Abstract
Dopamine D1, dopamine D2, and adenosine A2A receptors are highly expressed in striatal medium-sized spiny neurons. We have examined, in vivo, the influence of these receptors on the state of phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32). DARPP-32 is a potent endogenous inhibitor of protein phosphatase-1, which plays an obligatory role in dopaminergic transmission. A dose-dependent increase in the state of phosphorylation of DARPP-32 occurred in mouse striatum after systemic administration of the D2 receptor antagonist eticlopride (0.1–2.0 mg/kg). This effect was abolished in mice in which the gene coding for the adenosine A2A receptor was disrupted by homologous recombination. A reduction was also observed in mice that had been pretreated with the selective A2A receptor antagonist SCH 58261 (10 mg/kg). The eticlopride-induced increase in DARPP-32 phosphorylation was also decreased by pretreatment with the D1 receptor antagonist SCH 23390 (0.125 and 0.25 mg/kg) and completely reversed by combined pretreatment with SCH 23390 (0.25 mg/kg) plus SCH 58261 (10 mg/kg). SCH 23390, but not SCH 58261, abolished the increase in DARPP-32 caused by cocaine (15 mg/kg). The results indicate that, in vivo, the state of phosphorylation of DARPP-32 and, by implication, the activity of protein phosphatase-1 are regulated by tonic activation of D1, D2, and A2A receptors. The results also underscore the fact that the adenosine system plays a role in the generation of responses to dopamine D2 antagonists in vivo.
The striatum, a major component of the basal ganglia, plays a critical role in the control of movement. About 95% of striatal neurons are medium-sized spiny neurons that project either directly or indirectly (i.e., via globus pallidus and subthalamic nucleus) to the substantia nigra/entopeduncular nuclei, the main output structures of the basal ganglia. Based on this anatomical distinction, striatal projection neurons are generally referred to as belonging to the striatonigral (direct) or to the striatopallidal (indirect) pathway (1).
Medium-sized spiny neurons integrate glutamatergic excitatory inputs from cortex and thalamus with dopaminergic modulatory inputs from the mesencephalon. In the striatum, dopamine activates both D1 and D2 subtypes of dopamine receptors. Striatal projection neurons also express high levels of adenosine A2A receptors, which appear to be located on the striatopallidal neurons of the indirect pathway (2, 3) and interact antagonistically with dopamine D2 receptors (4).
Virtually all striatal projection neurons express high levels of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32; refs. 5–7). cAMP-dependent protein kinase catalyzes phosphorylation of DARPP-32 on a single threonyl residue (corresponding to Thr-34 of the rat sequence), converting it into a potent and selective inhibitor of protein phosphatase-1 (8). The DARPP-32/protein phosphatase-1 pathway is involved in regulation of the state of phosphorylation and activity of a large number of phosphoproteins, such as voltage-dependent sodium and calcium channels, the electrogenic pump Na+,K+-ATPase, and neurotransmitter receptors (9, 10).
Recent data obtained in vitro by using rat striatal slices showed that stimulation of either dopamine D1 or adenosine A2A receptors causes the phosphorylation and activation of DARPP-32 (11). Moreover, studies performed in striatal slices from rat and mouse showed that activation of D2 receptors inhibits the increase in DARPP-32 phosphorylation induced by either D1 receptor or A2A receptor agonists (12, 13). Nothing, however, is known about the contribution of dopamine and adenosine receptors to the regulation of the state of phosphorylation of DARPP-32 in intact animals.
In this study, we have established a technique to measure changes in the levels of phosphoproteins in vivo and used it to examine responses to pharmacological treatments or genetic inactivation that alter dopaminergic and purinergic transmission. Our results indicate that, in the intact striatum, blockade of tonic dopamine D2 receptor activation increases DARPP-32 phosphorylation and that this effect is counteracted by blockade of either adenosine A2A or dopamine D1 receptor-mediated transmission.
Materials and Methods
Chemicals.
Eticlopride and cocaine were purchased from Research Biochemicals (Natick, MA). SCH 23390 and SCH 58261 were gifts from E. Ongini (Schering–Plough, Milan, Italy). Goat anti-mouse and goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies and a BCA protein detection kit were purchased from Pierce, and an enhanced chemiluminescence (ECL Plus) detection kit was purchased from Amersham Pharmacia.
Experimental Animals.
Male C57/BL6 mice (20–30 g) were obtained from M & B (Ry, Denmark). Mice heterozygous for the invalidated A2A receptor gene (14) were bred for eight generations on a CD1 background to dilute the genetic background of the embryonic stem cells (R1 line) derived from the 129 mouse strain. Heterozygotes were bred to generate the A2A receptor knockout mice and their wild-type littermate controls that were used in this study.
Tissue Preparation.
Mice were injected i.p. with saline or drugs and killed by decapitation 15 min later. When combinations of two drugs were used, the mice were killed 15 min after the second injection. The heads of the animals were immediately immersed in liquid nitrogen for 6 s. The brains were then removed, and the striata were rapidly (20 s) excised on an ice-cold surface, sonicated in 750 μl of 1% SDS, and boiled for 10 min.
Determination of the Levels of ATP.
ATP levels were measured by bioluminescence with the CytoLux kit provided by Wallac Oy (Turku, Finland) in a Wallac luminometer.
Determination of Phosphoproteins.
Aliquots (3 μl) of the homogenate were used for protein determination. Equal amounts of protein (40 μg; containing equal amounts of DARPP-32) from each sample were loaded onto 10% polyacrylamide gels. The proteins were separated by SDS/PAGE, and transferred to poly(vinylidene difluoride) membranes (Amersham Pharmacia) by the method of Towbin et al. (15). Phospho[Thr-34]-DARPP-32 was detected by using mAb-23 (1:1,000 dilution; ref. 16), and phospho[Ser-133]-cAMP response element-binding protein (CREB) was detected by using a commercial polyclonal antibody (Upstate Biotechnology, Lake Placid, NY; 1:2,000 dilution). Antibody binding was revealed by using goat anti-mouse horseradish peroxidase-linked IgG (diluted 1:10,000) for phospho-DARPP-32 or goat anti-rabbit horseradish peroxidase-linked IgG (diluted 1:20,000) for phospho-CREB as well as the enhanced chemiluminescence immunoblotting detection system. In some experiments, a mAb (C24–5a; diluted 1:10,000; ref. 17) generated against DARPP-32, which is not phosphorylation state specific, was used to estimate the total amount of DARPP-32 in wild-type and A2A receptor knockout mice. Chemiluminescence was detected by autoradiography. Quantification of the phospho-DARPP-32 and phospho-CREB bands was done by densitometry by using National Institutes of Health image (version 1.52) software.
Validation of Methodology.
A critical prerequisite for the present study was that dephosphorylation during sampling would be low and hence that the level of phosphoproteins measured ex vivo would reflect the in vivo situation. Because degradation of ATP is one of the most rapid dephosphorylation reactions occurring post mortem, in one series of experiments, we determined the levels of ATP in our striatal extracts. We measured 5.72 ± 0.86 mmol of ATP/g of tissue (n = 12), a value comparable to the values (2.75 ± 0.10 mmol/g of tissue) determined by using the best available methods of enzymatic inactivation, such as freeze-blowing (18), and considerably higher than the values seen in a freshly prepared brain slice (0.5 mmol/g of tissue; ref. 19). We also tested the effect of eticlopride (0.1 and 0.5 mg/kg, i.p.) on the phosphorylation of CREB. CREB is phosphorylated on Ser-133 in response to administration of dopamine D2 receptor antagonists in vivo, and phospho-CREB is very rapidly dephosphorylated post mortem (20). Using our extraction procedure, we could determine a 2-fold increase in the levels of phospho-CREB after administration of 0.1 or 0.5 mg/kg (i.p.) of eticlopride (data not shown). Taken together, these results indicate that dephosphorylation is limited during tissue preparation.
Results
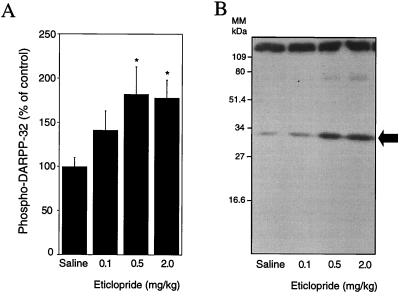
Administration of the dopamine D2 receptor antagonist eticlopride stimulated DARPP-32 phosphorylation in a dose-dependent manner (Fig. 1). A half-maximal increase in the level of phospho-DARPP-32 was reached at a dose of 0.1 mg/kg, and a maximal increase was reached at a dose of 0.5 mg/kg.
Figure 1.
Pharmacological blockade of dopamine D2 receptors increases DARPP-32 phosphorylation in vivo. Mice were treated i.p. with the indicated doses of eticlopride and killed by decapitation 15 min later. (A) Eticlopride increases phospho-DARPP-32 in a dose-dependent manner. The amount of phospho-DARPP-32 is expressed as percentage of that measured in saline-treated mice. Data represent means ± SEM of three experiments carried out in triplicate (n = 9–12). *, P < 0.05 vs. saline-treated group (ANOVA followed by Newman–Keuls test). (B) Autoradiogram obtained from one experiment. MM, molecular mass.
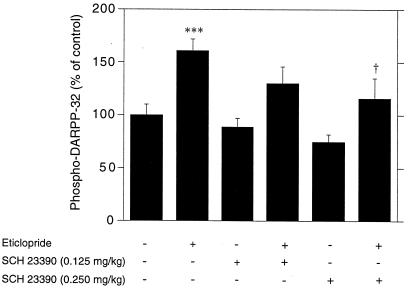
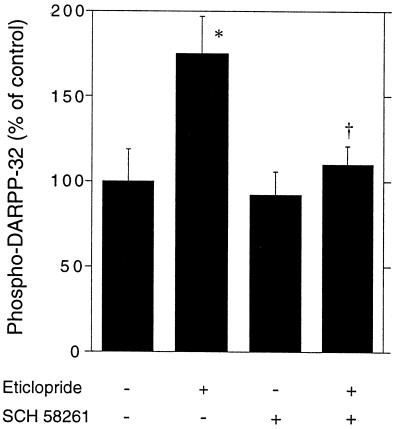
Previous data showed that, in striatal slices, activation of either dopamine D1 or adenosine A2A receptors increased the level of phospho-DARPP-32 (11). To determine whether DARPP-32 phosphorylation in intact mice is tonically regulated by these receptors, we administered selective antagonists i.p. 5 min before eticlopride. The selective dopamine D1 receptor antagonist SCH 23390 (0.125 and 0.25 mg/kg, i.p.) reduced the increase in DARPP-32 phosphorylation caused by the D2 receptor antagonist (Fig. 2). Similarly, the selective adenosine A2A receptor antagonist SCH 58261, used at a dose of 10 mg/kg and known to block A2A receptors in vivo, also reduced the increase in DARPP-32 phosphorylation caused by eticlopride (Fig. 3).
Figure 2.
Dopamine D2 receptor antagonist-induced phosphorylation of DARPP-32 depends on activation of dopamine D1 receptors. Mice were treated with the D1 receptor antagonist SCH 23390 (0.125 or 0.25 mg/kg, i.p) 5 min before receiving saline or eticlopride (0.5 mg/kg, i.p.) and killed 15 min later. Data represent means ± SEM. The number of animals per group varied from 11 to 28. ***, P < 0.001 vs. control; †, P < 0.05 vs. eticlopride (ANOVA followed by Newman–Keuls test).
Figure 3.
Dopamine D2 receptor antagonist-induced phosphorylation of DARPP-32 depends on activation of A2A receptors. Mice were treated with the A2A receptor antagonist SCH 58261 (10 mg/kg, i.p.) 5 min before receiving saline or eticlopride (0.5 mg/kg, i.p.) and killed 15 min later. Data represent means ± SEM. Each group consisted of nine animals. *, P < 0.05 vs. control; †, P < 0.05 vs. eticlopride (ANOVA followed by Newman–Keuls test).
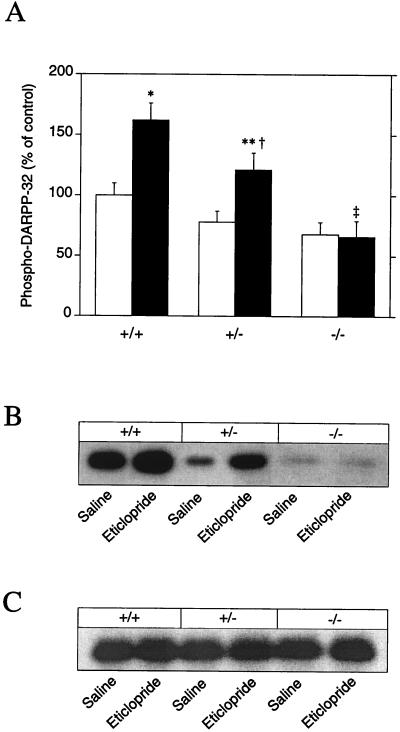
We also tested the ability of eticlopride to stimulate DARPP-32 phosphorylation in mice in which the gene coding for the adenosine A2A receptor was disrupted by homologous recombination. Eticlopride (0.5 mg/kg) failed to cause a significant increase in phospho-DARPP-32 when administered to A2A receptor knockout mice, in contrast to its effect in wild-type control mice (Fig. 4 A and B). Interestingly, eticlopride-treated mice heterozygous for the targeted A2A receptor allele showed a response intermediate to those of the +/+ and −/− mice (Fig. 4 A and B), indicating the absence of compensatory up-regulation of the remaining normal allele. To exclude that the effects observed were due to reduced expression of DARPP-32, the total amounts of DARPP-32 were estimated in the different genotypes after injection of saline or eticlopride. The levels of DARPP-32 were identical in the wild-type and A2A receptor knockout mice (Fig. 4C).
Figure 4.
Blockade of dopamine D2 receptors fails to induce DARPP-32 phosphorylation in adenosine A2A receptor knockout mice. Wild-type (+/+), heterozygote (+/−), or adenosine A2A receptor knockout (−/−) mice were treated with saline (open bars) or eticlopride (0.5 mg/kg, i.p.; closed bars) and killed 15 min later. The amount of phospho-DARPP-32 is expressed as percentage of that measured in saline-treated mice; data represent means ± SEM. The number of animals per group varied from four to seven. *, P < 0.05 vs. saline-treated +/+ mice; **, P < 0.01 vs. saline-treated +/− mice; †, P < 0.01 vs. eticlopride-treated −/− mice; ‡, P < 0.001 vs. eticlopride-treated +/+ mice (ANOVA followed by Newman–Keuls test). Autoradiograms obtained from one experiment with a mAb against phospho[Thr-34]-DARPP-32 (B) and a mAb generated against DARPP-32 (C), which is not phosphorylation state specific (see Materials and Methods).
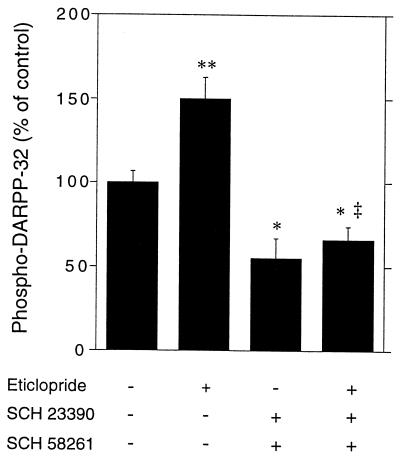
When administered alone, neither SCH 23390 nor SCH 58261 caused a statistically significant decrease in DARPP-32 phosphorylation (Figs. 2 and 3). In contrast, when combined, the two drugs not only completely reversed the stimulation of DARPP-32 phosphorylation induced by eticlopride but also caused a significant decrease in the basal levels of phospho-DARPP-32 (Fig. 5).
Figure 5.
Effect of combined blockade of D1 and A2A receptors on the basal and eticlopride-induced phosphorylation of DARPP-32. Mice were treated with the D1 receptor antagonist SCH 23390 (0.25 mg/kg, i.p.) plus the A2A receptor antagonist SCH 58261 (10 mg/kg, i.p.) 5 min before receiving saline or eticlopride (15 mg/kg, i.p.) and killed 15 min later. Data represent means ± SEM. The number of animals per group varied from 9 to 10. **, P < 0.01; *, P < 0.05 vs. control; ‡, P < 0.001 vs. eticlopride (ANOVA followed by Newman–Keuls test).
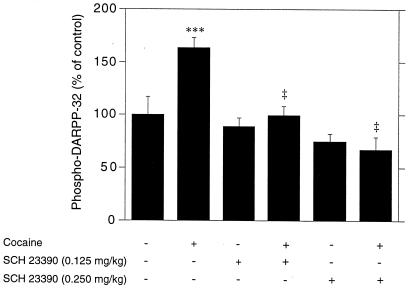
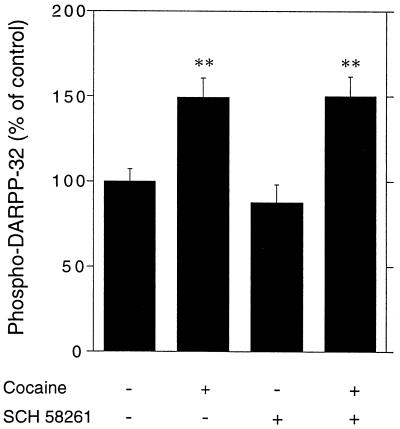
Cocaine, a drug that increases the extracellular concentration of dopamine by interfering with its reuptake, administered at a dose of 15 mg/kg, i.p., stimulated the phosphorylation of DARPP-32 (Figs. 6 and 7). This effect was abolished by pretreatment with SCH 23390 (Fig. 6). In contrast, SCH 58261 did not affect the increase in phospho-DARPP-32 caused by cocaine (Fig. 7).
Figure 6.
Cocaine-induced phosphorylation of DARPP-32 depends on activation of dopamine D1 receptors. Mice were treated with the D1 receptor antagonist SCH 23390 (0.125 or 0.25 mg/kg, i.p) 5 min before receiving saline or cocaine (15 mg/kg, i.p.) and killed 15 min later. Data represent means ± SEM. The number of animals per group varied from 5 to 28. ***, P < 0.001 vs. eticlopride; ‡, P < 0.001 vs. cocaine (ANOVA followed by Newman–Keuls test).
Figure 7.
Cocaine-induced phosphorylation of DARPP-32 does not depend on activation of A2A receptors. Mice were treated with the A2A receptor antagonist SCH 58261 (10 mg/kg, i.p.) 5 min before receiving saline or cocaine (15 mg/kg, i.p.) and killed 15 min later. Data represent means ± SEM. The number of animals per group varied from 13 to 19. **, P < 0.01 vs. control (ANOVA followed by Newman–Keuls test).
Discussion
In the present study, we have demonstrated that systemic administration of the D2 receptor antagonist eticlopride increases the levels of phospho-DARPP-32 in the striatum. These results indicate that, in vivo, DARPP-32 phosphorylation is kept low by tonic activation of dopamine D2 receptors. Regulation of the state of phosphorylation of DARPP-32 may account for some of the behavioral effects observed after blockade of D2 receptors. Indeed, recent data have shown that catalepsy induced by raclopride, a selective D2 receptor antagonist with neuroleptic properties, is strongly attenuated in DARPP-32 knockout mice (9).
The effect of eticlopride might be explained in either of two, not mutually exclusive ways. Tonic activity at dopamine D2 receptors might depress the release of dopamine by reducing firing rate or release at the nerve terminals. Hence, blockade of D2 receptors might increase the release of dopamine, which in turn could activate DARPP-32 phosphorylation via dopamine D1 receptors. Alternatively, eticlopride might prevent the decrease in DARPP-32 phosphorylation that results from the activation of dopamine D2 receptors present on medium sized-spiny neurons.
The basal level of DARPP-32 phosphorylation seen in untreated animals seems to represent a balance between the opposing effects of D2 receptor activation and D1/A2A receptor activation. The finding of a marked effect of eticlopride on DARPP-32 phosphorylation provides evidence that tonic activity of the D2 receptor signal transduction system maintains basal phospho-DARPP-32 at a relatively low level. Conversely, the increase in phospho-DARPP-32 seen in the presence of the D2 antagonist was reduced by dopamine D1 receptor and adenosine A2A receptor antagonists. In addition, combined blockade of dopamine D1 and adenosine A2A receptors significantly reduced the basal levels of phospho-DARPP-32, indicating the involvement of these two pathways in the regulation of DARPP-32 in vivo.
The increase in extracellular dopamine induced by cocaine activates not only dopamine D1 but also D2 receptors. Administration of cocaine also stimulates DARPP-32 phosphorylation in vivo. This effect was prevented by preadministration of SCH 23390, indicating that it is mediated via activation of dopamine D1 receptors. These results are in agreement with several other studies indicating that many effects of dopamine seem to be mediated by activation of D1 receptors. For example, the expression of immediate early genes induced by treatments that elevate extracellular dopamine, such as amphetamine, cocaine, and electrical stimulation of dopaminergic fibers, is blocked by D1 receptor antagonists (21–23). Likewise, changes in the firing rate of striatal projection neurons induced by elevated levels of extracellular dopamine are reversed by SCH 23390 (24, 25).
Our results indicate that SCH 23390 was more effective in preventing the cocaine-induced increase in DARPP-32 phosphorylation than the increase induced by eticlopride. This difference can be explained by considering the specific cell populations that might be affected by these drugs. The majority of D1 receptors are expressed in striatonigral neurons of the direct pathway (1, 26–28). Cocaine increases DARPP-32 phosphorylation via activation of D1 receptors and therefore is likely to affect mostly striatonigral neurons. In contrast, as discussed above, the effect of eticlopride is likely to occur (i) via blockade of presynaptic autoreceptors with a resultant increased release of dopamine and (ii) via blockade of postsynaptic D2 receptors that are located predominantly in striatopallidal neurons (1, 29). The latter effect would be expected to be relatively insensitive to SCH 23390.
An important conclusion of this study is that, in vivo, a basal, tonic activation of adenosine A2A receptors is required to generate the increase in DARPP-32 phosphorylation observed during blockade of dopamine D2 receptors. This requirement is clearly shown by the inability of eticlopride to stimulate DARPP-32 phosphorylation in A2A receptor knockout mice. In addition, preadministration of the specific A2A receptor antagonist SCH 58261 markedly reduced the effect of eticlopride.
Adenosine A2A receptors are enriched in striatopallidal neurons where they are coexpressed with D2 receptors (2, 3, 30). The two receptors exert opposite effects on molecular and physiological responses, such as immediate early gene expression, neurotransmitter release, and motor behavior (4). The opposite regulation exerted by A2A receptors and D2 receptors on DARPP-32 phosphorylation could explain some of these mutually antagonistic actions.
Changes in dopaminergic transmission seem to be involved in the pathogenesis of disorders such as Parkinson's disease and schizophrenia. Depletion of striatal dopamine represents the main cause of Parkinson's disease, which is treated by administering l-DOPA often in combination with D2 receptor agonists. In contrast, neuroleptic drugs that act by blocking dopamine D2 receptors are used in the treatment of schizophrenia. Previous studies have indicated that an A2A receptor antagonist exerts beneficial actions when tested in rodent and primate models of Parkinson's disease (31–33). On the other hand, A2A receptor agonists have been proposed to possess antipsychotic-like properties (34, 35). The ability of A2A receptors to regulate the state of phosphorylation of DARPP-32 in vivo is compatible with these suggestions that their pharmacological manipulation may be of potential clinical significance in the treatment of dopamine-related disorders.
Acknowledgments
This work was funded by U.S. Public Health Service Grants MH 40899 and DA 10044 (to P.G.), by Åke Wibergs Stiftelse (to P.S. and G.F.), by Stiftelsen Lars Hiertas Minne (to P.S.), by the Swedish Medical Research Council (to B.F. and G.F.), by Harald Jeanssons samt Harald och Greta Jeanssons Stiftelse (to G.F.), and by the Swedish Society for Medical Research (to M.L.).
Abbreviations
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- CREB
cAMP response element-binding protein
References
- 1.Gerfen C R, Engber T M, Mahan L C, Susel Z, Chase T N, Monsma F J, Sibley D R. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 2.Fink J S, Weaver D R, Rivkees S A, Peterfreund R A, Pollack A E, Adler E M, Reppert S M. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman S N, Vanderhaeghen J-J. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferré S, Fredholm B B, Morelli M, Popoli P, Fuxe K. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 5.Walaas S I, Greengard P. J Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langley K C, Bergson C, Greengard P, Ouimet C C. Neuroscience. 1997;78:977–983. doi: 10.1016/s0306-4522(96)00583-0. [DOI] [PubMed] [Google Scholar]
- 7.Ouimet C C, Langley-Guillion K-C, Greengard P. Brain Res. 1998;808:8–12. doi: 10.1016/s0006-8993(98)00724-0. [DOI] [PubMed] [Google Scholar]
- 8.Hemmings H C, Jr, Greengard P, Tung H Y L, Cohen P. Nature (London) 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 9.Fienberg A A, Hiroi N, Mermelstein P, Song W-J, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D B, Cole D G, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 10.Greengard P, Allen P B, Nairn A. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 11.Svenningsson P, Lindskog M, Rognoni F, Fredholm B B, Greengard P, Fisone G. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 12.Nishi A, Snyder G L, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindskog M, Svenningsson P, Fredholm B B, Greengard P, Fisone G. Neuroscience. 1999;88:1005–1008. doi: 10.1016/s0306-4522(98)00411-4. [DOI] [PubMed] [Google Scholar]
- 14.Ledent C, Vaugeois J M, Schiffman S N, Pedrazzini T, El Yacoubi M, Vanderhaegen J J, Costentin J, Heath J K, Vassart G, Parmentier M. Nature (London) 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder G L, Girault J-A, Chen J, Czernik A J, Kebabian J W, Nathanson J A, Greengard P. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmings H C J, Greengard P. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winn H R, Rubio R, Berne R M. Circ Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- 19.Fredholm B B, Dunwiddie T V, Bergman B, Lindström K. Brain Res. 1984;295:127–136. doi: 10.1016/0006-8993(84)90823-0. [DOI] [PubMed] [Google Scholar]
- 20.Konradi C, Cole R L, Heckers S, Hyman S E. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graybiel A M, Moratalla R, Robertson H A. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young S T, Porrino L J, Iadarola M J. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chergui K, Svenningsson P, Nomikos G G, Gonon F, Fredholm B B, Svensson T H. Eur J Neurosci. 1997;9:2370–2382. doi: 10.1111/j.1460-9568.1997.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 24.Gonon F. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyatkin E A, Rebec G V. J Neurosci. 1999;19:3594–3609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Moine C, Normand E, Bloch B. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hersh S M, Ciliax B J, Gutekunst C A, Rees H D, Heilman C J, Yung K K L, Bolam J P, Ince E, Yi H, Levey A I. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yung K K L, Smith A D, Levey A I, Bolam J P. Eur J Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 29.Le Moine C, Bloch B. J Comp Neurol. 1995;355:418–427. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 30.Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm B B. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 31.Kanda T, Jackson M J, Smith L A, Pearce R K, Nakamura J, Kase H, Kuwana Y, Jenner P. Ann Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- 32.Kanda T, Shiozaki S, Shimada J, Suzuki F, Nakamura J. Eur J Pharmacol. 1994;256:263–268. doi: 10.1016/0014-2999(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 33.Fenu S, Pinna A, Ongini E, Morelli M. Eur J Pharmacol. 1997;321:143–147. doi: 10.1016/s0014-2999(96)00944-2. [DOI] [PubMed] [Google Scholar]
- 34.Kafka S H, Corbett R. Eur J Pharmacol. 1996;295:147–154. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- 35.Rimondini R, Ferré S, Ögren S-O, Fuxe K. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]