clinically localized PCa are treated with radical prostatectomy which provides excellent cancer control and minimal surgical complications. In a series of 1,997 patients treated with radical prostatectomy at Johns Hopkins Hospital, Pound et al. 2 identified 304 men who developed PSA recurrence (15%) and were monitored without hormone therapy prior to demonstration of metastasis. Of the 304 men, 34% developed distant metastases over a median period of 8 years from the time of the first postoperative PSA elevation. An algorithm was developed by combining Gleason score, PSA doubling time and time to biochemical progression to predict actuarial metastasis-free survival. Subsequently, Han et al. 3updated this study cohort and reported 360 recurrences (17%) out of 2,091 men with PCa and they used three preoperative or post-operative parameters to create nomograms to assess biochemical recurrence free survival probabilities.
Another major dilemma for PCa management is over diagnosis and over treatment due to screen-detected PCa cases that are low stage, low grade and low volume tumors 4, 5. The current prognostic tools [e.g. Kattan nomogram 6 and Partin Tables7] rely exclusively on clinical and pathologic parameters. While these nomograms provide useful prediction tools of clinical states and outcome, the need for further refinement of risk stratification has been acknowledged 8.
In the search of new biomarkers to predict prognosis of men with PCa, several potential serologic and histologic biomarkers have been evaluated 9-13. At the tissue level, Gleason grade and extent of disease (stage) are predictors of progression and metastasis in men with PCa 14, 15. Likewise, DNA ploidy measured using a semi-automated, computer-assisted imaging system can detect abnormal DNA content representing large scale chromosomal alterations (i.e., tetraploidy, aneuploidy, hyperploidy, etc.) and reflect later stage changes of genetic instability in tumor cells 16. The gene encoding the Her-2/neu oncoprotein is amplified in 20 % to 30% of breast and ovarian cancer patients and is associated with poor prognosis 17-19. Our group previously in a cohort of 124 patients with mean follow-up duration of 6.6 years reported that Her-2/neu expression is a predictor of PCa progression 20. However, prognostic value of Her-2/neu expression in PCa is controversial, and most studies have limited number of cases with short-term followup 21-27. The current study utilizing PCa tissue sections has assessed the prognostic value of Her-2/neu expression and DNA content for prediction of progression, metastasis and PCa-specific death after radical prostatectomy in 252 men with mean follow-up duration of 15.3 years.
MATERIAL AND METHODS
Patients Sample
Five μm paraffin embedded tissue sections were cut sequentially from the archival radical prostatectomy blocks selected by a single pathologist (J.I.E.) of 252 clinically localized PCa patients treated by a single surgeon (P.C.W) at the Johns Hopkins Hospital during 1980-94. Sequential sections were stained with H & E, Feulgen and monoclonal antibody (Ab3) to Her-2/neu oncogene. Progression was defined as a rise in postoperative PSA of >0.2ng/ml and/or needle biopsy proved local or distant failure, and/or metastatic surveys by bone scan. A positive bone scan result or histologic evidence of distant failure was used for the diagnosis of distant metastasis. PCa-specific death was defined as death in patients with metastasis showing any progression following hormonal therapy. No patient received neoadjuvant therapy and adjuvant hormonal therapy prior to documented distant metastasis. All patients were followed-up every 3 months for the first year, semiannually for the second year and annually thereafter as described previously 2. Table 1 shows a summary of the information available in this patient cohort. The interpretation of the pathological specimen was done by a single pathologist (J.I.E.) at the Johns Hopkins Hospital. Of these 252 patients, 121, 110 and 21 patients presented with Gleason score < 7, 7 and > 7, respectively. As the number of cases with Gleason score > 7 were few, this category was combined with Gleason score 7 to form Gleason score ≥ 7 for analyses. The numbers of patients with clinical stage T1b, T1c, T2a, T2b and T2c were 2, 11, 95, 114 and 30, respectively. As the number of cases with T1b and T1c were few, these two categories were merged and considered as T1b/c.
Table 1.
Prostate Cancer Patients Demographic (N = 252)
| Variable | Progression | Metastasis | PCa specific Death | |||
|---|---|---|---|---|---|---|
| No (N = 115) | Yes (N = 137) | No (N = 192) | Yes (N = 60) | No (N = 216) | Yes (N = 36) | |
| Age | 58.8 ± 6.5 | 60.4 ± 6.4 | 59.5 ± 6.5 | 60.3 ± 6.5 | 59.68 ± 6.4 | 59.58 ± 7.1 |
| Gleason Score (%) | ||||||
| <7 | 76 (66.1) | 45 (32.8) | 107 (55.7) | 14 (23.3) | 114 (52.8) | 7 (19.4) |
| ≥7 | 39 (33.9) | 92 (67.2) | 85 (44.3) | 46 (76.7) | 102 (47.2) | 29 (80.6) |
| TNM Stage (%) | ||||||
| T1b/c | 10 (8.7) | 3 (2.2) | 12 (6.3) | 1 (1.7) | 13 (6.0) | |
| T2a | 32 (27.8) | 63 (46.0) | 69 (35.9) | 26 (43.3) | 81 (37.5) | 14 (38.9) |
| T2b | 56 (48.7) | 58 (42.3) | 87 (45.3) | 27 (45.0) | 96 (44.5) | 18 (50.0) |
| T2c | 17 (14.8) | 13 (9.5) | 24 (12.5) | 6 (10.0) | 26 (12.0) | 4 (11.1) |
| Organ Confined (%) | ||||||
| Yes | 41 (35.7) | 19 (13.9) | 51 (26.6) | 9 (15.0) | 54 (25.0) | 6 (16.7) |
| No | 74 (64.3) | 118 (86.1) | 141 (73.4) | 51 (85.0) | 162 (75.0) | 30 (83.3) |
| Focal Extra-Prostatic Extension (%) | ||||||
| Absent | 46 (40.0) | 33 (24.1) | 66 (34.4) | 13 (21.7) | 71 (32.9) | 8 (22.2) |
| Present | 69 (60.0) | 104 (75.9) | 126 (65.6) | 47 (78.3) | 145 (67.1) | 28 (77.8) |
| Non-Focal Extra-Prostatic Extension (%) | ||||||
| Absent | 70 (60.9) | 45 (32.8) | 100 (52.1) | 15 (25.0) | 106 (49.1) | 9 (25.0) |
| Present | 45 (39.1) | 92 (67.2) | 92 (47.9) | 45 (75.0) | 110 (50.9) | 27 (75.0) |
| Surgical Margin (%) | ||||||
| Negative | 100 (87.0) | 75 (54.8) | 140 (72.9) | 35 (58.3) | 158 (73.2) | 17 (47.2) |
| Positive | 15 (13.0) | 62 (45.2) | 52 (24.1) | 25 (41.7) | 58 (26.8) | 19 (52.8) |
| Seminal Vesicle (%) | ||||||
| Negative | 109 (94.8) | 108 (78.8) | 178 (92.7) | 39 (65.0) | 193 (89.3) | 24 (66.7) |
| Positive | 6 (5.2) | 29 (21.2) | 14 (7.3) | 21 (35.0) | 23 (10.7) | 12 (33.3) |
| Lymph Node (%) | ||||||
| Negative | 113 (98.3) | 117 (85.4) | 182 (94.8) | 48 (80.0) | 204 (94.4) | 26 (72.2) |
| Positive | 2 (1.7) | 20 (14.6) | 10 (5.2) | 12 (20.0) | 12 (5.6) | 10 (27.8) |
DNA Content Analysis
A minimum of ∼125 intact, Feulgen-stained nuclei were captured from the cancer area for each patients. DNA content measurements were calculated after generating two reference points to define calibration curve using rat hepatocyte standard. Normal DNA ploidy and abnormal DNA ploidy status were defined by using our previously determined criteria 28, 29 on CAS-200 imaging system [Bacus Labs, Lombard, IL] with Cell Sheet v2.0 software generated histograms by a pathologist. Next, we used the variance of the DNA mass (pg) and DNA index of the Feulgen-stained nuclei captured from each case to generate continuous variables. DNA index was multiplied by 100 for each case and was labeled as %DNA index. These DNA content measurements i.e. DNA ploidy, DNA mass (pg DNA) and %DNA index were used for analysis and %DNA index was found to be the best among these. Hence all the data for %DNA index [range: 0.5-84%; median 8.7%] is presented as a computer-generated imaging variable in the results and discussion sections of this manuscript.
Measurement of Her-2/neu Expression
To determine Her-2/neu (c-erbB-2) expression in tissue sections, monoclonal antibody (Ab-3) provided by Oncogene Science, Inc. (Uniondale, NY) was used 20. The supersensitive MultiLinkTM kit (BioGenex Inc., San Ramon, CA), which employs the streptavidin-biotin complex alkaline phosphatase labeling method, was used for monoclonal antibody detection. All staining was performed with the MicroProbeTM manual staining system (Fisher Scientific, Pittsburgh, PA). Incubation temperature for monoclonal antibody was 40 C overnight and remaining staining methods followed the recommended procedure of the BioGenex MultiLinkTM kit. Scoring was performed in the pathologist-confirmed cancerous areas of each archival specimen in 1994-96 by two experts, with a third expert used if there was a >10% discrepancy between the results of the two experts. Interpretation of results utilized a scoring method developed by J.I.E. and categorized patients as Her-2/neu negative if there was staining in <5% of the marked tumor area, as focal Her-2/neu staining if 5-30% of the marked tumor area showed staining, and diffuse Her-2/neu staining if >30% of the marked tumor area showed staining.
Statistical Analysis
All data was analyzed using StataTM v10.0 statistical analyses software (Stata Corporation, College Station, TX). The nptrend test was used for trend across non-progressors, progressors without metastasis, and progressors with metastasis, and for trend across death due to another cause without progression, another cause with progression and PCa-specific death. We determined optimal cutoffs to dichotomize %DNA index data [≤7 vs. >7] using the classification and regression tree (CART) method. Univariate Cox proportional hazard regression was used to identify significant prognostic factors for prediction of progression free-survival, time to metastasis and time to PCa-specific death. Harrell's C concordance index (c index) was calculated for all significant variables. Ties were handled by the Breslow method. The proportional hazard assumption was verified by examination of residual plots. Univariately significant variables were further assessed using in multivariate Cox regression. Logrank and Logrank trend test were used to test equality of survivor functions across two groups and to test trend of the survivor function across three or more ordered groups respectively. Statistical significance in this study was set as p ≤ 0.050.
RESULTS
The demographic, clinical and pathologic information of cohort are shown in Table 1. Of the 137 patients with evidence of disease progression, 60 (43.8%) showed evidence of distant metastasis and 36 (26.3%) died due to PCa. Mean follow-up time after radical prostatectomy for all patients was 15.3 years (range: 1-25 years, median: 17 years), with approximately 83% (209/252) patients having ≥10 years of follow-up. Although the median time to progression was 12 years, 11 % (28 of 252) and 3.5% (9 of 252) patients developed PCa progression at ≥10 years and ≥15 years of follow-up, respectively..
The proportions of Her-2/neu positive tumors significantly increased from non-progressors (53%; 61 of 115) to progressors without metastasis (70%; 54 of 77) to progressor with metastasis (87%; 52 of 60) (p < 0.0001). The proportions of high %DNA index tumors also significantly increased from non-progressor (41%; 47 of 115) to progressor without metastasis (69%; 53 of 77) to progressor with metastasis (80%; 48 of 60) (p < 0.0001). Further, the proportions of Her-2/neu positive tumors significantly increased from patients who died from another cause without progression (62%; 18 of 29) to those who died from another cause with progression (72%; 16 of 22) to those who died a PCa-specific death (86%; 31 of 36) (p = 0.027). Similarly, the proportions of high %DNA index tumors significantly increased from patients who died from another cause without progression (38%; 11 of 29) to those who died from another cause with progression (64%; 14 of 22) to those who died due to a PCa-specific death (83%; 30 of 36) (p <0.0001).
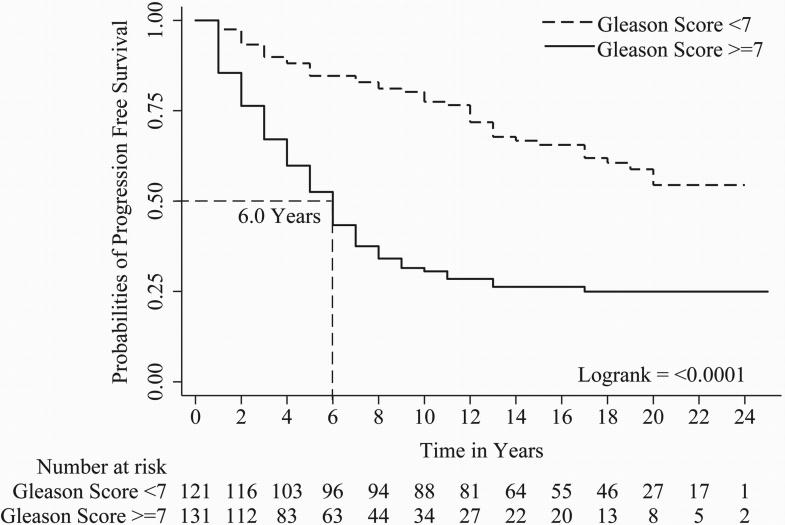
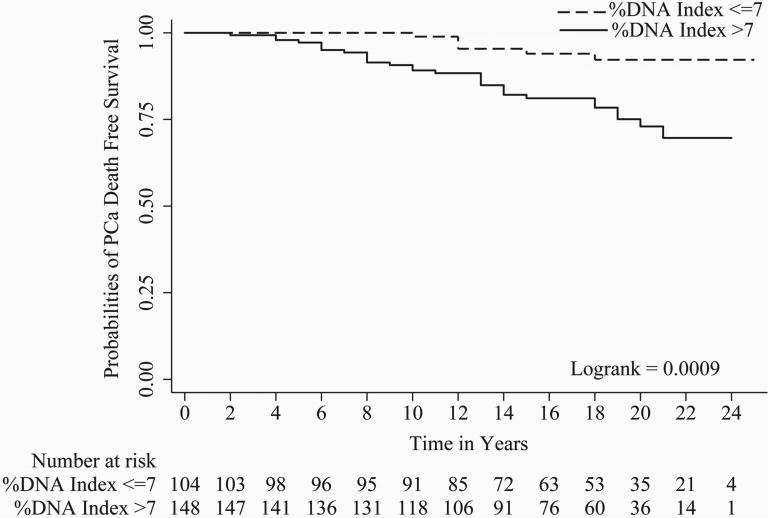
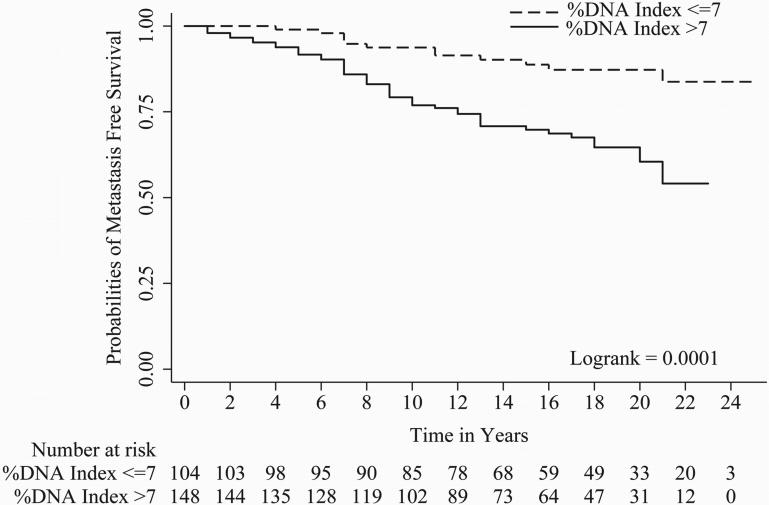
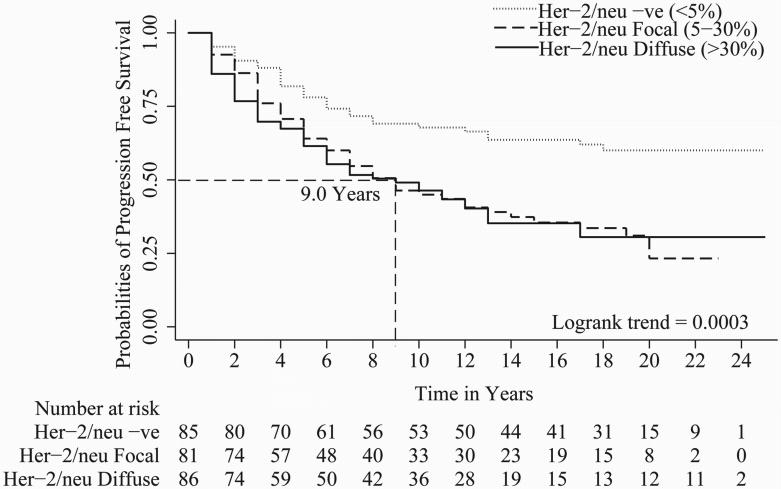
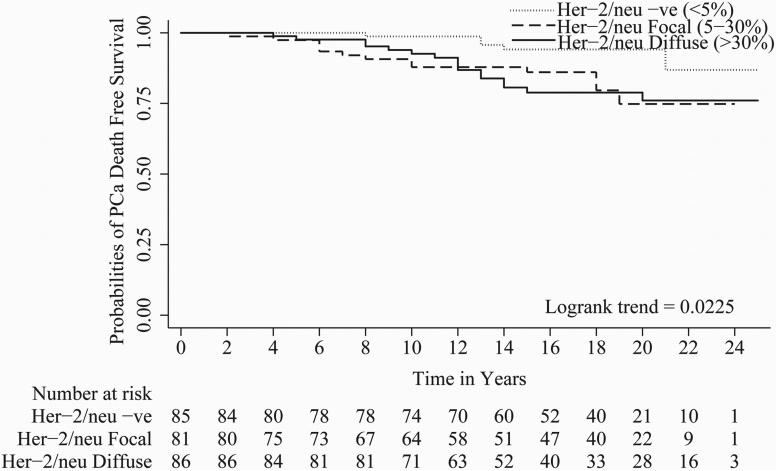
In univariate Cox regression, all parameters evaluated except tumor stage i.e. Gleason score (c index = 0.67), organ confined status (c index = 0.58), focal extra-prostatic extension (c index = 0.55), non-focal extra-prostatic extension (c index = 0.62), surgical margin status (c index = 0.62), seminal vesicle involvement (c index = 0.57), lymph node involvement (c index = 0.56), Her-2/neu expression (c index = 0.59), and %DNA index (c index = 0.62) were statistically significant predictors of PCa progression-free survival (Table 2). For metastasis-free survival, Gleason score (c index = 0.71), organ confined status (c index = 0.57), focal extra-prostatic extension (c index = 0.57), non-focal extra-prostatic extension (c index = 0.65), surgical margin status (c index = 0.58), seminal vesicle involvement (c index = 0.63), lymph node involvement (c index = 0.60), Her-2/neu expression (c index = 0.62), and %DNA index (c index = 0.65) were statistically significant predictors in univariate analyses (Table 2). For time to PCa-specific death, Gleason score (c index = 0.70), non-focal extra-prostatic extension (c index = 0.63), surgical margin status (c index = 0.62), seminal vesicle involvement (c index = 0.62), lymph node involvement (c index = 0.63), Her-2/neu expression (c index = 0.62), and %DNA index (c index = 0.67) were statistically significant predictors in univariate analyses (Table 2). Please note, %DNA index when assessed as a continuous predictive marker for progression free survival [1.02 (1.01 - 1.04), p<0.0001], metastasis free survival [1.03 (1.02 - 1.05), p<0.0001] and PCa-specific death [1.04 (1.02 - 1.06), p<0.0001] was also significant. Kaplan-Meier survival curves for progression-free survival; metastasis-free survival and time to PCa-specific death are presented in Figures 1, 2 and 3 of Gleason score, %DNA index and Her-2/neu expression, respectively. As seen in Figures 3A, 3B, and 3C, the survival curves for focal and diffuse Her-2/neu expression were similar, so for the multivariate analyses, these two categories were combined and Her-2/neu was categorized as positive or negative.
Table 2.
Univariate Cox Proportional Hazards Regression analysis
| Variable | N | Progression | Metastasis | PCa specific Death | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | ||
| Gleason Score | |||||||
| <7 | 121 | 1.00 | <0.0001* | 1.00 | <0.0001* | 1.00 | <0.0001* |
| ≥7 | 131 | 3.12 (2.17-4.49) |
4.28 (2.34-7.85) |
5.08 (2.21-11.66) |
|||
| TNM Stage | |||||||
| T1b/c | 13 | 1.00 | 1.00 | ***** | |||
| T2a | 95 | 3.48 (1.10-11.1) |
0.035 | 2.94 (0.40-21.7) |
0.290 | ||
| T2b | 114 | 2.55 (0.8-8.14) |
0.114 | 2.87 (0.39-21.17) |
0.301 | ||
| T2c | 30 | 2.36 (0.67-8.31) |
0.179 | 2.67 (0.32-22.24) |
0.362 | ||
| Organ Confined | |||||||
| Yes | 60 | 1.00 | <0.0001* | 1.00 | 0.037* | 1.00 | 0.178 |
| No | 192 | 2.59 (1.59-4.21) |
2.13 (1.05-4.32) |
1.83 (0.76-4.39) |
|||
| Focal Extra-Prostatic Extension | |||||||
| No | 79 | 1.00 | 0.008* | 1.00 | 0.042* | 1.00 | 0.155 |
| Yes | 173 | 1.70 (1.15-2.52) |
1.89 (1.02-3.49) |
1.77 (0.80-3.88) |
|||
| Non-Focal Extra-Prostatic Extension | |||||||
| No | 115 | 1.00 | <0.0001* | 1.00 | <0.0001* | 1.00 | 0.005* |
| Yes | 137 | 2.35 (1.64-3.37) |
3.19 (1.77-5.73) |
2.96 (1.39-6.31) |
|||
| Surgical Margin | |||||||
| Negative | 175 | 1.00 | <0.0001* | 1.00 | 0.028* | 1.00 | 0.003* |
| Positive | 77 | 2.63 (1.87-3.69) |
1.78 (1.06-2.97) |
2.68 (1.39-5.15) |
|||
| Seminal Vesicle | |||||||
| Negative | 217 | 1.00 | <0.0001* | 1.00 | <0.0001* | 1.00 | <0.0001* |
| Positive | 35 | 2.82 (1.86-4.28) |
5.03 (2.93-8.64) |
3.79 (1.89-7.60) |
|||
| Lymph Node | |||||||
| Negative | 230 | 1.00 | <0.0001* | 1.00 | <0.0001* | 1.00 | <0.0001* |
| Positive | 22 | 3.59 (2.22-5.82) |
4.75 (2.49-9.09) |
6.90 (3.24-14.71) |
|||
| Her-2/neu | |||||||
| Negative (<5%) |
85 | 1.00 | 1.00 | 1.00 | |||
| Focal (5%-30%) |
81 | 2.17 (1.93-3.39) |
0.001* | 3.85 (1.74-8.50) |
0.001* | 3.30 (1.20-9.09) |
0.021* |
| Diffuse (>30%) |
87 | 2.21 (1.42-3.45) |
<0.0001* | 3.41 (1.54-7.55) |
0.002* | 3.28 (1.20-8.96) |
0.021* |
| %DNA Index | |||||||
| ≤7 | 104 | 1.00 | <0.0001* | 1.00 | <0.0001* | 1.00 | 0.002* |
| >7 | 148 | 2.69 (1.83-3.94) |
3.32 (1.76-6.26) |
3.94 (1.64-9.47) |
|||
Significant
No event in T1b/c
Figure 1.
A, B and C shows Kaplan-Meier curves of Gleason score [<7 and ≥7] for progression, metastasis and PCa-specific death.
Figure 2.
A, B and C shows Kaplan-Meier curves of %DNA Index [≤7 and >7] for progression, metastasis and PCa-specific death.
Figure 3.
A, B and C shows Kaplan-Meier curves of Her-2/neu expression [Negative staining (<5%), focal staining (5-30%) and diffuse staining (>30%)] for progression, metastasis and PCa-specific death. Focal and diffuse Her-2/neu expression have similar hazards ratio and Kaplan-Meier survival curves for progression, metastasis and PCa-specific death
When all univariately significant variables were evaluated using multivariate Cox regression, Gleason score, surgical margin status, %DNA index and Her-2/neu expression were multivariately significant predictors of progression-free survival (Table 3). For metastasis-free survival, Gleason score, %DNA index and Her-2/neu expression were multivariately significant (Table 3). Gleason score, lymph node involvement, %DNA index and Her-2/neu expression were multivariately significant for prediction of time to PCa-specific death (Table 3). Both %DNA index and Her-2/neu expression were multivariately significant parameters for progression, metastasis, PCa-specific death free survivals (Table 3).
Table 3.
Multivariate Cox Proportional Hazards analysis
| Variable | N | Progression | Metastasis | PCa-specific Death | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | ||
| Gleason Score | |||||||
| <7 | 121 | 1.00 | <0.0001* | 1.00 | 0.019* | 1.00 | 0.022* |
| ≥7 | 131 | 2.40 (1.60-3.57) |
2.24 (1.14-4.40) |
2.96 (1.17-7.49) |
|||
| Organ Confined | |||||||
| Yes | 60 | 1.00 | 0.192 | 1.00 | 0.156 | ||
| No | 192 | 1.73 (0.76-3.97) |
0.34 (0.07-1.51) |
||||
| Focal Extra-Prostatic Extension | |||||||
| No | 79 | 1.00 | 0.409 | 1.00 | 0.238 | ||
| Yes | 173 | 0.79 (0.44-1.39) |
1.88 (0.67-5.42) |
||||
| Non-Focal Extra-Prostatic Extension | |||||||
| No | 115 | 1.00 | 0.539 | 1.00 | 0.063 | 1.00 | 0.589 |
| Yes | 137 | 1.16 (0.72-1.86) |
2.37 (0.95-5.87) |
1.28 (0.53-3.10) |
|||
| Surgical Margin | |||||||
| Negative | 175 | 1.00 | 0.001* | 1.00 | 0.501 | 1.00 | 0.057 |
| Positive | 77 | 1.91 (1.31-2.81) |
1.21 (0.68-2.16) |
1.98 (0.98-4.02) |
|||
| Seminal Vesicle | |||||||
| Negative | 217 | 1.00 | 0.849 | 1.00 | 0.056 | 1.00 | 0.994 |
| Positive | 35 | 1.05 (0.65-1.70) |
1.87 (0.98-3.54) |
1.00 (0.44-2.25) |
|||
| Lymph Node | |||||||
| Negative | 230 | 1.00 | 0.078 | 1.00 | 0.061 | 1.00 | 0.003* |
| Positive | 22 | 1.62 (0.94-2.78) |
1.96 (0.97-3.97) |
3.57 (1.54-8.30) |
|||
| Her-2/neu | |||||||
| Negative | 85 | 1.00 | 0.001* | 1.00 | 0.001* | 1.00 | 0.012* |
| Positive | 167 | 2.06 (1.36-3.10) |
3.57 (1.68-7.59) |
3.39 (1.30-8.83) |
|||
| %DNA Index | |||||||
| ≤7 | 104 | 1.00 | <0.0001* | 1.00 | 0.014* | 1.00 | 0.039* |
| >7 | 148 | 2.23 (1.49-3.32) |
2.29 (1.18-4.42) |
2.60 (1.04-6.45) |
|||
Significant
Next, a bootstrap resampling procedure employing 200 replications was used to perform backward stepwise Cox analyses. The goal was to identify important parameters for progression, metastasis, and PCa-specific death free survivals. Each variable in the replicated models were counted with significance level of Pe ≤0.01 for a variable that entered the model and Pr ≤0.05 (variable selection cut-off) to remain in the model. Table 4 shows inclusion frequencies of all evaluated parameters for progression, metastasis and PCa-specific death free survivals. Using a cut-off criterion of at least 50% inclusion frequency, Gleason score, surgical margin, lymph node involvement, Her-2/neu expression and %DNA index yielded a model with c index of 0.76 for progression free survival prediction. For metastasis free survival prediction, Gleason score, non-focal extra-prostatic extension, lymph node involvement, seminal vesicle involvement, Her-2/neu expression and %DNA index resulted a model with c index of 0.80. Gleason score, surgical margin, lymph node involvement, Her-2/neu expression and %DNA index yielded a model with c index of 0.81 for PCa-specific death free survival prediction. Of note, Gleason score, lymph node involvement, Her-2/neu expression and %DNA index had inclusion frequencies ≥50% for all three endpoint i.e. progression, metastasis, and PCa-specific death free survivals predictions. The increment in c-index for progression, metastasis and PCa-specific death free survival models by addition of either Her-2/neu or DNA index was ∼2% and of both biomarkers was ∼3%.
Table 4.
Inclusion Frequencies of Evaluated parameters
| Variable | Progression | Metastasis | PCa-specific Death |
|---|---|---|---|
| Gleason Score | 100 | 78 | 69 |
| TNM Stage | 11 | 9.5 | 12.5 |
| Organ Confined | 37.5 | 18.5 | 15 |
| Focal Extra-Prostatic Extension | 16 | 17.5 | 8.5 |
| Non-Focal Extra-Prostatic Extension | 17.5 | 52.5 | 14 |
| Surgical Margin | 94.5 | 12 | 61.5 |
| Seminal Vesicle | 5 | 56 | 7.5 |
| Lymph Node | 50 | 59 | 82.5 |
| Her-2/neu | 95.5 | 94.5 | 76.5 |
| %DNA Index | 98 | 78.5 | 67 |
Preoperative PSA information was able for 150 cases only because of lack of availability of PSA assay during early 80s since the PSA was not FDA approved until 1987 and then became routine in the 1990s. In this subset of 150 cases, PSA (0-4, 4.1-10 and >10ng/ml) was not a significant predictor of progression-free survival (p = 0.671; 0.021), metastasis-free survival (p = 0.624; 0.072) or time to PCa-specific death (p = 0.693; 0.633) in univariate Cox regression analyses (data not shown).
DISCUSSION
Slamon et al. 17 were the first to show that Her-2/neu gene amplification and protein overexpression is associated with an increase risk of relapse and death in patients with breast cancer. Since then several investigators 21-27, 30-41 have explored the role of Her-2/neu oncogene in PCa progression and metastasis.
In the current study, we demonstrated that the proportions of Her-2/neu positive and high %DNA index tumors significantly increased from non-progressors to progressors without metastasis to progressors with metastasis. Further, the proportions of Her-2/neu positive and high %DNA index tumors were significantly increased from patients who died from another cause without progression to those who died from another cause with progression to those who died with PCa-specific death. Her-2/neu expression and %DNA index were significant predictors of progression-free survival, metastasis-free survival and time to PCa-specific death (Table 2, Figures 2and 3). Survival curves for patients with focal (5-30%) and diffuse (>30%) Her-2/neu protein expressions overlapped and were not significantly different (Figures 3A, 3B, and 3C). Both Her-2/neu expression and %DNA index were multivariately significant for the prediction of progression-free survival, metastasis-free survival and time to PCa-specific death (Table 3). Of note, Gleason score, lymph node involvement, Her-2/neu expression and %DNA index had inclusion frequencies ≥50% for all three endpoint i.e. progression, metastasis, and PCa-specific death free survivals predictions (Table 4). However, when all other clinicopathologic information is available, the increment in c-index by addition of either Her-2/neu or DNA index was only ∼2% and of both biomarkers was ∼3% for progression, metastasis and PCa-specific death free survival models. Clearly the importance of these two biomarkers in PCa pathogenesis have been demonstrated and the role they may be playing in the disease progression should not be minimized 12-13, 16, 20-25.
Myers et al. 42 suggested that increased expression and changes in the subcellular distribution of Her-2/neu is an early event in the development and progression of PCa. Signoretti et al. 24 showed that PCa progression towards androgen independence is characterized by increased Her-2/neu expression by tumor cells. However, others have found no increase or decrease in Her-2/neu expression in androgen independent tumors 21, 22, 30. The difference in the results may be due to technical assay differences, including variability in tissue fixation protocols, antibodies to different Her-2/neu epitopes, antibody production species, lack of standard operating immunohistochemistry protocols, and different scoring methodologies. Studies on Her-2/neu amplification at the molecular level showed that amplification of this gene in PCa is a rare event 22-24. However, Her-2/neu protein overexpression in PCa tissue sections have been shown to be associated with significantly decreased survival for androgen independent tumors 30, 31.
The extracellular domain of Her-2/neu is frequently cleaved and released into the circulation, where they can be detected by enzyme linked immunosorbant assays (ELISA). Similar to tissue levels, circulating levels of Her-2/neu have been associated with disease progression and aggressive clinical outcome in PCa 32, 33. Also, circulating levels of Her-2/neu have been shown to correlate with gene amplification and tissue Her-2/neu protein overexpression 33, 34.
Her-2/neu overexpression promotes the activation of the androgen receptor (AR), enhances the binding of AR to the promoters of androgen-regulated genes and protects AR from proteasome-mediated degradation 35, 36. Her-2/neu induces mitogen activated protein kinase (MAPK) and phosphatidylinositol 3 kinase (PI3K)-AKT pathways and promotes androgen-independent PCa cell proliferation and survival 37, 38. Casimiro et al. 39 showed that cyclin D1 is a target of Her-2/neu in both androgens dependent and independent PCa which is critical for controlling progression through G1 to S phase of the cell cycle. Her-2/neu is also an important component of IL-6 signaling through MAPK pathways which regulate the growth of many tumors including PCa 43. Further, Her-2/neu induce nuclear factor-κB nuclear activity through PI3K-AKT pathway 40, which may represent prognostic indicator of tumor grade 41.
Notably, the Her-2/neu monoclonal antibody and small molecule kinase inhibitors have failed to show significant activity in PCa trials to date 44, 45. Insufficient target inhibition, not used in combination with the other agents, rare Her-2/neu gene amplification, and commonly occurring PTEN gene loss in PCa are among the reasons cited for failure of these therapeutic modalities 46.
Several investigators have assessed prognostic value of DNA content of tumors for PCa progression and metastasis 16, 20, 47-57. Tumors with normal DNA content are slow growing, less likely to disseminate, have better prognosis than non-diploid tumors and have independent prognostic value for PCa progression prediction 16, 20, 47-53. Tribukait et al. 53 in a prospective study evaluated 287 patients that were under active surveillance and 309 hormonally treated patients with minimum of 10 years of follow-up showed that nuclear DNA ploidy have independent prognostic value, particularly in low-grade, low-stage tumors in which other known variables did not provide any prognostic value. However, several investigators 54-57 have raised doubt about independent prognostic value of DNA ploidy in presence of routine clinicopathologic parameters. In our cohort, DNA ploidy assessed by histogram interpretation by a pathologist was significant univariately for progression, metastasis and PCa-specific death free survivals prediction. DNA ploidy became insignificant when it was considered with other clinicopathologic parameters in multivariate models for progression, metastasis and PCa-specific death free survival prediction, (data not shown). Although %DNA index was significant both univariately and multivariately for progression, metastasis and PCa-specific death free survival predictions, but the actual increment in c-index by its addition in multivariate models was only ∼2%. The importance of %DNA index in PCa pathogenesis can be realized by the fact that it maintained inclusion frequency ≥ 50% for all three end points i.e. progression, metastasis, and PCa-specific death free survival prediction.
In conclusion, clinically localized PCa patients with Her-2/neu positive and high %DNA index are at a higher risk for disease progression, metastasis and PCa-specific death. Further, Her-2/neu expression and %DNA index can be used with clinicopathologic parameters for prediction of long-term prognosis in PCa.
Acknowledgements
Funding for this project was provided by The Johns Hopkins University Prostate Cancer SPORE (Grant number: P50CA58236), Early Detection Research Network (EDRN) NCI/NIH (Grant number CA086323-06), Prostate Cancer Foundation, and the Patana Fund.
Reference
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. The Journal of urology. 2003;169:517–23. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 4.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. Journal of the National Cancer Institute. 2006;98:355–7. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, Epstein JI. Expectant Management of Prostate Cancer With Curative Intent: An Update of The Johns Hopkins Experience. The Journal of urology. 2007 doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr., Dotan ZA, DiBlasio CJ, Reuther A, Klein EA, Kattan MW. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–12. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, Partin AW. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Smith A, Kattan MW, Satagopan J, Reuter VE, Scardino PT, Gerald WL. Integration of gene expression profiling and clinical variables to predict prostate carcinoma recurrence after radical prostatectomy. Cancer. 2005;104:290–8. doi: 10.1002/cncr.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–64. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 10.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. The New England journal of medicine. 2003;349:366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex Biomarker Approach for Determining Risk of Prostate-Specific Antigen-Defined Recurrence of Prostate Cancer. Journal of the National Cancer Institute. 2003;95:661–68. doi: 10.1093/jnci/95.9.661. [DOI] [PubMed] [Google Scholar]
- 12.Veltri RW. Molecular biology of serum biomarkers of prostate cancer. In: Kirby RS, Partin AW, Feneley MR, Parsons JK, editors. Prostate cancer: Principles and Practiceed. Taylor & Francis; London & New York: 2006. pp. 269–84. [Google Scholar]
- 13.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10:3943–53. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 14.Gleason DF. Histologic grading of prostate cancer: a perspective. Human pathology. 1992;23:273–9. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Allsbrook WC, Jr., Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American journal of surgical pathology. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael MJ, Veltri RW, Partin AW, Miller MC, Walsh PC, Epstein JI. Deoxyribonucleic acid ploidy analysis as a predictor of recurrence following radical prostatectomy for stage T2 disease. The Journal of urology. 1995;153:1015–9. [PubMed] [Google Scholar]
- 17.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Science. Vol. 235. New York, N.Y: 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene; pp. 177–82. [DOI] [PubMed] [Google Scholar]
- 18.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Science. Vol. 244. New York, N.Y: 1989. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer; pp. 707–12. [DOI] [PubMed] [Google Scholar]
- 19.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer research. 1990;50:4087–91. [PubMed] [Google Scholar]
- 20.Veltri RW, Partin AW, Epstein JE, Marley GM, Miller CM, Singer DS, Patton KP, Criley SR, Coffey DS. Quantitative nuclear morphometry, Markovian texture descriptors, and DNA content captured on a CAS-200 Image analysis system, combined with PCNA and HER-2/neu immunohistochemistry for prediction of prostate cancer progression. Journal of cellular biochemistry. 1994;19:249–58. [PubMed] [Google Scholar]
- 21.Savinainen KJ, Saramaki OR, Linja MJ, Bratt O, Tammela TL, Isola JJ, Visakorpi T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. The American journal of pathology. 2002;160:339–45. doi: 10.1016/S0002-9440(10)64377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo BF, Levine AM, Marcos M, Collins QF, Iacocca MV, Caskey LS, Gregory CW, Lin Y, Whang YE, Earp HS, Mohler JL. Human epidermal receptor-2 expression in prostate cancer. Clin Cancer Res. 2003;9:1087–97. [PubMed] [Google Scholar]
- 23.Reese DM, Small EJ, Magrane G, Waldman FM, Chew K, Sudilovsky D. HER2 protein expression and gene amplification in androgen-independent prostate cancer. American journal of clinical pathology. 2001;116:234–9. doi: 10.1309/VXKK-YVRH-9B11-YDPT. [DOI] [PubMed] [Google Scholar]
- 24.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. Journal of the National Cancer Institute. 2000;92:1918–25. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 25.Sadasivan R, Morgan R, Jennings S, Austenfeld M, Van Veldhuizen P, Stephens R, Noble M. Overexpression of Her-2/neu may be an indicator of poor prognosis in prostate cancer. The Journal of urology. 1993;150:126–31. doi: 10.1016/s0022-5347(17)35413-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz S, Jr., Caceres C, Morote J, De Torres I, Rodriguez-Vallejo JM, Gonzalez J, Reventos J. Over-expression of epidermal growth factor receptor and c-erbB2/neu but not of int-2 genes in benign prostatic hyperplasia by means of semi-quantitative PCR. International journal of cancer. 1998;76:464–7. doi: 10.1002/(sici)1097-0215(19980518)76:4<464::aid-ijc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn EJ, Kurnot RA, Sesterhenn IA, Chang EH, Moul JW. Expression of the c-erbB-2 (HER-2/neu) oncoprotein in human prostatic carcinoma. The Journal of urology. 1993;150:1427–33. doi: 10.1016/s0022-5347(17)35799-3. [DOI] [PubMed] [Google Scholar]
- 28.Badalament RA, Miller MC, Peller PA, Young DC, Bahn DK, Kochie P, O'Dowd GJ, Veltri RW. An algorithm for predicting nonorgan confined prostate cancer using the results obtained from sextant core biopsies with prostate specific antigen level. The Journal of urology. 1996;156:1375–80. [PubMed] [Google Scholar]
- 29.Veltri RW, O'Dowd GJ, Orozco R, Miller MC. The role of biopsy pathology, quantitative nuclear morphometry, and biomarkers in the preoperative prediction of prostate cancer staging and prognosis. Seminars in urologic oncology. 1998;16:106–17. [PubMed] [Google Scholar]
- 30.Hernes E, Fossa SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. British journal of cancer. 2004;90:449–54. doi: 10.1038/sj.bjc.6601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards J, Traynor P, Munro AF, Pirret CF, Dunne B, Bartlett JM. The role of HER1-HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin Cancer Res. 2006;12:123–30. doi: 10.1158/1078-0432.CCR-05-1445. [DOI] [PubMed] [Google Scholar]
- 32.Osman I, Mikhail M, Shuch B, Clute M, Cheli CD, Ghani F, Thiel RP, Taneja SS. Serum levels of shed Her2/neu protein in men with prostate cancer correlate with disease progression. The Journal of urology. 2005;174:2174–7. doi: 10.1097/01.ju.0000181205.23233.65. [DOI] [PubMed] [Google Scholar]
- 33.Domingo-Domenech J, Fernandez PL, Filella X, Martinez-Fernandez A, Molina R, Fernandez E, Alcaraz A, Codony J, Gascon P, Mellado B. Serum HER2 extracellular domain predicts an aggressive clinical outcome and biological PSA response in hormone-independent prostate cancer patients treated with docetaxel. Ann Oncol. 2007 doi: 10.1093/annonc/mdm490. [DOI] [PubMed] [Google Scholar]
- 34.Andersen TI, Paus E, Nesland JM, McKenzie SJ, Borresen AL. Detection of c-erbB-2 related protein in sera from breast cancer patients. Relationship to ERBB2 gene amplification and c-erbB-2 protein overexpression in tumour. Acta oncologica (Stockholm, Sweden) 1995;34:499–504. doi: 10.3109/02841869509094014. [DOI] [PubMed] [Google Scholar]
- 35.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nature medicine. 1999;5:280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 36.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer cell. 2004;6:517–27. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5458–63. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer research. 2000;60:6841–5. [PubMed] [Google Scholar]
- 39.Casimiro M, Rodriguez O, Pootrakul L, Aventian M, Lushina N, Cromelin C, Ferzli G, Johnson K, Fricke S, Diba F, Kallakury B, Ohanyerenwa C, et al. ErbB-2 induces the cyclin D1 gene in prostate epithelial cells in vitro and in vivo. Cancer research. 2007;67:4364–72. doi: 10.1158/0008-5472.CAN-06-1898. [DOI] [PubMed] [Google Scholar]
- 40.Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–99. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- 41.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU international. 2003;91:417–20. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 42.Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160erbB-3 and p185erbB-2 in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Journal of the National Cancer Institute. 1994;86:1140–5. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- 43.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 44.Canil CM, Moore MJ, Winquist E, Baetz T, Pollak M, Chi KN, Berry S, Ernst DS, Douglas L, Brundage M, Fisher B, McKenna A, et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol. 2005;23:455–60. doi: 10.1200/JCO.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 45.de Bono JS, Bellmunt J, Attard G, Droz JP, Miller K, Flechon A, Sternberg C, Parker C, Zugmaier G, Hersberger-Gimenez V, Cockey L, Mason M, et al. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol. 2007;25:257–62. doi: 10.1200/JCO.2006.07.0888. [DOI] [PubMed] [Google Scholar]
- 46.Solit DB, Rosen N. Targeting HER2 in prostate cancer: where to next? J Clin Oncol. 2007;25:241–3. doi: 10.1200/JCO.2006.08.8187. [DOI] [PubMed] [Google Scholar]
- 47.Badalament RA, O'Toole RV, Young DC, Drago JR. DNA ploidy and prostate-specific antigen as prognostic factors in clinically resectable prostate cancer. Cancer. 1991;67:3014–23. doi: 10.1002/1097-0142(19910615)67:12<3014::aid-cncr2820671215>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzato M, Rey D, Durlach A, Bouttens D, Birembaut P, Staerman F. DNA image cytometry on biopsies can help the detection of localized Gleason 3+3 prostate cancers. The Journal of urology. 2004;172:1311–3. doi: 10.1097/01.ju.0000139375.52611.0e. [DOI] [PubMed] [Google Scholar]
- 49.Koss LG. Localized prostate cancer and DNA ploidy. Jama. 2005;294:1207. doi: 10.1001/jama.294.10.1207-a. author reply 07-8. [DOI] [PubMed] [Google Scholar]
- 50.Adolfsson J, Ronstrom L, Hedlund PO, Lowhagen T, Carstensen J, Tribukait B. The prognostic value of modal deoxyribonucleic acid in low grade, low stage untreated prostate cancer. The Journal of urology. 1990;144:1404–6. doi: 10.1016/s0022-5347(17)39754-9. discussion 06-7. [DOI] [PubMed] [Google Scholar]
- 51.Ahlgren G, Falkmer U, Gadaleanu V, Abrahamsson PA. Evaluation of DNA ploidy combined with a cytometric proliferation index of imprints from core needle biopsies in prostate cancer. European urology. 1999;36:314–9. doi: 10.1159/000020011. [DOI] [PubMed] [Google Scholar]
- 52.Borre M, Hoyer M, Nerstrom B, Overgaard J. DNA ploidy and survival of patients with clinically localized prostate cancer treated without intent to cure. The Prostate. 1998;36:244–9. doi: 10.1002/(sici)1097-0045(19980901)36:4<244::aid-pros5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 53.Tribukait B. Nuclear deoxyribonucleic acid determination in patients with prostate carcinomas: clinical research and application. European urology. 1993;23(Suppl 2):64–76. doi: 10.1159/000474709. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Jabaloyas JM, Ruiz-Cerda JL, Hernandez M, Jimenez A, Jimenez-Cruz F. Prognostic value of DNA ploidy and nuclear morphometry in prostate cancer treated with androgen deprivation. Urology. 2002;59:715–20. doi: 10.1016/s0090-4295(02)01530-3. [DOI] [PubMed] [Google Scholar]
- 55.Sebo TJ, Cheville JC, Riehle DL, Lohse CM, Pankratz VS, Myers RP, Blute ML, Zincke H. Predicting prostate carcinoma volume and stage at radical prostatectomy by assessing needle biopsy specimens for percent surface area and cores positive for carcinoma, perineural invasion, Gleason score, DNA ploidy and proliferation, and preoperative serum prostate specific antigen: a report of 454 cases. Cancer. 2001;91:2196–204. doi: 10.1002/1097-0142(20010601)91:11<2196::aid-cncr1249>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Stamey TA. DNA ploidy status. Urology. 1995;45:563–5. doi: 10.1016/S0090-4295(99)80043-0. [DOI] [PubMed] [Google Scholar]
- 57.Thompson RH, Blute ML, Slezak JM, Bergstralh EJ, Leibovich BC. Is the GPSM scoring algorithm for patients with prostate cancer valid in the contemporary era? The Journal of urology. 2007;178:459–63. doi: 10.1016/j.juro.2007.03.124. discussion 63. [DOI] [PubMed] [Google Scholar]