Abstract
Background and Aims
Capsule endoscopy (CE) and double balloon enteroscopy (DBE) allow complete small bowel examination but consume additional healthcare resources. A cost-minimization analysis determined the optimal initial management strategy for obscure occult gastrointestinal bleeding (OGIB).
Methods
We compared 5 strategies: initial small bowel follow through, enteroclysis, push enteroscopy (PE), CE, or DBE. Incorporation of multiple tests was allowed with costs taken from a third-party payer perspective. We modeled medically refractory disease with two separate model endpoints where treatment or definitive diagnosis was necessary and where visual diagnosis was sufficient to suspend testing. Sensitivity analyses included variations in parameter estimates, Monte Carlo simulation, and structural variations in the model in which DBE was not available as an initial strategy.
Results
When treatment or definitive diagnosis was necessary, the optimal strategy was initial DBE at a cost of $3,824. An initial CE strategy costs an incremental $440. CE was preferred when DBE exceeded $1,849 or when the sensitivity of DBE dropped below 68%. If DBE were unavailable as an initial test CE was preferred to PE unless CE exceeded $1,190, capsule retention was greater than 3%, or 64% of lesions were within reach of PE. When visual diagnosis was sufficient, initial CE was preferred.
Conclusions
For OGIB, initial DBE may be the least expensive strategy when treatment or definitive diagnosis is necessary and initial CE may be preferred when visual identification is sufficient. In settings where DBE is not available as an initial test, initial CE may be the preferred strategy.
BACKGROUND
Obscure gastrointestinal bleeding (OGIB) is defined as persistent or recurrent gastrointestinal bleeding in the setting of a negative standard endoscopic evaluation (e.g., esophagogastroduodenoscopy and ileocolonoscopy). OGIB may be either overt or occult hemorrhage, and is most often due to small bowel lesions.1–4 OGIB is associated with increased medical expenditures and resource utilization, including prolonged hospitalizations and procedures.5, 6
Investigating occult OGIB involves identification of the underlying lesion(s) and may include directed therapy. Non-invasive modalities include traditional radiographic studies, such as small bowel follow through (SBFT) and enteroclysis (EN). SBFT and EN are often utilized as the test to exclude luminal mass lesions (e.g., tumors) but cannot detect flat lesions (e.g., vascular, inflammatory).7,8 The advent of push enteroscopy in the early 1990’s allowed extended, but incomplete examination of the small bowel. New technologies, such as capsule endoscopy (CE) and double balloon enteroscopy (DBE) allow full examination of the small bowel but consume additional time and costs. It is unclear how these technologies should be incorporated into current clinical practice.
The least costly approach to evaluate occult OGIB remains uncertain. Therefore, we performed this study to compare the economic impact of competing strategies for the diagnosis and management of OGIB.
METHODS
In general, evaluation of occult OGIB requires the use of multiple tests performed in a sequential manner. The clinical scenario was limited to occult OGIB because the management differs from overt OGIB, which may require more frequent hospitalizations and use of angiography. In order to mimic this process for occult OGIB, we created a model in which we examined different initial tests but allowed use of additional tests if required for diagnostic or therapeutic purposes. Since each strategy could utilize one or eventually all available tests, the overall diagnostic and therapeutic yield is similar between strategies. Therefore, this study was designed as a cost-minimization analysis to determine the optimal, or least costly, strategy for patients with occult OGIB in whom endoscopy and ileocolonoscopy are negative.
We evaluated two scenarios with unique model endpoints. One scenario assumed that endoscopic or surgical management was necessary. Evaluation was pursued until the lesion(s) were treated or biopsied for histopathological diagnosis. We considered an alternative scenario in which endoscopic or surgical intervention was not necessary to control hemorrhage; in this case visual diagnosis of a lesion was deemed sufficient to direct medical therapy. Finally, we recognized that DBE is not widely available in most practice settings and capacity to conduct this test may be limited to referral centers. We therefore created a third model in which DBE would not be available as an initial diagnostic test but could be pursued as a secondary test if the primary diagnostic modality failed to reveal a small bowel bleeding source.
Literature Review
To assess key variables, we searched the MEDLINE database using the terms gastrointestinal hemorrhage [MeSH] AND (obscure [kw] OR unknown [kw]). Limiting the search to the English language and clinical trials or comparative and evaluation studies yielded 90 studies. Model inputs were based on studies that reported the diagnostic yield of CE or DBE and if applicable, comparison to another modality. We excluded small studies (less than 20 patients), pediatric studies, and those not pertaining to obscure bleeding e.g. chronic diarrhea or Crohn’s disease. Since these studies did not incorporate a true gold standard, determination of diagnostic yield based on comparative strategies offered the best data. Among studies with obscure bleeding, most studies included both overt and occult bleeding although some studies did not specify the patient presentation. Where available, we extracted outcomes specific to occult bleeding understanding that data limitations would be reflected in the range used for the sensitivity analysis. If no data existed for a specific transition, author consensus (MS, IMG, JMI) for the parameter estimate was utilized in the base-case scenario.
Decision Analytic Model
Patients
Using a decision analysis software (TreeAge Pro Suite 2006 Release 0.3, Williamstown, MA), we evaluated a hypothetical cohort of patients who had experienced recurrent and refractory occult gastrointestinal bleeding and in whom upper endoscopy and ileocolonoscopy were negative. Both endoscopy and ileocolonoscopy were assumed to effectively exclude lesions within the reach of either endoscope. Therefore, lesions modeled were defined to be located distal to the reach of an upper endoscopy and proximal to the terminal ileum.
Model Endpoint: Therapy or Definitive Diagnosis of Lesion
In the first scenario the goal of all competing strategies was management of the bleeding source including therapy or histological diagnosis of the lesion. For bleeding vascular ectasias or ulcers this implied application of endoscopic or surgical therapy, while for neoplastic or inflammatory lesions this implied biopsy of the lesion. In this model, visual identification of the lesion by CE, SBFT or EN was not sufficient to suspend further testing. Based on published data it was assumed that among subjects with OGIB, 30% would have no identifiable lesion,2, 9–14 and 15% of lesions that were identified and treated would experience recurrent bleeding. It was assumed that 100% of missed lesions would have recurrent bleeding.
Test Characteristics of Strategies Modeled and Prevalence of Small Bowel Lesions
Figure 1 shows a simplified schematic of the decision model. The 5 strategies modeled included initial evaluation with SBFT, EN, PE, CE, or DBE. Etiologies of OGIB were divided into four categories (base-case values): vascular ectasia (35%), ulcer or inflammatory lesions (25%), neoplasia (10%), and no identifiable lesion (30%) (Table 1).2, 10, 11, 13–15 The sensitivity of radiographic studies, SBFT and EN, was dependent upon the type of lesion.7, 8, 16, 17 We estimated that EN identified 40% of ulcers and neoplasias, but was not able to identify flat mucosal lesions i.e. vascular ectasias. Similarly, SBFT identified 20% of ulcers/neoplasias but also did not identify flat mucosal lesions. The sensitivity of CE was estimated to be 75% for all small bowel mucosal lesions.10, 13, 18, 19 It was assumed that PE and DBE were able to detect 90% of lesions within the reach of the scope.18 However, the diagnostic yield of DBE and PE was dependent on the extent of small bowel evaluated. That is, despite equal sensitivity, PE has a lower diagnostic yield given its limited examination of the small bowel relative to DBE. Procedural complications such as capsule retention and DBE perforation have been described and were modeled at 1% and 0.5%, respectively.20–22
Figure 1.
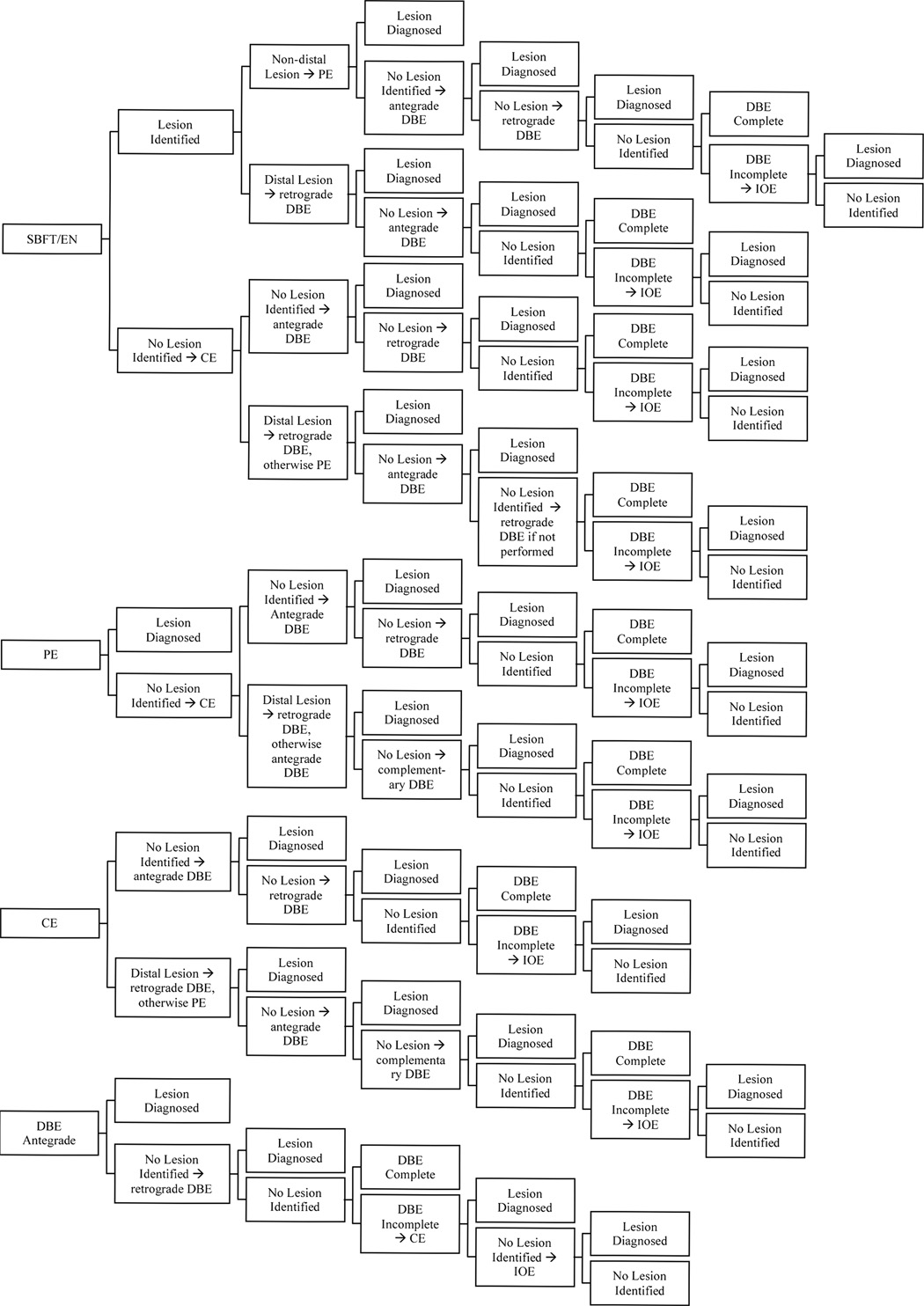
Simplified model excluding complications of five competing strategies for diagnosing and managing OGIB when therapy or definitive diagnosis was intended.
Table 1.
Clinical Estimates and Cost Inputs
| Variable | Base Case | Rangea | Reference |
|---|---|---|---|
| Prevalence of Lesionb | 2, 9–16, 21, 22, 25, 26, 35,36 | ||
| Vascular ectasia | 0.35 | 0.10 – 0.65 | |
| Ulcer | 0.25 | 0.05 – 0.55 | |
| Neoplasia | 0.10 | 0.05 – 0.40 | |
| No lesion | 0.30 | 0 – 0.50 | |
| Test Sensitivityc | |||
| EN for ulcer/neoplasm | 0.40 | 0 – 0.80 | 7, 8, 16 |
| EN for AVM | 0 | ||
| SBFT for ulcer/neoplasm | 0.20 | 0 – 0.50 | 16, 17, 37, 38 |
| SBFT for AVM | 0 | ||
| PE for lesion within reach | 0.90 | 0.70 – 1.0 | 9–12, 18, 25, 26, 35, 36 |
| CE | 0.75 | 0.50 – 1.0 | 9–12, 16–19, 25, 26, 34–36 |
| DBE | 0.90 | 0.50 – 1.0 | 2, 14, 15, 19, 22, 39 |
| IOE | 0.90 | 0.70 – 1.0 | 21, 34, 40, 41 |
| Distribution of Lesionsd | |||
| Proximal lesion | 0.60 | 0 – 1.0 | 9–12, 25, 26, 35, 36 |
| Complications | |||
| DBE incompletion rate | 0.35 | 0.15 – 0.75 | 2, 22 |
| Rebleeding after treatment | 0.15 | 0.05 – 0.50 | 14, 42–44 |
| DBE perforation | 0.005 | 0.001 – 0.01 | 21, 22 |
| Capsule retention | 0.01 | 0.001 – 0.05 | 10, 20, 45 |
| Cost, $e | |||
| Biopsy | 135 | ||
| SBFT | 164 | 150 – 750 | |
| EN | 225 | 100 – 500 | |
| PE | 875 | 500 – 2000 | |
| CE | 750 | 250 – 1500 | |
| DBE | 875 | 500 – 3000 | |
| IOEf | 2,000 | 1000 – 5000 | |
| GIBg, DRG 175 | 8,691 | 5000 – 20000 | |
| Perforation, DRG 149 | 22,471 | ||
AVM: arteriovenous malformation; CE: capsule endoscopy; DBE: double balloon enteroscopy; DRG: diagnosis related groups; EN: enteroclysis; GIB: gastrointestinal bleeding; IOE: intraoperative enteroscopy; PE: push enteroscopy; SBFT: small bowel follow through
Range used in Monte Carlo simulation and sensitivity analysis.
Prevalence of lesions were derived from CE and DBE studies.
Sensitivity of tests were derived from CE and DBE studies in comparison other diagnostic studies.
Distribution of lesions were derived from comparative studies between CE and PE.
Data from the Center for Medicare and Medicaid Services 2006.
Author’s consensus.
Median 2005 Medicare reimbursement based on AHRQ http://hcupnet.ahrq.gov/
The small bowel was divided into three anatomic segments. Prior studies suggest that PE examines the small bowel to approximately 100 cm beyond the ligament of Treitz.23, 24 Lesions up to this point were defined as “proximal.” Based on literature comparing PE to CE, 60% of lesions were assumed to be distributed within the reach of PE. 9–12, 25, 26 Despite reports of successful antegrade DBE extending to the colon, retrograde DBE is frequently required to complete the small bowel examination.22 The antegrade DBE has been reported to have an average insertion length of 250 – 350 cm beyond the pylorus.2, 15, 21 Therefore we assumed that an antegrade DBE was able to extend examination beyond a PE to the distal jejunum; however, the remainder of the small bowel required further evaluation with a retrograde DBE. Lesions located in the ileum requiring retrograde DBE to visualize were considered “distal.” The small intestine beyond the reach of PE, but within reach of the antegrade DBE was defined to be the “intervening segment.” The proportion of lesions beyond the reach of PE was divided equally between the intervening and distal segments.
Competing Strategies
1. SBFT
SBFT categorized lesion location into distal or non-distal lesions. If initial SBFT identified a distal lesion, a retrograde DBE examination was performed, followed by antegrade DBE if no lesions were identified (Figure 1). SBFT identification of a lesion in any other segment prompted a PE, followed by antegrade DBE if PE was negative. If antegrade DBE was negative, India ink was submucosally injected to mark the distal extent of examination and a retrograde DBE was performed. No further studies were necessary if the retrograde DBE identified a lesion since appropriate therapy or biopsy could be performed. Failure to reach the India ink marker in the absence of a lesion was defined as an incomplete DBE examination.2, 15, 21, 22 Incomplete DBE examination prompted an intra-operative enteroscopy (IOE). IOE resulted in either diagnosis of a lesion, or failure to identify a lesion. In the latter case, no additional tests were performed and the subject was defined as having no identifiable lesion. In the case where no lesions were noted on the initial SBFT, CE was performed. Distal lesions on CE led to retrograde DBE, followed by antegrade DBE if retrograde examination was negative. Non-distal lesions prompted a PE. If PE failed to identify the lesion the DBE protocol was followed as described above.
2. EN
The algorithm for EN is identical to SBFT.
3. PE
PE was assumed to extend 100 cm distal to the ligament of Treitz.23, 24 If PE diagnosed a lesion, no further studies were necessary since appropriate therapy or biopsy could be performed. A negative PE led to CE. A distal lesion on CE prompted a retrograde DBE; otherwise an antegrade DBE was performed. The algorithm following retrograde and antegrade DBE are described above.
4. CE
If initial CE identified a distal lesion, a retrograde DBE was performed. Non-distal lesions were examined with a PE. If the CE failed to identify a lesion or if PE did not identify a lesion, an antegrade DBE was performed. The protocol following DBE is described above.
5. DBE
Subjects in this strategy were initially evaluated with an antegrade DBE. No further studies were performed if the antegrade DBE identified a lesion. The protocol following antegrade DBE is described above. The exception to the protocol follows an incomplete DBE, which in this strategy led to CE. Negative CE led to an IOE. IOE resulted in either diagnosis of a lesion, or failure to identify a lesion. In the latter case, no additional tests were performed and the subject was defined as having no identifiable lesion.
Costs
Costs, not charges, were the basis for inputs used in this analysis (Table 1). A third-party payer perspective was utilized, where costs included direct cost of care for delivery of services that were derived from published literature and 2006 data from the Center for Medicare and Medicaid Services (CMS). Inpatient resource utilization was based on diagnosis-related groups and Current Procedural Terminology codes, whereas outpatient data were based on ambulatory payment classification and Current Procedural Terminology codes. The cost of antegrade or retrograde DBE was estimated by using the Common Procedural Terminology code designated for small bowel enteroscopy (44376) and was based on the reimbursement from CMS.
Model Assumptions
Upper endoscopy and ileocolonoscopy effectively excluded lesions within the reach of these endoscopic evaluations. All lesions were assumed to be in the small bowel.
All subjects were candidates for all tests
Specificity was not modeled due to the paucity of published literature evaluating the false positive rates of various diagnostic modalities.
Sensitivity Analysis
One-Way and Two-Way Sensitivity Analysis
All assumptions for the model were varied over a wide range of values in a series of one-way sensitivity analyses (Table 1, Table 2). We analyzed a range of costs and probability estimates that exceeded the published ranges from the literature. In the absence of published literature, baseline rates were set at extreme values with adjustment by consensus from the authors. We evaluated the impact of sensitivity analysis on the incremental costs between strategies. Variables that affected preferences for a strategy were combined in two-way sensitivity analyses.
Table 2.
Cost-Minimization Analysis with Therapy or Definitive Diagnosis as Model Endpoint
| Rank | Strategy | Value, $ | Incremental Cost, $ |
|---|---|---|---|
| 1 | DBE | 3,824 | 0 |
| 2 | CE | 4,263 | 440 |
| 3 | SBFT | 4,311 | 488 |
| 4 | EN | 4,331 | 508 |
| 5 | PE | 4,408 | 585 |
Monte Carlo Simulation
Monte Carlo simulation creates a multi-way sensitivity analysis. The base-case decision tree was repeatedly analyzed using inputs with appropriate distributions to determine the proportion of runs each strategy was identified as the least costly initial strategy. The following variables were used in the simulation: costs and sensitivity of tests; rate of procedural complications; incomplete DBE rate; and distribution of lesions. Table 1 and Table 2 illustrate the distribution of the inputs for the Monte Carlo simulation, which can be described as an expected value with a maximum and minimum value. Each simulation performed included 100,000 trials.
Alternative Model Structure: DBE Not Available as an Initial Strategy
In order to reflect the fact that DBE is not widely available in clinical practice we modified the model structure to exclude the strategy using DBE as an initial test. In this analysis, however, DBE was considered to be available if other initial tests were negative or DBE was required to provide therapeutic or diagnostic intervention. Sensitivity analysis, including Monte Carlo simulation, was performed to evaluate the preference for alternative strategies. We utilized therapy and histological diagnosis as the model endpoint in this model structure.
Model Endpoint: Lesion Identification without Endoscopic Intervention
Our first scenario was a cohort of patients with refractory occult OGIB. Therefore, the goal of the model was endoscopic treatment or biopsy of lesions and included the potential for IOE in all strategies. The “therapy or histological diagnosis” endpoint contrasts clinically from many subjects who present with OGIB who do not require blood transfusions or hospitalization and are rarely referred for IOE. In order to model a cohort with lower disease severity, the model was truncated with lesion identification being the desired clinical endpoint. In this model, identification of a lesion by CE, SBFT or EN was deemed adequate to direct medical therapy and endoscopic therapy was not required. Furthermore, the extent of evaluation was limited to DBE rather than extending it to IOE.
RESULTS
Model Endpoint: Therapy or Definitive Diagnosis of Lesion
In the evaluation of subjects with OGIB, the least costly strategy was initial DBE with a cost of $3,824 (Table 2). Initial CE cost an incremental $440, while SBFT and EN cost an additional $488 and $508 relative to DBE. The cost of a strategy utilizing initial PE was most expensive with an incremental cost of $585 relative to DBE.
Sensitivity Analysis
One-Way Sensitivity Analysis
The strategy employing initial DBE examination was sensitive to the cost and test characteristic for DBE. Figure 2a is a tornado diagram depicting the effect on cost by varying each of the examined variables. Only two variables affect the preference for DBE: cost of DBE and sensitivity of DBE. CE became the preferred strategy when the cost of antegrade or retrograde DBE exceeded $1,849 (Figure 2b) or when the sensitivity of DBE dropped below 68% (Figure 2c). When the cost of DBE exceeded $2,473, PE became a viable initial strategy. Variations in other assumptions, including the sensitivity of CE, costs, and complications related to CE did not change the order of preferred strategies.
Figure 2.
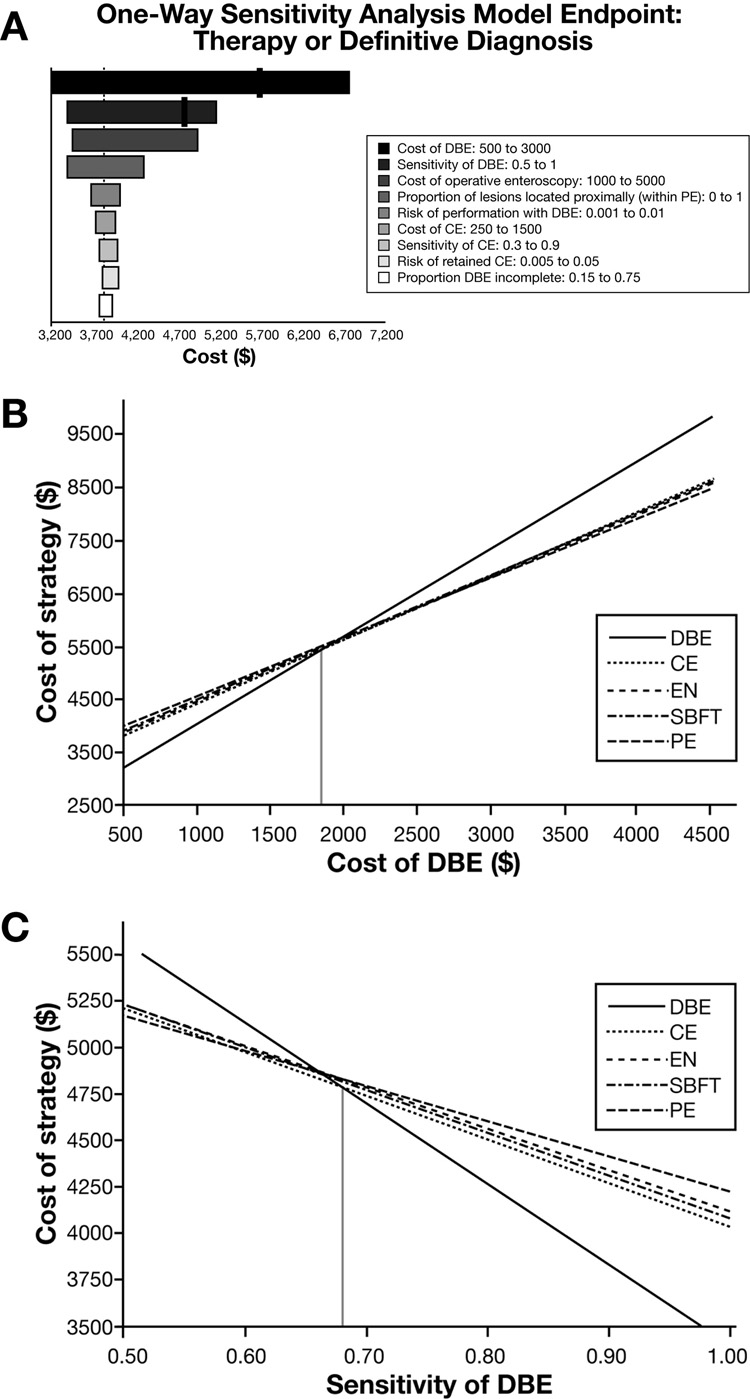
Figure 2a. One-way sensitivity analyses. Tornado diagram for therapy or histological diagnosis as model endpoint depicts the effect on costs by varying each of the examined variables. DBE is preferred unless a threshold is identified (black line). Preference for CE occurs when cost of DBE exceeds $1,849 and PE preferred when DBE cost exceeds $2,473. CE was preferred when sensitivity of DBE was less than 68%. Only variables displaying a change in preferred strategy or that were considered clinically important parameters are depicted. DBE: double balloon enteroscopy; CE: capsule endoscopy; PE: push enteroscopy
Figure 2b. Sensitivity analysis on the cost of DBE. Using a model with the goal of endoscopic therapy or definitive diagnosis, preference for DBE was sensitive to the cost of DBE. When either antegrade or retrograde DBE cost exceeded $1,849, CE became the preferred initial strategy (dotted vertical line – left).
Figure 2c. Sensitivity analysis on the sensitivity of DBE. Using a model with the goal of endoscopic therapy or definitive diagnosis, preference for DBE changed to CE when DBE identified less than 68% of lesion.
Two-Way Sensitivity Analysis
Figure 3a depicts a two-way sensitivity analysis varying the sensitivity of CE and the cost of DBE. Assuming a 90% sensitivity for CE, CE became a viable alternative when the cost of DBE exceeded $1,650. However, CE was no longer a viable strategy once the sensitivity fell below 66%. PE became a viable alternative when DBE costs exceeded $1950. Varying the cost of CE with the cost of DBE, Figure 3b reveals that CE was not a viable strategy whenever CE costs exceeded $875 or when the cost of DBE was less than $975. PE was only viable when the cost of DBE exceeded $2,000 and the cost of CE exceeded $600.
Figure 3.
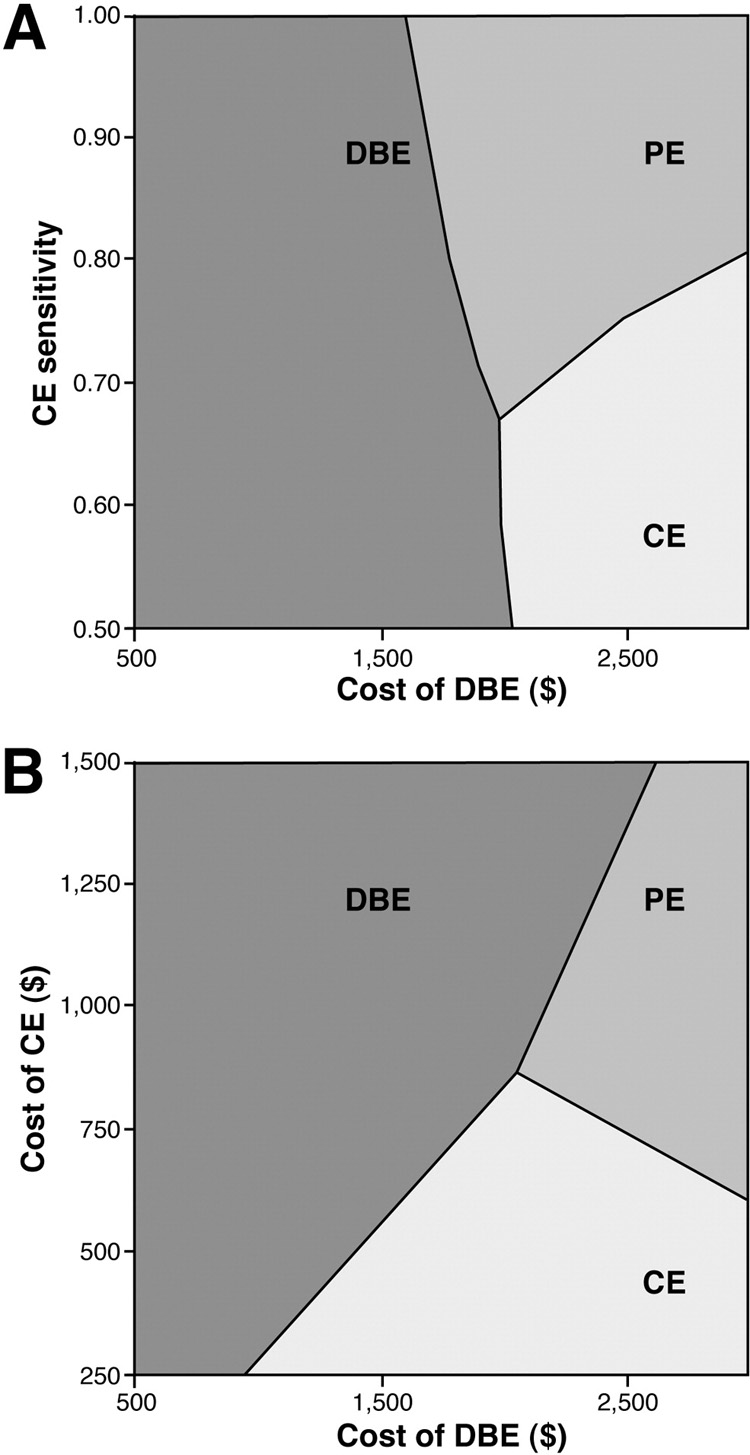
Figure 3a. Two-way sensitivity analysis varying DBE cost and sensitivity of CE. At optimal CE performance (sensitivity > 90%), CE was preferred as long as the cost of DBE exceeded $1,650. However, CE was no longer a viable strategy once its sensitivity fell below 66%.
Figure 3b. Two-way sensitivity analysis varying DBE cost and CE cost. CE was not a viable strategy whenever CE costs exceeded $875 or when the cost of DBE was less than $975. PE became viable when cost of DBE exceeded $2,000 and cost of CE exceeded $600.
Monte Carlo Simulation
The Monte Carlo simulation demonstrated that when therapy or histological diagnosis was the model endpoint, DBE was the preferred initial strategy in 77% of trials (Figure 5a). Initial CE was preferred in 9% of the simulated trials, while PE was preferred in 8%. EN and SBFT was preferred in a combined 6% of trials.
Figure 5.
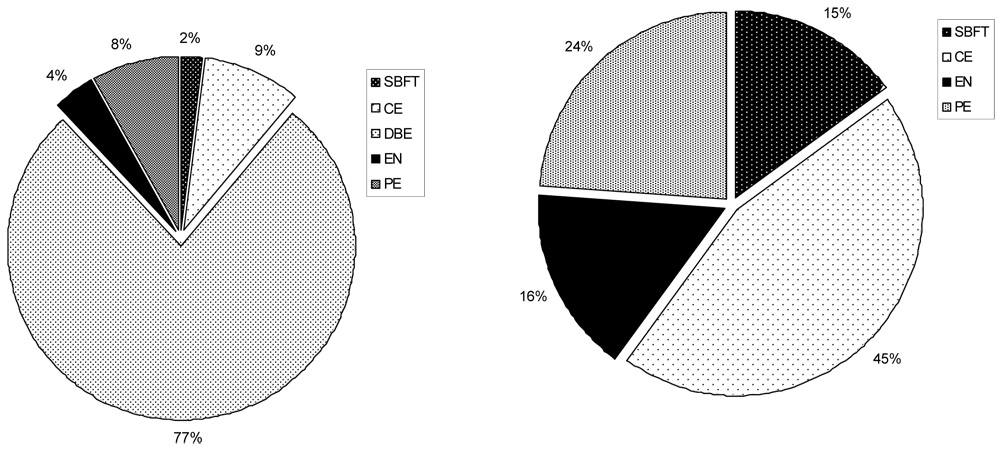
Figure 5a. Preferred initial testing strategy when therapy or definitive diagnosis is the model endpoint. Using a Monte Carlo simulation of 100,000 trials, 77% of trials favored DBE as the initial diagnostic strategy. CE was preferred in 9% of simulated trials, while PE was preferred as the initial test in 8%. EN and SBFT accounted for 6% of total trials.
Figure 5b. Preferred initial testing strategy when therapy or definitive diagnosis is the model endpoint and DBE is not available as an initial strategy. Using a Monte Carlo simulation of 100,000 trials, 45% of trials favored CE as the initial diagnostic strategy. PE was preferred in 24% of simulated trials, while EN and SBFT were preferred as the initial test in 16% and 15% of trials respectively.
Alternative Model Structure: No Available DBE
If DBE was not available as the initial diagnostic test, CE was the preferred strategy. Preference for CE was sensitive to the cost of CE with a threshold of $1,190 (Figure 4a) and a 3% capsule retention rate, above which PE became the preferred strategy. Furthermore, PE became the preferred strategy if over 64% of the lesions were located within the reach of PE (Figure 4b). Variation in other inputs did not change the order of preferred strategies including the sensitivity of CE, primarily because variation in this variable affected all strategies. Monte Carlo simulation revealed that if DBE were unavailable, CE was the preferred strategy in 45% of the trials (Figure 5b) while PE was preferred in 24%. EN and SBFT were preferred in 16% and 15% of simulated trials, respectively.
Figure 4.
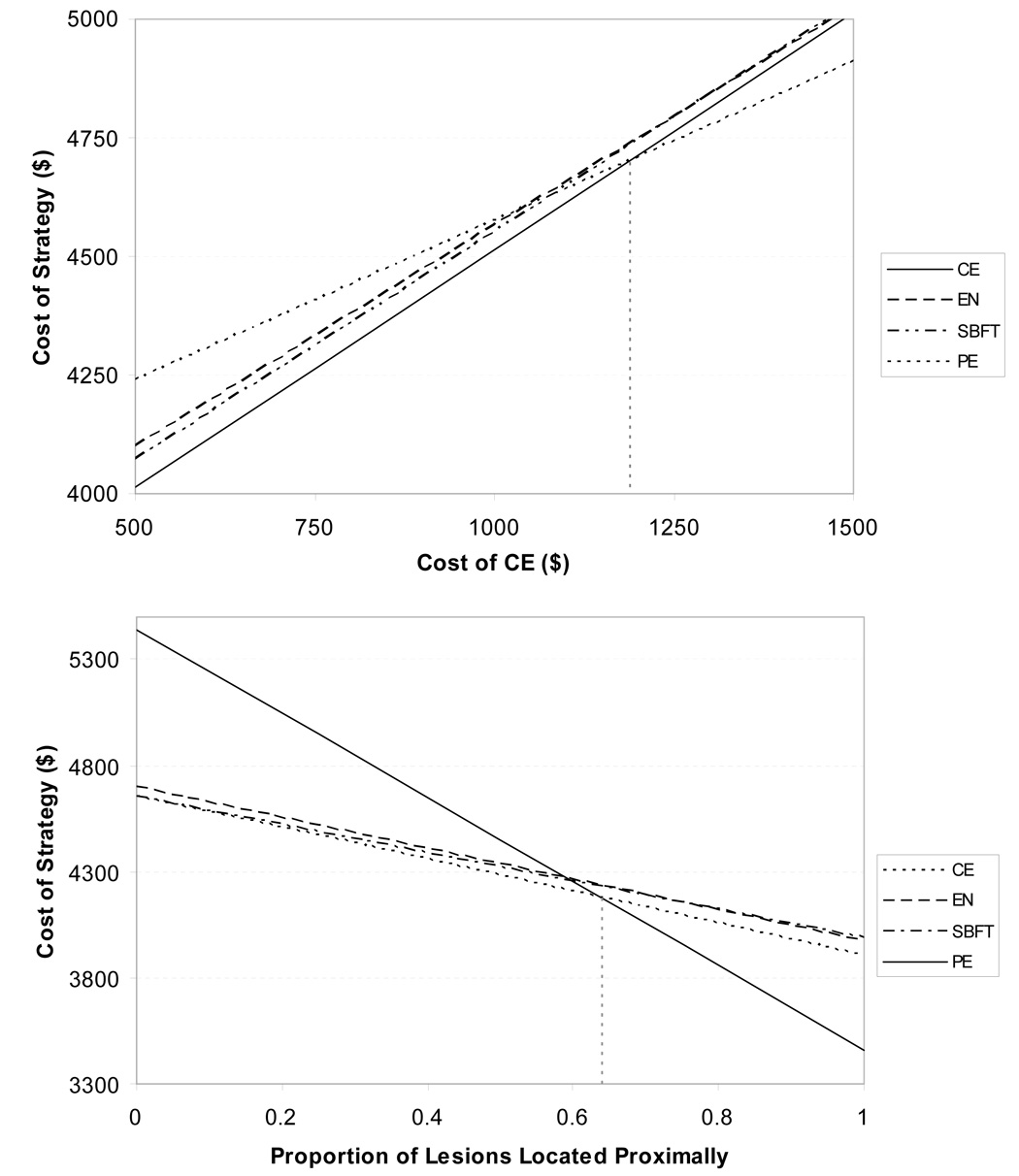
Figure 4a. Sensitivity analysis on the cost of CE. Using a model where DBE was unavailable as an initial strategy, preference for CE was sensitive to the cost of CE. When CE cost exceeded $1,190, PE became the preferred strategy (dotted vertical line).
Figure 4b. Sensitivity analysis of the proportion of lesions located proximally. Using a model where DBE was unavailable as an initial strategy, preference for CE was sensitive to the proportion of lesions located proximally, which is defined as within the reach of a PE. When the proportion of proximal lesions exceeded 64%, then PE became the preferred strategy (dotted vertical line).
Model Endpoint: Lesion Identification without Endoscopic Intervention
Changing the model structure to lesion identification as the endpoint, CE was the least costly strategy at $1,826 per subject evaluated for OGIB (Table 3). The radiographic tests, EN and SBFT, followed as preferred strategies and DBE and PE as least preferred strategies. DBE was preferred when the cost of CE exceeded $936 or when the cost of DBE was less than $716. DBE was also preferred when the sensitivity of CE was less than 63%, when capsule retention rates exceeded 1.8%, or when more than 78% of lesions were located in the proximal segment of the small bowel (Table 4). Preference for SBFT was achieved when its sensitivity for non-flat lesions exceeded 35% while preference for EN occurred when its cost was less than $190 or when the sensitivity for non-flat lesions exceeded 50%. PE was only preferred when its cost dropped below $409. Increases in the prevalence of non-flat lesions, such as the probability of neoplasia greater than 16% or probability of ulcer greater than 31%, resulted in a preference for EN as the initial test.
Table 3.
Cost-Minimization Analysis with Lesion Identification as Model Endpoint
| Rank | Strategy | Value $ | Incremental Cost |
|---|---|---|---|
| 1 | CE | 1,826 | 0 |
| 2 | EN | 1,860 | 35 |
| 3 | SBFT | 1,894 | 69 |
| 4 | DBE | 1,948 | 122 |
| 5 | PE | 2,291 | 466 |
Table 4.
Sensitivity Analysis with Lesion Identification as Model Endpoint
| Variable | Threshold | Preference for CE Changes to |
|---|---|---|
| CE cost | > $936 | DBE |
| CE sensitivity | < 63% | DBE |
| DBE cost | < $716 | DBE |
| EN sensitivity for ulcer/neoplasm | > 50% | EN |
| SBFT sensitivity for ulcer/neoplasm | > 35% | SBFT |
| EN cost | < 190 | EN |
| PE cost | < 409 | PE |
| Capsule Retention | > 1.8% | DBE |
| Proportion of lesion located proximally | > 78% | DBE |
DISCUSSION
Occult OGIB remains a diagnostic challenge. New technologies such as CE and DBE allow examination of the entire small bowel. It is unclear how these new diagnostic and therapeutic strategies should be incorporated into our current armamentarium. This cost-minimization analysis suggests that the optimal strategy to manage patients with occult OGIB depends on whether endoscopic or surgical intervention is required versus visual diagnosis of lesions. For patients in whom endoscopic or surgical intervention is necessary, a strategy that employs DBE as the initial test may be optimal. However, if only visual diagnosis of a lesion is necessary, a strategy employing initial CE is preferred. Additionally, it is recognized that capacity for performance of DBE in most practice settings is insufficient to allow it to be conducted in every patient with OGIB. In this case, CE appears to be the optimal initial test.
Despite the increased interest in CE and DBE in the evaluation of OGIB, few studies address the optimal initial testing strategy. Lewis and Goldfarb argued for initial CE evaluation of OGIB based upon clinical reasoning and lower medical costs.5 A recent meta-analysis found that CE offered a superior diagnostic yield when compared to PE, SBFT, and EN;27, 28 however, neither study provided comparison with DBE. To date, there are few comparative studies between CE and DBE. One study of 35 patients reported superior performance with CE while a case series of four subjects described missed lesions with CE subsequently found on DBE.19, 29 Several articles have noted the need for an economic analysis of CE and DBE in the evaluation of OGIB.29–31 This cost-minimization analysis illustrates that either CE or DBE may constitute economically sound initial testing strategies. The preferred initial test depends upon the patient presentation and whether endoscopic or surgical therapy is required. Most likely these are complementary procedures for use in this patient population.
A major limitation of this analysis stems from the type of data available in published literature. Although IOE and DBE may be considered gold standards, both tests have not been routinely applied to all subjects in published case series. Therefore, the data are fragmented with individual test characteristics and few studies employ simultaneous testing strategies. Studies that compared multiple diagnostic tests were preferentially used for inputs in the base-case scenarios. The diagnostic yield of various strategies in the literature is partly confounded by the indication for that testing strategy. That is, there is inherently greater yield associated with tests such as DBE given that subjects referred for such studies often have been refractory to conventional therapy. In contrast, the diagnostic yield of CE may be biased towards lower performance since the pretest probability of disease is typically lower than among subjects referred for DBE. Furthermore, it should be noted that the distribution of lesion type is dependent upon the patient demographics. It is known that patients with renal failure will have more vascular ectasias as their source of OGIB.32, 33 Several studies have also suggested that the diagnostic yield is dependent on timing of the diagnostic test such that administration of a diagnostic test early in the course of disease offered higher yield than delayed testing.34 Our model assumed a static lesion that was unaffected by the order of testing. Because of these shortcomings in the literature, we used sensitivity analyses to test a wide range of inputs.
Our cost-analysis was limited to a third-party payer perspective, although there are additional relevant perspectives. For example, the cost to the endoscopist and their capacity to deliver care was not evaluated. In the case of DBE, incomplete upper DBE examinations warrant performing the retrograde procedure another day; it is not uncommon that the antegrade procedure takes 90 minutes while the retrograde procedure is an additional 70 minutes.15, 21, 22 The willingness of endoscopists to provide DBE will likely depend on reimbursement; if greater revenue can be generated per unit time from performance of other procedures it is unlikely that DBE capacity will meet demand. Specifically, if current reimbursement for DBE remains at $875, the amount reimbursed by CMS for small bowel enteroscopy, it is unlikely that DBE will gain wide adoption into clinical practice.
Future studies should include concomitant testing of patients with CE and DBE, which will allow better understanding of the diagnostic yields of these new tests. Additionally, the national capacity for DBE has not been evaluated. Because of the initial capital investment, the steep learning curve associated with the procedure, and issues related to provider reimbursement relative to time consumption of the procedure, DBE may not be widely available in clinical gastroenterology practice. Beyond the direct health care costs, opportunity costs incurred by the gastroenterologist as well as potential costs to patients required to endure longer waits in the queue should be identified in order to provide more accurate overall health care costs.
In conclusion, a strategy using initial DBE to evaluate occult OGIB after negative endoscopy and ileocolonoscopy appears to be least costly if the goal of testing is treatment or definitive diagnosis. However, initial CE is preferred if the capacity of DBE is insufficient to meet demand or if the cost of either antegrade or retrograde DBE exceeds $1,849 or if the sensitivity of DBE is less than 68%. Alternatively, if the goal is limited to visual identification, initial CE may be preferred. Given the current reimbursement for DBE relative to its prolonged procedure time, the fixed cost of capital investment, and the technical skill required to perform the procedure, DBE capacity will likely remain low. It is therefore likely that CE will be viewed as a viable initial test in the evaluation of OGIB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
John M. Inadomi and Ian M. Gralnek are consultants to Given Imaging, Ltd.
REFERENCES
- 1.Zuckerman GR, Prakash C, Askin MP, Lewis BS. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201–221. doi: 10.1016/s0016-5085(00)70430-6. [DOI] [PubMed] [Google Scholar]
- 2.Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 3.Prakash C, Zuckerman GR. Acute small bowel bleeding: a distinct entity with significantly different economic implications compared with GI bleeding from other locations. Gastrointest Endosc. 2003;58:330–335. doi: 10.1016/s0016-5107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 4.Lewis BS. Small intestinal bleeding. Gastroenterol Clin North Am. 1994;23:67–91. [PubMed] [Google Scholar]
- 5.Lewis B, Goldfarb N. Review article: The advent of capsule endoscopy--a not-so-futuristic approach to obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2003;17:1085–1096. doi: 10.1046/j.1365-2036.2003.01556.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfeld RS, Smith TP, Schneider AM, Rockey DC. Provocative angiography in patients with gastrointestinal hemorrhage of obscure origin. Am J Gastroenterol. 2000;95:2807–2812. doi: 10.1111/j.1572-0241.2000.03191.x. [DOI] [PubMed] [Google Scholar]
- 7.Moch A, Herlinger H, Kochman ML, Levine MS, Rubesin SE, Laufer I. Enteroclysis in the evaluation of obscure gastrointestinal bleeding. AJR Am J Roentgenol. 1994;163:1381–1384. doi: 10.2214/ajr.163.6.7992733. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Lappas JC, Maglinte DD, Malczewski MC, Kopecky KA, Cockerill EM. Enteroclysis in the evaluation of suspected small intestinal bleeding. Gastroenterology. 1989;97:58–60. doi: 10.1016/0016-5085(89)91415-7. [DOI] [PubMed] [Google Scholar]
- 9.Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T, Florent C, Gay G. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576–584. doi: 10.1055/s-2003-40244. [DOI] [PubMed] [Google Scholar]
- 10.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 11.Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut. 2003;52:1122–1126. doi: 10.1136/gut.52.8.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mata A, Bordas JM, Feu F, Gines A, Pellise M, Fernandez-Esparrach G, Balaguer F, Pique JM, Llach J. Wireless capsule endoscopy in patients with obscure gastrointestinal bleeding: a comparative study with push enteroscopy. Aliment Pharmacol Ther. 2004;20:189–194. doi: 10.1111/j.1365-2036.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 13.Fireman Z, Eliakim R, Adler S, Scapa E. Capsule endoscopy in real life: a four-centre experience of 160 consecutive patients in Israel. Eur J Gastroenterol Hepatol. 2004;16:927–931. doi: 10.1097/00042737-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, Wu Y, Zhong J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:2011–2015. doi: 10.1111/j.1572-0241.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 15.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 16.Hara AK, Leighton JA, Sharma VK, Fleischer DE. Small bowel: preliminary comparison of capsule endoscopy with barium study and CT. Radiology. 2004;230:260–265. doi: 10.1148/radiol.2301021535. [DOI] [PubMed] [Google Scholar]
- 17.Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999–1005. doi: 10.1053/gast.2002.35988. [DOI] [PubMed] [Google Scholar]
- 18.Appleyard M, Fireman Z, Glukhovsky A, Jacob H, Shreiver R, Kadirkamanathan S, Lavy A, Lewkowicz S, Scapa E, Shofti R, Swain P, Zaretsky A. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431–1438. doi: 10.1053/gast.2000.20844. [DOI] [PubMed] [Google Scholar]
- 19.Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52–57. doi: 10.1111/j.1572-0241.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 20.Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065–1067. doi: 10.1055/s-2005-870264. [DOI] [PubMed] [Google Scholar]
- 21.Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM, Binmoeller K, Waxman I, Kozarek R, Lo SK. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740–750. doi: 10.1016/j.gie.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A, Ajibe H, Ido K, Sugano K. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010–1016. doi: 10.1016/s1542-3565(04)00453-7. [DOI] [PubMed] [Google Scholar]
- 23.Chong J, Tagle M, Barkin JS, Reiner DK. Small bowel push-type fiberoptic enteroscopy for patients with occult gastrointestinal bleeding or suspected small bowel pathology. Am J Gastroenterol. 1994;89:2143–2146. [PubMed] [Google Scholar]
- 24.Landi B, Tkoub M, Gaudric M, Guimbaud R, Cervoni JP, Chaussade S, Couturier D, Barbier JP, Cellier C. Diagnostic yield of push-type enteroscopy in relation to indication. Gut. 1998;42:421–425. doi: 10.1136/gut.42.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685–689. doi: 10.1055/s-2002-33446. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann D, Schilling D, Bolz G, Hahne M, Jakobs R, Siegel E, Weickert U, Adamek HE, Riemann JF. Capsule endoscopy versus push enteroscopy in patients with occult gastrointestinal bleeding. Z Gastroenterol. 2003;41:377–382. doi: 10.1055/s-2003-39330. [DOI] [PubMed] [Google Scholar]
- 27.Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 28.Marmo R, Rotondano G, Piscopo R, Bianco MA, Cipolletta L. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther. 2005;22:595–604. doi: 10.1111/j.1365-2036.2005.02625.x. [DOI] [PubMed] [Google Scholar]
- 29.Chong AK, Chin BW, Meredith CG. Clinically significant small-bowel pathology identified by double-balloon enteroscopy but missed by capsule endoscopy. Gastrointest Endosc. 2006;64:445–449. doi: 10.1016/j.gie.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34:679–698. doi: 10.1016/j.gtc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34:699–718. doi: 10.1016/j.gtc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Karagiannis S, Goulas S, Kosmadakis G, Galanis P, Arvanitis D, Boletis J, Georgiou E, Mavrogiannis C. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data) World J Gastroenterol. 2006;12:5182–5185. doi: 10.3748/wjg.v12.i32.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807–818. [PubMed] [Google Scholar]
- 34.Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Moller K, Jakobs R, Reitzig P, Weickert U, Gellert K, Schultz H, Guenther K, Hollerbuhl H, Schoenleben K, Schulz HJ, Riemann JF. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005;61:826–832. doi: 10.1016/s0016-5107(05)00372-x. [DOI] [PubMed] [Google Scholar]
- 35.Adler DG, Knipschield M, Gostout C. A prospective comparison of capsule endoscopy and push enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2004;59:492–498. doi: 10.1016/s0016-5107(03)02862-1. [DOI] [PubMed] [Google Scholar]
- 36.Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc. 2002;56:349–353. doi: 10.1016/s0016-5107(02)70037-0. [DOI] [PubMed] [Google Scholar]
- 37.Rabe FE, Becker GJ, Besozzi MJ, Miller RE. Efficacy study of the small-bowel examination. Radiology. 1981;140:47–50. doi: 10.1148/radiology.140.1.7244242. [DOI] [PubMed] [Google Scholar]
- 38.Fried AM, Poulos A, Hatfield DR. The effectiveness of the incidental small-bowel series. Radiology. 1981;140:45–46. doi: 10.1148/radiology.140.1.6787661. [DOI] [PubMed] [Google Scholar]
- 39.Manabe N, Tanaka S, Fukumoto A, Nakao M, Kamino D, Chayama K. Double-balloon enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2006;64:135–140. doi: 10.1016/j.gie.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Kendrick ML, Buttar NS, Anderson MA, Lutzke LS, Peia D, Wang KK, Sarr MG. Contribution of intraoperative enteroscopy in the management of obscure gastrointestinal bleeding. J Gastrointest Surg. 2001;5:162–167. doi: 10.1016/s1091-255x(01)80029-9. [DOI] [PubMed] [Google Scholar]
- 41.Douard R, Wind P, Panis Y, Marteau P, Bouhnik Y, Cellier C, Cugnenc P, Valleur P. Intraoperative enteroscopy for diagnosis and management of unexplained gastrointestinal bleeding. Am J Surg. 2000;180:181–184. doi: 10.1016/s0002-9610(00)00447-5. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann D, Schmidt H, Schilling D, Kinze F, Eickhoff A, Weickert U, Schulz HJ, Riemann JF. Follow-up of patients with obscure gastrointestinal bleeding after capsule endoscopy and intraoperative enteroscopy. Hepatogastroenterology. 2007;54:780–783. [PubMed] [Google Scholar]
- 43.Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304–309. doi: 10.1016/j.gie.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 44.Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224–1228. doi: 10.1111/j.1572-0241.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 45.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716. doi: 10.1016/j.gie.2005.05.002. [DOI] [PubMed] [Google Scholar]


