Abstract
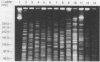
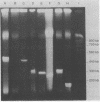
Pulsed-field gel electrophoresis and restriction endonucleases with rare recognition sites were used to generate large restriction fragment (LRF) patterns of genomic DNA from 48 isolates of Mycobacterium fortuitum biovariant fortuitum. Epidemiologically unrelated isolates gave highly diverse patterns when AsnI, HpaI, AflII, DraI, NdeI, XbaI, SpeI, or SspI was used. Epidemiologically related isolates produced identical or minimally different LRF patterns. Minor variations in LRF patterns were seen in two epidemic isolates digested with XbaI, suggesting that genetic alteration had occurred. LRF patterns were used to study three cardiac surgery wound infection outbreaks and one respiratory disease nosocomial outbreak. In two outbreaks, LRF patterns confirmed the reported clustering of isolates on the basis of multiple phenotyping methods. In the remaining two outbreaks, isolates which could not be separated by prior typing methods were easily distinguished by LRF pattern analysis. Environmental water isolates from two outbreaks had LRF patterns identical to those of the disease-producing strains, confirming that the local environment was the source of infection. Pulsed-field gel electrophoresis of LRFs of genomic DNA offers great promise as an epidemiologic tool for the study of M. fortuitum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Band J. D., Ward J. I., Fraser D. W., Peterson N. J., Silcox V. A., Good R. C., Ostroy P. R., Kennedy J. Peritonitis due to a mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982 Jan;145(1):9–17. doi: 10.1093/infdis/145.1.9. [DOI] [PubMed] [Google Scholar]
- Bolan G., Reingold A. L., Carson L. A., Silcox V. A., Woodley C. L., Hayes P. S., Hightower A. W., McFarland L., Brown J. W., 3rd, Petersen N. J. Infections with Mycobacterium chelonei in patients receiving dialysis and using processed hemodialyzers. J Infect Dis. 1985 Nov;152(5):1013–1019. doi: 10.1093/infdis/152.5.1013. [DOI] [PubMed] [Google Scholar]
- Burns D. N., Wallace R. J., Jr, Schultz M. E., Zhang Y. S., Zubairi S. Q., Pang Y. J., Gibert C. L., Brown B. A., Noel E. S., Gordin F. M. Nosocomial outbreak of respiratory tract colonization with Mycobacterium fortuitum: demonstration of the usefulness of pulsed-field gel electrophoresis in an epidemiologic investigation. Am Rev Respir Dis. 1991 Nov;144(5):1153–1159. doi: 10.1164/ajrccm/144.5.1153. [DOI] [PubMed] [Google Scholar]
- Foz A., Roy C., Jurado J., Arteaga E., Ruiz J. M., Moragas A. Mycobacterium chelonei iatrogenic infections. J Clin Microbiol. 1978 Mar;7(3):319–321. doi: 10.1128/jcm.7.3.319-321.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm L. N., Branstrom A. A., Warren R. L. Detection of restriction fragment length polymorphisms in clinical isolates and serially passaged Pseudomonas aeruginosa strains. J Clin Microbiol. 1990 Oct;28(10):2178–2182. doi: 10.1128/jcm.28.10.2178-2182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. C., Fraser D. W., Robicsek F., O'Bar P. R., Mauney C. U. Two outbreaks of sternal wound infection due to organisms of the Mycobacterium fortuitum complex. J Infect Dis. 1981 Apr;143(4):533–542. doi: 10.1093/infdis/143.4.533. [DOI] [PubMed] [Google Scholar]
- Hoy J., Rolston K., Hopfer R. L. Pseudoepidemic of Mycobacterium fortuitum in bone marrow cultures. Am J Infect Control. 1987 Dec;15(6):268–271. doi: 10.1016/0196-6553(87)90121-0. [DOI] [PubMed] [Google Scholar]
- Inman P. M., Beck A., Brown A. E., Stanford J. L. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969 Aug;100(2):141–147. [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritsky J. N., Bullen M. G., Broome C. V., Silcox V. A., Good R. C., Wallace R. J., Jr Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med. 1983 Jun;98(6):938–939. doi: 10.7326/0003-4819-98-6-938. [DOI] [PubMed] [Google Scholar]
- Laussucq S., Baltch A. L., Smith R. P., Smithwick R. W., Davis B. J., Desjardin E. K., Silcox V. A., Spellacy A. B., Zeimis R. T., Gruft H. M. Nosocomial Mycobacterium fortuitum colonization from a contaminated ice machine. Am Rev Respir Dis. 1988 Oct;138(4):891–894. doi: 10.1164/ajrccm/138.4.891. [DOI] [PubMed] [Google Scholar]
- Lowry P. W., Beck-Sague C. M., Bland L. A., Aguero S. M., Arduino M. J., Minuth A. N., Murray R. A., Swenson J. M., Jarvis W. R. Mycobacterium chelonae infection among patients receiving high-flux dialysis in a hemodialysis clinic in California. J Infect Dis. 1990 Jan;161(1):85–90. doi: 10.1093/infdis/161.1.85. [DOI] [PubMed] [Google Scholar]
- Lowry P. W., Jarvis W. R., Oberle A. D., Bland L. A., Silberman R., Bocchini J. A., Jr, Dean H. D., Swenson J. M., Wallace R. J., Jr Mycobacterium chelonae causing otitis media in an ear-nose-and-throat practice. N Engl J Med. 1988 Oct 13;319(15):978–982. doi: 10.1056/NEJM198810133191504. [DOI] [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Thorel M. F., Varnerot A., Gicquel B. DNA polymorphism in Mycobacterium paratuberculosis, "wood pigeon mycobacteria," and related mycobacteria analyzed by field inversion gel electrophoresis. J Clin Microbiol. 1989 Dec;27(12):2823–2826. doi: 10.1128/jcm.27.12.2823-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Falkinham J. O., 3rd Plasmid-encoded mercuric reductase in Mycobacterium scrofulaceum. J Bacteriol. 1984 Feb;157(2):669–672. doi: 10.1128/jb.157.2.669-672.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Kvach J. T., Mounts P. Isolation and restriction endonuclease analysis of mycobacterial DNA. J Gen Microbiol. 1986 Feb;132(2):541–551. doi: 10.1099/00221287-132-2-541. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Pyle L. E., Stemke G. W., Finch L. R. Human ureaplasmas show diverse genome sizes by pulsed-field electrophoresis. Nucleic Acids Res. 1990 Mar 25;18(6):1451–1455. doi: 10.1093/nar/18.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safranek T. J., Jarvis W. R., Carson L. A., Cusick L. B., Bland L. A., Swenson J. M., Silcox V. A. Mycobacterium chelonae wound infections after plastic surgery employing contaminated gentian violet skin-marking solution. N Engl J Med. 1987 Jul 23;317(4):197–201. doi: 10.1056/NEJM198707233170403. [DOI] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musser J. M., Hull S. I., Silcox V. A., Steele L. C., Forrester G. D., Labidi A., Selander R. K. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989 Apr;159(4):708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Steele L. C., Labidi A., Silcox V. A. Heterogeneity among isolates of rapidly growing mycobacteria responsible for infections following augmentation mammaplasty despite case clustering in Texas and other southern coastal states. J Infect Dis. 1989 Aug;160(2):281–288. doi: 10.1093/infdis/160.2.281. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple D. L., Le Febvre R. B., Andrews R. E., Jr, Thiermann A. B. Isolation and analysis of restriction endonuclease digestive patterns of chromosomal DNA from Mycobacterium paratuberculosis and other Mycobacterium species. J Clin Microbiol. 1987 Aug;25(8):1511–1515. doi: 10.1128/jcm.25.8.1511-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]