Summary
How do animals integrate internal drives and external environmental cues to coordinate behaviours? We address this question studying mate-searching behaviour in C. elegans. C. elgans males explore their environment in search of mates (hermaphrodites) and will leave food if mating partners are absent. However, when mates and food coincide, male exploratory behaviour is suppressed and males are retained on the food source. We show that the drive to explore is stimulated by male specific neurons in the tail, the ray neurons. Periodic contact with the hermaphrodite detected through ray neurons changes the male’s behaviour during periods of no contact and prevents the male from leaving the food source. The hermaphrodite signal is conveyed by male-specific interneurons that are post-synaptic to the rays and that send processes to the major integrative center in the head. This study identifies key parts of the neural circuit that regulates a sexual appetitive behaviour in C. elegans.
Results
C. elegans male mate-searching behaviour is inhibited after contact with an hermaphrodite
Mate-searching behaviour of the C. elegans male is inhibited by the presence of hermaphrodites [1]. How do males detect the hermaphrodite’s presence? C. elegans males accumulate at sources of hermaphrodite-secreted pheromones [2] [3] [4] (supplemental Fig. 1A,B). However, hermaphrodite pheromones alone failed to retain males on food when we tested males in the leaving assay [1] (see Experimental Procedures; supplemental Fig.1 C). This suggests that hermaphrodite cues different from those that cause males to accumulate with them are required for the inhibition of male exploratory behaviour.
We therefore asked whether males detect hermaphrodites through physical contact. We placed dead, paraformaldehyde (PFA)-fixed hermaphrodites on the food source and tested their effect on male exploratory behaviour. Males were fully retained by fixed hermaphrodites (Cr [coefficient of retention, see Fig. 1 legend] = 0.93; for living hermaphrodites Cr=1.0; no retention Cr=0) (Fig.1A). Because production and release of hermaphrodite pheromones into the environment is likely to require active metabolism, this result suggests that contact cues alone are sufficient for retention. Furthermore, when contact and mating between the male and the fixed hermaphrodites was blocked by a thin layer of agarose covering the hermaphrodite, males were not retained (Cr= 0.06). These observations suggest that contact is both necessary and sufficient for retention (Fig. 1A).
Fig 1. Mate searching behaviour is inhibited after contact with a hermaphrodite.
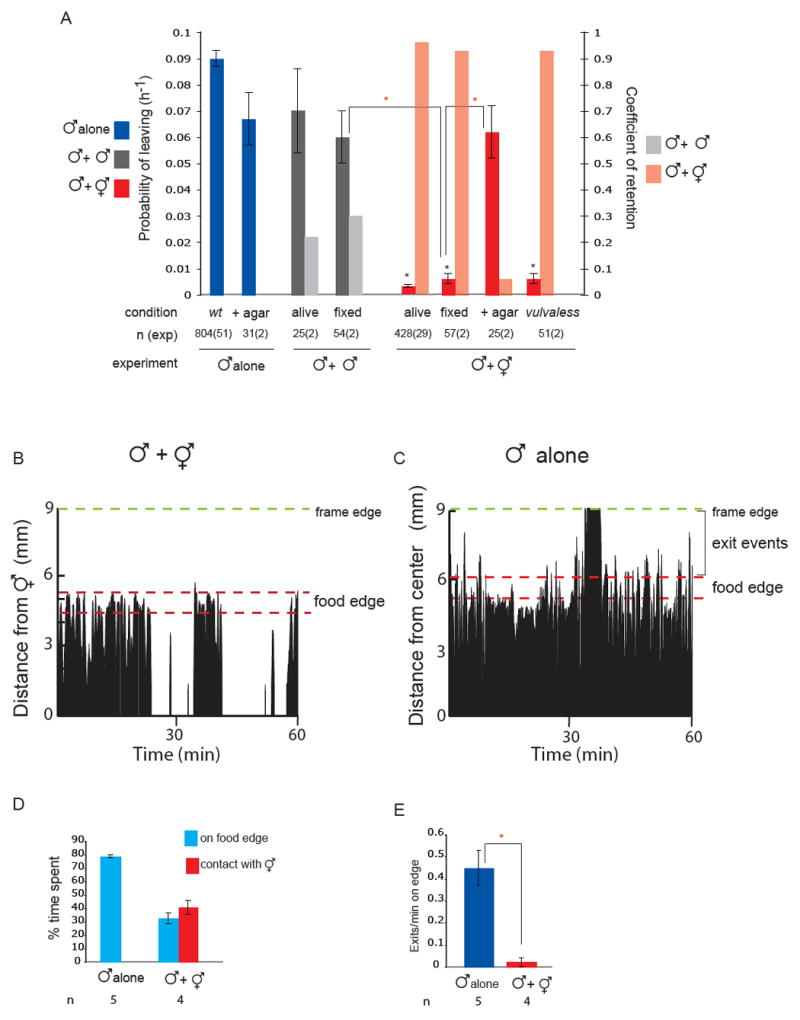
(A) Contact with a hermaphrodite is required to retain males. The hermaphrodite vulva is not required to retain males. PL values (probability of leaving food per worm per hour) and Cr (coefficient of retention) for males alone or in the presence of agar, with paralysed living or PFA-fixed males, with paralysed living or PFA-fixed hermaphrodites, PFA-fixed hermaphrodites covered in agar or paralysed vulvaless (lin-39 n1760) hermaphrodites. The number of worms assayed and the number of independent assays is indicated under each bar as n (exp). Replicas from living males, fixed males and fixed hermaphrodites were performed in parallel on the same day 2 different days. Fixation was performed in fresh 4% PFA at 4°C overnight and worms were rinsed in PBS before the assay. Assays with PFA-fixed hermaphrodites covered in agar were performed in parallel and compared with assays of males alone in the presence of agar. Retention levels, as the proportion in PL reduction, are represented with the coefficient of retention, Cr = [PL(alone) - PL(with hermaphrodites)]/ PL(alone). Cr=1 indicates full retention (PL(alone) >0 and PL(with hermaphrodites)= 0), Cr= 0 indicates no retention [PL(alone)=PL(with hermaphrodites)]. Males were fully retained by all hermaphrodites except by those covered in agar, and only partially retained by males. * indicates p< 0.001 when PL values are compared to wild type males alone;  indicates p< 0.001 when PL values are compared to each-other.
indicates p< 0.001 when PL values are compared to each-other.
(B) Male behaviour with a hermaphrodite. The position is shown of a single male on a food patch during an hour interval. In the presence of hermaphrodites, a male alternates between bouts of contact and bouts on the food edge. The graph plots a male’s distance from one hermaphrodite in the center of the plate (in mm) against time (min). Position was scored every 5 seconds. The food edge and the frame edge are marked.
(C) Male behaviour alone. A male alone spends most of its time on the food edge and beyond. Events scored as exits are indicated by the brackets. The graph plots a male’s distance from the center of the plate (in mm) against time (min). The food edge and the frame edge are marked.
(D) Males spend a significant amount of time on the food edge when they are in the presence of hermaphrodites but less time than when they are alone. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes.
(E) Males produce significantly more exit events per minute on the food edge when alone than when in the presence of hermaphrodites. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes.  indicates p< 0.003 compared to each-other (t-test).
indicates p< 0.003 compared to each-other (t-test).
Notably, males can discriminate between PFA-fixed males and fixed hermaphrodites since, just as with living males in parallel experiments, males still left in the presence of fixed males (Cr= 0.30) (Fig.1A). This sexual discrimination is not due to the absence of a vulva in the male since males are fully retained by lin-39(n1760) vulvaless hermaphrodites [5] (Cr=0.93) (Fig.1A). Contact-dependent sexual discrimination suggests that chemical as well as mechanical cues may be detected by the male when it touches the hermaphrodite.
A male may respond to contact with another worm by beginning the mating sequence, pressing its tail against the other worm’s body and backing up along it. During the time interval a male is thus responding, it cannot be exploring, and hence this contact response is expected to result in a decrease in the rate of leaving (partial retention). However, contact with a hermaphrodite stops the male from leaving the food altogether. We examined the behaviour of males in the presence of hermaphrodites on a food source. We found that the male spends 40.7 ± 5.2 % of its time in contact backing along the hermaphrodite body, 32.5 ± 4.0% of its time at the food edge (defined as a ring 1mm wide at the perimeter of the food), and the remaining 27% of its time exploring other areas of the patch of food (Fig.1B,D). Thus the male spends a significant proportion of its time away from the hermaphrodite. This suggests that hermaphrodite contact has some effect on male behaviour during periods of non-contact that inhibits exploratory activity.
To examine this possibility, we compared the behaviour of a male alone and a male with hermaphrodites during periods of non-contact. This revealed a significant difference in male behaviour at the food edge. A male alone spends 78.7 ±1.0% of its time at the food edge (Fig. 1C,D). During this time, the male periodically exits the lawn and immediately returns. An exit event was scored when the whole body of the worm came out of the food. A male alone produces on average 0.45±0.08 such exit events per minute spent at the food edge (Fig.C,E). In contrast, when hermaphrodites are present, a male produces only 0.02±0.02 exit events per minute spent at the food edge (Fig.1B,E). The suppression of exits was not due to a change in the distribution of lengths of bouts on edge or a consequence of ejaculation (supplemental Figure 1 D, E and F).
The male specific ray neurons regulate mate searching behaviour both in the presence and in the absence of hermaphrodites
We sought to identify the male sensory neurons responsible for hermaphrodite detection. The male specific CEM neurons in the head are involved in male attraction to hermaphrodite pheromones [3],[4]. Consistent with our observation that hermaphrodite secreted cues do not play a major role in inhibition of male exploratory behaviour, retention of males on food by hermaphrodites does not require the CEM neurons (supplemental Fig. 2). Likewise, examination of touch-insensitive mutants showed that retention does not require the mechanoreceptors present in both males and hermaphrodites (data not shown).
We found that the sensory neurons required to detect the hermaphrodite were the male specific ray sensory neurons in the tail. Two mutants with defects in the development of the male tail, including the rays, lin-32(e1926) and mab-3(mu15) [6, 7], displayed significantly reduced male leaving behaviour compared to wild type males and, as previously reported for mab-3 [8], were strongly defective in retention by hermaphrodites (Cr= 0.40 and Cr= 0.25 respectively) (Fig. 2A). The male tail includes 4 sets of sensilla with putative mechanosensory and chemosensory neurons: the 9 bilateral pairs of rays, the spicules, the hook and the post-cloacal sensilla [9] (Fig.2E). To establish which of these sensilla are important for the regulation of mate searching behaviour, we carried out laser ablations of each tail sensillum in wild type males and tested the operated animals in the leaving assay with and without hermaphrodites at adulthood.
Fig 2. The male specific ray neurons stimulate male exploratory behaviour away from food and retention by hermaphrodites.
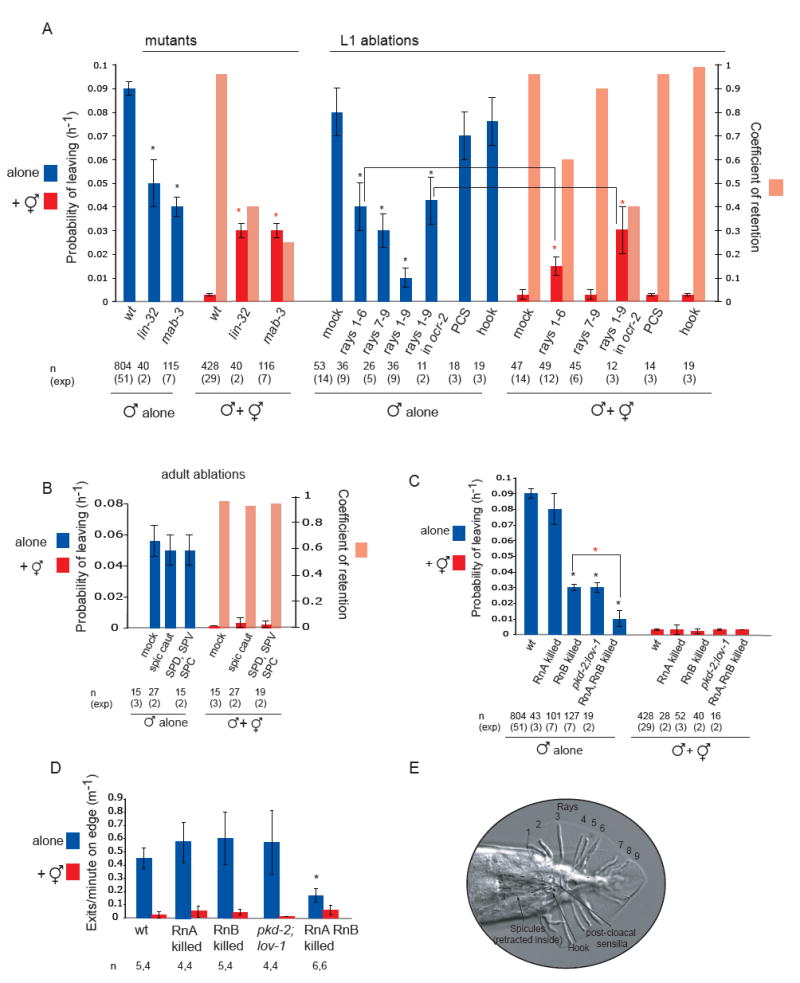
(A) The rays, but not other tail sensilla, are required to stimulate exploratory behaviour and for retention by hermaphrodites. The decrease in retention observed in rays 1-6 ablated wild type males indicates that activity of rays 1-6 is required to inhibit exploratory activity induced by rays 7-9. This suggests that the exploration and retention signals are two different signals that can be produced by different ray neurons. PL and Cr values for wt, mutant and operated males alone and in the presence of hermaphrodites. Mutant strains used were mab-3(mu15) and lin-32(e1926). Laser ablations of wt and ocr-2(ak47) males were performed at the L1 or L2 stage: rays 1 to 6 were removed by bi-lateral ablation of V5p L-R and V6p L-R; rays 7-9 were removed by bi-lateral ablation of Tap L-R; p9p and p10p were ablated to remove the hook sensillum; Y was ablated to remove the post-cloaca sensilla. * indicates p< 0.01 when PL values are compared to controls alone (wt for mutants and mock ablated wt and ocr-2 for ablations);  indicates p< 0.002 when PL values are compared to controls (wt or mock ablated wt and ocr-2 males with hermaphrodites)
indicates p< 0.002 when PL values are compared to controls (wt or mock ablated wt and ocr-2 males with hermaphrodites)
(B) The spicule neurons are not required for male exploratory behaviour or for retention. PL and Cr values alone and in the presence of hermaphrodites for males with intact, cauterized, or ablated spicule-associated sensory neurons (SPD, SPV and SPC). Ablations were performed in young adults in a syIs33( P(gpa-1)∷gfp) background to visualize the spicule sensory neurons. Mock animals underwent the same manipulations as ablated animals with the exception of not being shot with the laser microbeam. These manipulations slightly reduced the rate of leaving alone compared to non-manipulated males.
(C) RnA and RnB neurons stimulate male exploratory behaviour away from food. PL values, alone and in the presence of hermaphrodites, for males with and without RnA or RnB neurons and pkd-2(sy606);lov-1(sy582) double mutants. Only males in which all RnB neurons were dead were assayed. RnA death was not complete. Strains used: bxIs14( P(pkd-2)∷gfp); bxEx136 [P(pkd-2)∷ICE+P(unc-122)∷gfp] for RnB genetic ablations; bxEx137[P(trp-4)∷caspase3-NZ+P(grd-13)∷CZ-caspase3+P(elt-2)∷gfp] or bxEx138[P(trp-4)∷ ICE+P(elt-2)∷gfp] for RnA genetic ablations; and bxEx136;bxEx137 or bxEx136;bxEx138 for RnA,RnB genetic ablations. * indicates p< 0.001 when PL values are compared to wt males alone;  indicates p< 0.02 when PL values are compared to each-other.
indicates p< 0.02 when PL values are compared to each-other.
(D) Loss of all RnB neurons and most RnA neurons results in a reduction of exit events at the food edge. Bars plot the average of exit events per minute spent on food edge per worm in 1h 30 min observation periods alone and in the presence of hermaphrodites; * indicates p< 0.05 compared to intact males alone (t-test).
(E) DIC photograph of a male tail with the ventral side in focus; posterior is oriented toward the left. All the male specific sensilla are labelled. Dashed lines indicate the position of the spicules, which remain retracted inside the gubernaculum.
Males without rays 1-6 or without rays 7-9 showed a significantly reduced rate of male exploratory behaviour compared to mock ablated males (Fig.2A). These effects appeared to be additive since ablation of all rays almost completely abolished male exploratory behaviour (Fig. 2A). Thus, exploratory behaviour is induced by the ray neurons.
In the presence of hermaphrodites, males without rays 1-6 displayed partial defects in retention (Cr= 0.6), whereas males without rays 7-9 were fully retained (Cr= 0.9) (Fig.2A). Due to the extremely low rate at which males with no rays left food, retention could not be meaningfully assessed in these animals. To circumvent this problem we sought a genetic background in which leaving rates are strongly enhanced. The ocr-2(ak47) mutation increases the rate at which males leave food, presumably because of decreased food attraction (see below and Fig. 4). Thus, in an ocr-2(ak47) mutant background, males lacking all rays left food at a high enough rate for retention to be assessed (Fig.2A). In the presence of hermaphrodites, intact ocr-2 males were fully retained (Cr= 0.96), whereas in ocr-2 males lacking all rays retention was severely impaired (Cr= 0.4) (Fig.2A). Thus, the rays are largely responsible for retention of males by hermaphrodites, although other sensory neurons may contribute.
Fig 4. Amphid neurons modulate male exploratory behaviour and contribute to retention.
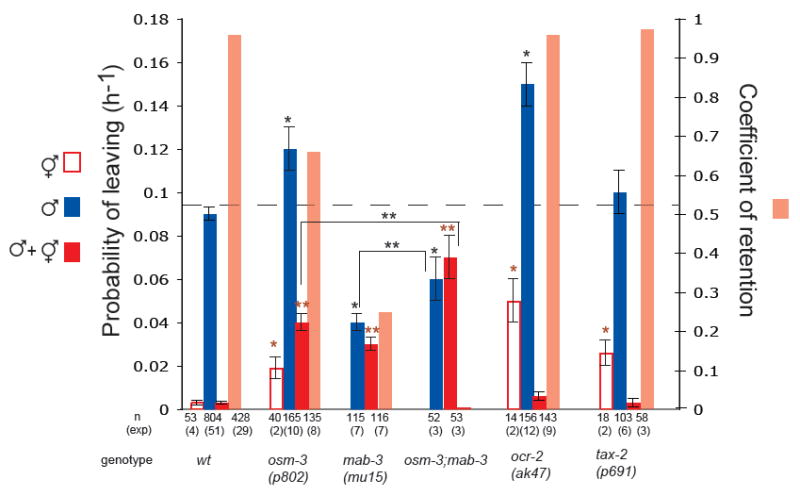
Mutants in food sensation display higher rate of exploratory behaviour but are fully retained by hermaphrodites; mutants defective for retention have defects in neurons involved in both food sensation and hermaphrodite detection. Unlike wild type hermaphrodites, which remain on food, osm-3(p802), tax-2(p691) and ocr-2(ak47) hermaphrodites left food at measurable rates, indicative of the role of these genes in food sensation. Examination of tax-2 and ocr-2 canonical reporter lines in males revealed no expression in ray neurons [18] [19]. Only osm-3 is expressed and disrupts the cilia of RnB neurons, as well as the other male specific ciliated neurons exposed to the outside [24]. Bars show the PL and Cr values of hermaphrodites alone, males alone and males with hermaphrodites for wt and mutants in gustatory amphid and ray neurons. Dashed line indicates the rate of exploratory behaviour in wild type males. Strains used: wt, osm-3(p802), mab-3(mu15), mab-3(mu15);osm-3(p802), tax-2(p691) and ocr-2(ak47); * indicates p< 0.001 when PL values are compared to wt males alone;  indicates p< 0.001 when PL values are compared to wt hermaphrodites;
indicates p< 0.001 when PL values are compared to wt hermaphrodites;  indicates p< 0.001 when PL values are compared to wt males with hermaphrodites; * * indicates p< 0.03 when PL values are compared to each other.
indicates p< 0.001 when PL values are compared to wt males with hermaphrodites; * * indicates p< 0.03 when PL values are compared to each other.
Disruption of male exploratory behaviour and retention by hermaphrodites was specific to the ablation of ray precursor cells. No defects were observed after ablation of the post-cloacal sensilla (Cr= 0.96) or the hook (Cr= 0.99) (Fig.2A). Similarly, cauterization of the spicule tips or ablation of the spicule associated sensory and motor neurons caused no disruption of male exploratory behaviour or retention (Cr= 0.94 and Cr= 0.96 respectively) (Fig. 2B). Therefore we conclude that male exploratory behaviour is regulated specifically by the rays, which are required both for stimulating exploratory behaviour in the absence of hermaphrodites and for retention in their presence.
Both RnA and RnB neurons stimulate male exploratory behaviour
Each ray sensillum contains 2 neurons, RnA and RnB, which differ in structure, neurotransmitters and post-synaptic targets [9] [10] (Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm). To assess the contribution to the regulation of mate searching behaviour made by each neuronal type, we ablated them separately by genetically targeted expression of a caspase (see Experimental Procedures).
RnB killed males displayed significantly reduced male exploratory behaviour compared to wild type animals and were completely retained by hermaphrodites (Fig. 2C). Due to the promoter used to genetically kill RnB neurons, the CEMs and the hook neuron HOB also undergo cell detah in these animals [11]. However, the phenotype observed can be attributed to lack of RnB neurons since, unlike ray ablation, lack of CEMs or hook does not result in defects in male exploratory behaviour (Fig. 2A and supplemental Fig. 2). We also tested the role of a calcium channel formed by the pkd-2 and lov-1 genes, members of the polycystins family of TRP channels, and required for RnB neuron function in mating behaviour [11, 12]. pkd-2(sy606);lov-1(sy582) double mutants displayed the same phenotype as RnB ablated males (Fig. 2C). Thus, RnB stimulation of male exploratory behaviour is dependent on the activity of the PKD-2/LOV-1 channel. Retention of RnB killed and pkd-2;lov-1 males indicates that RnA neurons are sufficient to detect hermaphrodites and inhibit leaving behaviour.
In contrast to males lacking RnB neurons, males lacking RnA neurons displayed normal exploratory behaviour (Fig. 2C). However, killing both RnA and RnB neurons by the caspase approach caused a higher reduction in exploratory behaviour than killing RnB alone and was similar to laser ablation of all rays (Fig. 2C). This indicates that RnA neurons also contribute to the stimulation of mate searching behaviour. RnA killed males were fully retained by hermaphrodites, but due to incomplete death of these neurons in our experimental technique, no conclusion can be drawn about the sufficiency of RnB neurons for retention (Fig. 2C).
In order to gain additional insight into the mechanisms by which ray neurons may regulate male exploratory behaviour, we compared the behaviour of intact males and males with genetically killed ray neurons at the food edge. Compared to intact males, males in which both RnA and RnB neuronal types were genetically killed produced significantly fewer exit events per minute at the food edge when alone (Fig. 2D). Moreover, in the presence of hermaphrodites, exits were not completely suppressed (Fig.2D). Thus, ray neurons regulate male behaviour at the food edge. In contrast, RnA killed, RnB killed and pkd-2(sy606);lov-1(sy582) males produced the same rate of exit events/min on food edge when alone as intact males and these exit events were suppressed by the presence of hermaphrodites (Fig.2D). Normal rate of food exits in backgrounds with slow leaving rate (RnB killed, pkd-2;lov-1) suggests the rays might alter additional aspects of male behaviour.
We compared the locomotion of intact males and males with genetically killed ray neurons on food and off food. We found speed on food to be similar in all males (supplemental Fig.3A). In contrast, we observed an increase in high angle turns (produced either by omega turns or long reversals) when immediately off food in all males with slow rate of leaving behaviour (supplemental Fig.3C and D). These observations suggest that ray neurons, in addition to stimulating food exits, may also inhibit the return to a food source by inhibiting high angle turns upon exiting food, thereby promoting exploratory behaviour away from the food source.
Male specific interneurons post-synaptic to ray neurons regulate retention by hermaphrodites
The male specific EF interneurons are major post-synaptic targets of A and B ray neurons in the pre-anal ganglion [9] (Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm). EF neurons extend processes along the ventral nerve cord into the nerve ring where they are likely to be pre-synaptic to neurons in the core nervous system (Fig.3F). These properties suggest that EF neurons might carry ray input to the core nervous system to affect male behaviour. We removed the EF interneurons and their lineage companions DX interneurons by laser ablation of their precursor cells [9]. Loss of EF/DX interneurons disrupted retention (Cr=0.6) but not exploratory behaviour of solitary males (Fig.3 A). The male’s tendency to mate was also not affected. There was no decrease in the time the male spent in contact with the hermaphrodite or the average length of contact bouts (i.e. response to contact and backing steps of mating behaviour) (Fig.3 C,E). This further demonstrates that mating alone cannot account for retention. Furthermore, similar to intact males alone, EF/DX ablated males produced an average of 0.39±0.1 exit events/min on edge in the presence of hermaphrodites (Fig.3 B,D). These results indicate that male specific interneurons, most likely the EF interneurons, are required for the behavioural changes in male mate searching behaviour associated with the presence of hermaphrodites.
Fig 3. Male specific interneurons regulate retention by hermaphrodites.
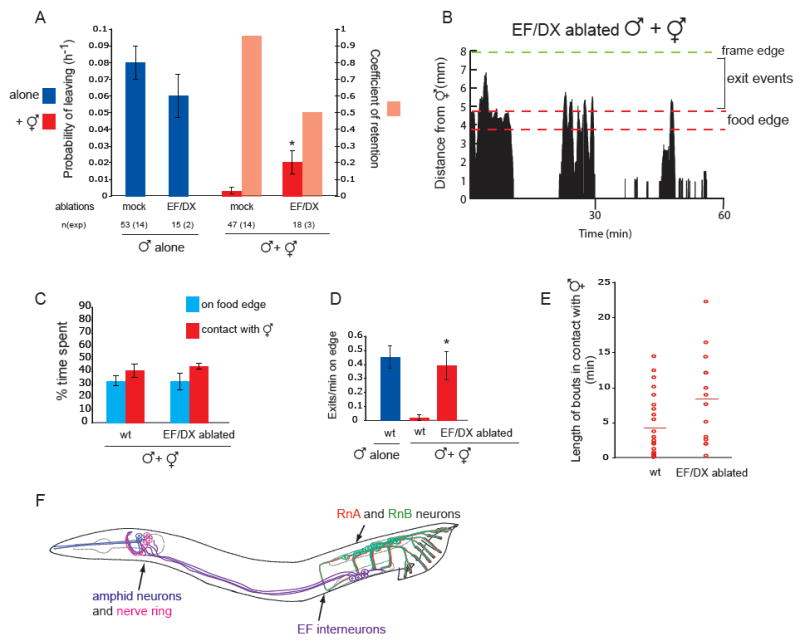
(A) Loss of EF/DX interneurons results in partial loss of retention by hermaphrodites. PL and Cr values for intact and operated males alone and in the presence of hermaphrodites. EF and DX interneurons were removed by laser ablation of the U and F cells at L1 stage. DX interneurons are post-synaptic mainly to hook neurons and pre-synaptic to post-cloacal sensilla (PCS) neurons, and may be involved in sperm transfer (Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm), [23]. We have shown that, unlike the rays, hook and PCS are not required for retention, making the DX interneurons unlikely candidates for conveying the hermaphrodite signal. EF/DX ablation did not disrupt retention as severely as ablation of all rays. This difference could be explained by the fact that, unlike EF ablated males, males with no rays spend little or no time in contact with the hermaphrodite and therefore, the proportion of time available to explore away from the food lawn is increased in ray ablated males. * indicates p< 0.001 when PL values are compared to mock ablated males with hermaphrodites.
(B) A male without EF and DX interneurons moves beyond the food edge after bouts of contact with a hermaphrodite. The graph plots the male’s distance from the hermaphrodites in the center (in mm) against time (min). The food and the frame edge are marked.
(C) EF/DX ablated males spend the same proportion of time on the food edge and in contact with hermaphrodites as intact males. Bars plot average percentage of time spent on food edge or in contact with a hermaphrodite per worm for intact and EF/DX ablated males. Same data for wild type male as in figure 1D and 2D; n=4 for each condition in 1h 30 min observation periods.
(D) EF/DX ablated males in the presence of hermaphrodites produce the same frequency of exit events as intact males alone. Bars plot average of exit events per minute spent on food edge per worm. Same data for wild type male as in figure 1E; n=4 for each condition (n=5 for wt alone) in 1h 30 min observation periods * indicates p< 0.03 compared to intact males with hermaphrodites, (t-test).
(E) Bouts of contact with a hermaphrodite are similar in length for EF/DX ablated and intact males. The duration (in minutes) of each contact bout produced by 4 individual intact or EF/DX ablated males in 1h 30 min observation periods is represented by a circle and the average is represented by a horizontal line.
(F) Male neurons that regulate mate-searching behaviour. Drawing of a male worm, anterior is oriented to the left. Neurons are labelled in colours: A and B type ray sensory neurons in the tail (red and green); EF interneurons post-synaptic to ray neurons in the pre-anal ganglion (purple) send processes to the nerve ring in the head (integration center, pink); amphid sensory neurons in the head (blue).
Amphid neurons modulate male exploratory behaviour and contribute to hermaphrodite detection
The amphids are the main chemosensory structures present in the head of both males and hermaphrodites [13]. Given the prominent role of amphid neurons in the regulation of exploratory behaviour in relation to food in the hermaphrodite [14-16] we asked whether they contribute to the regulation of exploratory behaviour in relation to food and mates in the male. In particular, we asked whether male leaving behaviour is regulated through modulation of the male’s response to food and whether amphid neurons detect hermaphrodite cues required for retention.
We used mutants that disrupt the function of different subsets of amphid neurons, namely mutants in the kinesin osm-3(p802), in the cyclic GMP-gated channel subunit tax-2(p691) and in the TRPV channel subunit ocr-2(ak47) [17], [18], [19]. In the absence of hermaphrodites, males mutant for these genes displayed a greater rate of exploratory behaviour than wild type males, indicating that amphid neurons inhibit male leaving behaviour, likely by promoting attraction to food (Fig. 4). Thus, a solitary male can still sense food and amphid neurons act antagonistically to ray neurons in the regulation of male exploratory behaviour. Consistent with this, killing RnA or RnB neurons in ocr-2 mutants results in reduction of male exploratory behaviour (supplemental Fig.4). Similarly, disruption of amphid function in mab-3 mutants by introducing the osm-3 mutation results in a significant increase in rate of exploratory behaviour (Fig.4).
We then asked whether amphid neurons regulate retention by hermaphrodites and, if so, is their effect because of their function in food sensation or is it because they function to directly sense hermaphrodite cues. In the presence of hermaphrodites, ocr-2 and tax-2 mutant males were completely retained (Cr=0.96 and Cr=0.97 respectively) (Fig.4). This indicates that loss of food attraction does not necessarily result in loss of retention, the presence of hermaphrodites overcoming the sensory deficit in these mutants. In contrast, osm-3 males, which have defects also in male-specific ciliated neurons including RnB, displayed reduced retention (Cr=0.66) consistent with hermaphrodite detection being also weakened (Fig. 4). The partial loss of retention in osm-3 males cannot be explained by the additive effect of reduced food sensation through amphids and reduced hermaphrodite detection through RnB neurons since killing RnB in other mutants in food sensation such as ocr-2 and tax-2 does not disrupt retention (supplemental Fig.4 and data not shown). Therefore, these results suggest that some gustatory amphid neurons impaired in osm-3 mutants may sense hermaphrodite cues directly to contribute to retention.
Discussion
In all sexual animals, a drive to reproduce is essential for species survival. We have investigated a sex-specific, appetitive reproductive behaviour in C. elegans and asked what neurons are responsible for it and how do they function. We have found that both the male’s drive to explore away from food and its ability to detect hermaphrodites are mediated by the ray sensory neurons, components of the male sexual organ. Both mate searching behaviour and ray neuron development occur upon maturation of the male to adulthood [1], [9]. Mechanistic coupling of the differentiation of sexual structures with behaviour would appear to be a natural way of coordinating diverse events of maturation.
The tendency of a male to explore away from a food source (leaving) depends on the balance of two competing needs: feeding and reproduction. Consistent with this, the rate of leaving is regulated by the opposing activity of food-sensing amphid neurons and sexual ray neurons. In hermaphrodites, amphid neurons are part of the circuit for navigation that regulates exploratory behaviour in response to food by modulating the frequency of runs and turns [14-16]. Stimulation of male exploratory behaviour away from food by ray neurons is regulated, at least in part, through the stimulation of exit events at the food edge and the inhibition of high angle turns immediately upon exiting a food source without mates. Our results show that ray neurons do not promote leaving by blocking the food-attraction pathway in amphid neurons. We propose that ray neurons are part of the male navigation circuit that stimulates mate-searching independently of food attraction and that together with the amphid neurons, allows the male to locate a source of both food and mates.
The presence of mates on the food patch is detected by contact through the ray sensilla. It has previously been shown that rays stimulate the response to contact and backing (along the hermaphrodite’s body) steps of mating [20]. We show that while the execution of these steps reduces the time a male spends exploring and reduces the probability of leaving, it is not sufficient to completely retain a male on food. Males execute these steps with both hermaphrodites and males. However, periodic backing along the hermaphrodite body produces a behavioural change in the male for an extended period of time, which correlates with a suppression of exit events at the food edge and prevents the male from leaving. Prior experience has been shown to alter C. elegans behaviour in several contexts [21], [22].
Since males can discriminate between fixed males and fixed hermaphrodites, we speculate that cuticle-bound chemicals may be detected upon contact to bring about the difference in behaviour. Another possibility is that bouts of contact need to be a minimum length of time to cause a lasting effect in the male. This would be consistent with the observation that bouts of contact with hermaphrodites are significantly longer than with males and that loss of retention due to lack of most rays also reduces the length of contact bouts. In this scenario, ray neurons would act as timers that register length of contact bouts.
Both, response to contact and retention are triggered by the same sensory sensilla, the rays. The circuit for retention, however, includes male specific interneurons that do not affect response to contact. The EF interneurons are major post-synaptic targets of ray neurons in the pre-anal ganglion and extend processes into the nerve ring in the head [9] (Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm). Thus, EF interneurons may provide a neural substrate for the integration of hermaphrodite signals, sensed by male specific neurons in the tail, into the male’s core nervous system where food signals are processed. Determination of the molecular mechanisms by which hermaphrodite signals are detected and male exploratory behaviour is produced, and the complete identification of the mate-searching circuit awaits further studies.
Supplementary Material
Acknowledgments
We thank R. Azevedo for help with the statistical analysis; M. Bernstein for being a blind eye; G. Kleemann and R. Gosh for discussions; R. Poole for advice, many helpful discussions and comments on the manuscript; Y. Sze and O. Hobert for comments on the manuscript; Charlie Smith and Josephine Di Mele for expert technical assistance; J. White and E. Jorgensen for sharing related data with us before publication; the Maricq lab for reagents and strains; the Chalfie lab for reagents; H. Schwartz for unpublished strains; the Zarkower lab, the Sternberg lab and the Greenwald lab for strains. Additional strains were provided by the Caenorhabditis Genetics Center. This research was supported by grants from the National Institute of Health to SWE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The Sensory Circuitry for Sexual Attraction in C. elegans Males. Curr Biol. 2007 doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloof JN, Kenyon C. The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development. 1998;125:181–190. doi: 10.1242/dev.125.2.181. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Emmons SW. A transcription factor controlling development of peripheral sense organs in C. elegans. Nature. 1995;373:74–78. doi: 10.1038/373074a0. [DOI] [PubMed] [Google Scholar]
- 7.Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- 8.Yi W, Ross JM, Zarkower D. Mab-3 is a direct tra-1 target gene regulating diverse aspects of C. elegans male sexual development and behavior. Development. 2000;127:4469–4480. doi: 10.1242/dev.127.20.4469. [DOI] [PubMed] [Google Scholar]
- 9.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 10.Lints R, Jia L, Kim K, Li C, Emmons SW. Axial patterning of C. elegans male sensilla identities by selector genes. Dev Biol. 2004;269:137–151. doi: 10.1016/j.ydbio.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 12.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 13.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 14.Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- 15.Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J Mol Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- 18.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 19.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 20.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 21.Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male’s copulatory spicules during mating. Cell. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- 22.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 23.Liu K. Sensory regulation of C elegans male mating behaviour Volume PhD in Biology. Pasadena, CA: California Institute of Technology; 1995. [Google Scholar]
- 24.Peden EM, Barr MM. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


