Abstract
Echocardiographic imaging has become the primary tool to evaluate cardiac structure and function in human and veterinary medicine. The cynomolgus monkey (Macaca fascicularis) is a nonhuman primate species frequently used in biomedical research, particularly for the study of human cardiovascular disease and toxicology, yet echocardiographic reference ranges are not available for this species. Using standard 2-dimensional and M-mode imaging, we performed echocardiographic evaluation of 118 female and 119 male cynomolgus monkeys under sedation with either ketamine hydrochloride (10 mg/kg IM) alone or with a combination of tiletamine hydrochloride and zolazepam (4.0 mg/kg IM) and atropine sulfate (0.015 mg/kg IM). Reference ranges were developed (tolerance interval methodology) separately for each gender for heart rate, left ventricular (LV) size (interventricular septum in diastole, LV internal diameter in diastole and systole, LV free wall in diastole), left atrial diameter, and aortic diameter. LV functional parameters (fractional shortening, aortic peak flow velocity, LV ejection time, and LV preejection period) and mitral valve E point to septal separation were also measured. After normalization for body weight (1.7 to 6.3 kg), the data were analyzed for gender- and sedation-associated differences. Using a large number of healthy subjects (118 of each gender), we have developed gender-specific echocardiographic reference ranges for cynomolgus monkeys.
Abbreviations: AO, aortic diameter; AVmax, aortic peak flow velocity; BW, body weight; EPSS, E point to septal separation; FS, shortening fraction; HR, heart rate; IVSd, interventricular septal diameter during diastole; LA, left atrium; LV, left ventricle; LVET, left ventricular ejection time; LVFWd, left ventricular free wall diameter during diastole; LVIDd, left ventricular internal diameter during diastole; LVIDs, left ventricular internal diameter during systole; LVPEP, left ventricular preejection period, RR, R-to-R interval
Cardiac ultrasonongraphy (echocardiography) is a widely used noninvasive imaging tool useful for serial evaluation of cardiac size and function. The cynomolgus monkey (Macaca fascicularis) is a nonhuman primate that is used frequently in biomedical research because of its similarity to human anatomy and physiology.2,4 In fact, cynomolgus monkeys are one of the most commonly used nonrodent species used in preclinical safety studies.6 In such trials, potential cardiac effects on the heart are an important concern, and most current studies focus on cardiac morphologic evaluation and histologic data available at necropsy.6 However, these assessments do not allow cardiac functional evaluation, unlike real-time echocardiography.
Only 3 small studies describing echocardiographic parameters in nonhuman primates have been published. One5 included 44 (male and female) cynomolgus monkeys, another10 evaluated 31 male cynomolgus monkeys, and the third7 was performed in 28 healthy rhesus monkeys (Macaca mulatta). For the current study, we used a large population of healthy cynomolgus monkeys (n > 100 per gender for most parameters) to develop more accurate echocardiographic reference ranges for this species.
Materials and Methods
Echocardiographic evaluation, using standard 2-dimensional and M-mode imaging, was performed on 237 cynomolgus monkeys (M. fascicularis). All monkeys were healthy according to physical examination and electrocardiographic assessment and were housed in a facility accredited by AAALAC International in compliance with the recommendations found in the Guide for the Care and Use of Laboratory Animals8 and as mandated by the Animal Welfare Act. All activities were consistent with the AALAS Position Statement on the Humane Care and Use of Laboratory Animals.1 All animals were provided various enrichment devices. Relative humidity was controlled at 30% to 70%, and a commercial monkey feed was offered once or twice daily, with water provided ad libitum. Lighting was provided on a 12:12-h light:dark cycle. Monkeys were housed in individual cages and, depending on their behavior, adjacent animals were allowed to comingle during a portion of the day. The origins and ages of the monkeys varied, but all monkeys were older than 2 y of age.
The same sonographer (MMS) performed all echocardiographic examinations, which were performed at 1 of 2 facilities. Prior to echocardiographic assessment, the monkeys were sedated with either ketamine hydrochloride (10 mg/kg IM; Ketaset, Fort Dodge Animal Health, Overland Park, KS; facility 1) or with a combination of tiletamine hydrochloride plus zolazepam (4.0 mg/kg IM; Telazol, Fort Dodge Animal Health) and atropine sulfate (0.015 mg/kg IM; Atropine SA, Butler Animal Health, Dublin, OH; facility 2).
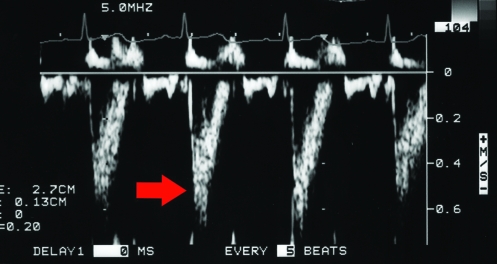
Echocardiographic evaluation was performed with the animal in right and left lateral recumbency by using a cut-out table and imaging from below. Standard 2-dimensional echocardiographic views were obtained from the right parasternal and left apical windows. Cardiac structures, including the interventricular septum during diastole (IVSd), left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs), left ventricular free wall in diastole (LVFWd), left atrial diameter (LA), and aortic diameter (AO) were measured using the leading-edge–to–leading-edge technique as previously described and recommended by the American Society of Echocardiography.9 Diastolic M-mode measurements were obtained at the beginning of the QRS interval, and the systolic dimension was obtained at the smallest LV dimension. The mitral valve E point to septal separation (EPSS) was measured as the distance between the E point of the mitral valve and the interventricular septum (Figure 1 A through C). The shortening fraction (FS) was calculated using the equation: (LVIDd-LVIDs)/LVIDd. The AO and LA measurements were obtained from the right parasternal short axis view at the heart base (Figure 2). These measurements were obtained in all monkeys at both sites (facility #1 and 2). Additional left ventricular (LV) functional parameters measured included the aortic peak flow velocity (AVmax), LV ejection time (LVET), and LV preejection period (LVPEP) at facility 1. These measurements were obtained from a pulsed wave Doppler tracing obtained at the aortic root (Figure 3) but were not possible with the equipment at facility 2.
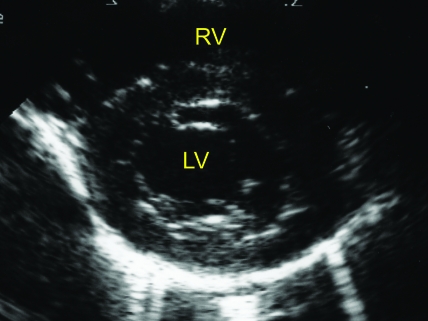
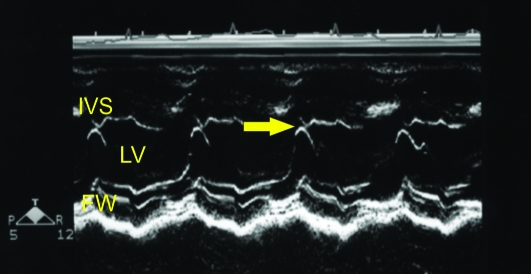
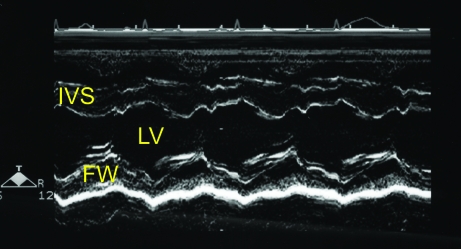
Figure 1.
(A) Right parasternal short axis echocardiogram obtained at the level of the mitral valve annulus. (B) M-mode image obtained from the level depicted in Figure 1A. The E point to septal separation would be obtained from this view (arrow). (C) M-mode image obtained at the ventricular level (slightly ventral to that depicted in Figure 1B). The ventricular dimensions and shortening fraction were obtained from M-mode imaging at this cardiac level. FW, left ventricular free wall. IVS- interventricular septum; LV, left ventricle; RV, right ventricle.
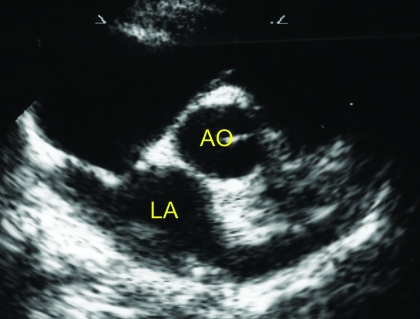
Figure 2.
Right parasternal short-axis view at the heart base. Ao, aorta; LA, left atrium.
Figure 3.
Pulsed-wave Doppler echocardiogram obtained from the left apical window with the sample volume placed in the ascending aorta. Note the laminar appearance of aortic blood flow (arrow).
At facility 1, monkeys were sedated with ketamine and were imaged with a Sonos 4500 ultrasound machine; a simultaneous lead II ECG was recorded and used for timing of cardiac events (Philips, Bothell, WA). At facility 2, animals sedated with tiletamine–zolazepam were evaluated with a Biosound Megas GPX ultrasound system (Indianapolis, IN). No other premedication was administered at either facility.
Descriptive statistics (mean, SD, and coefficient of variation [%CV]) were used to summarize the cardiac parameters separately by gender and type of sedation used. A Pearson correlation coefficient (r) was calculated for each parameter, separately by gender, to determine whether there was a significant relationship with body weight. A 1-way analysis of covariance (ANCOVA) model was used to assess gender-associated differences for each parameter, with body weight as the covariate to adjust for weight differences between genders. A similar model was used to evaluate differences in sedation; body weight was included in the ANCOVA model to adjust the sedation-type means for body weight differences. Reference ranges for the cardiac parameters were calculated by using 2-sided 95% tolerance intervals, with 99% coverage of the population.11 The normality of the distributions was verified prior to calculation of the reference ranges. The tolerance intervals give the range of values within which 99% of all future values should fall, with 95% confidence.
Results
Table 1 contains the echocardiographic parameters separately by gender; also included in the table are summary statistics for each parameter, the Pearson correlation coefficient describing the relationship between each parameter and body weight, and the reference range. The reference range for each parameter was determined by calculating a 95% tolerance interval, designed to cover 99% of all future values. Correlation coefficients that showed a statistically significant (P < 0.05) relationship between body weight and parameter are indicated. Weak but significant relationships with body weight for both genders were revealed for the following parameters: AO, LVET, LA:AO, LVIDd, and LVIDs. Weak but significant relationships were also noted in male monkeys for AVmax, heart rate (HR), LA, and LVFWd, and in female monkeys for body weight and EPSS. Gender-associated differences for each parameter were assessed by using an ANCOVA model, adjusting parameter means for possible body weight differences between genders. Based on the ANCOVA using the body-weight adjusted means, the following cardiac parameters demonstrated small but significant differences between genders: AO, LVET, LA:AO, and LVIDd (all P < 0.01) and IVSd (P < 0.05). In all cases, the differences were within 11%. Although the statistical analysis was performed by using body weight-adjusted means, arithmetic means are presented in Table 1.
Table 1.
Cardiac parameters of cynomolgus monkeys
| Female |
Male |
|||||||||||
| N | Mean | SD | CV (%) | r | Reference range | N | Mean | SD | CV (%) | r | Reference range | |
| BW (kg) | 118 | 2.56 | 0.42 | 16 | — | 1.47–3.67 | 119 | 3.49 | 0.71 | 20 | — | 1.63–5.36 |
| AO (cm) | 107 | 0.79c | 0.10 | 13 | 0.50a | 0.52–1.07 | 108 | 0.84 | 0.10 | 12 | 0.71a | 0.58–1.11 |
| AVmax (m/s) | 54 | 0.82 | 0.12 | 14 | –0.11 | 0.49–1.15 | 54 | 0.79 | 0.14 | 18 | –0.40a | 0.39–1.19 |
| LVET (s) | 118 | 0.19c | 0.03 | 15 | 0.27 | 0.12–0.27 | 119 | 0.20 | 0.03 | 15 | 0.45a | 0.12–0.28 |
| LVPEP (s) | 64 | 0.03 | 0.01 | 35 | 0.07 | 0.00–0.05 | 65 | 0.03 | 0.01 | 37 | 0.23 | 0.00–0.06 |
| LVPEP/ET | 64 | 0.14 | 0.04 | 31 | 0.09 | 0.02–0.26 | 65 | 0.15 | 0.04 | 30 | 0.21 | 0.02–0.27 |
| HR (bpm) | 117 | 161.36 | 28.24 | 18 | 0.12 | 86.52–236.20 | 119 | 152.39 | 28.39 | 19 | –0.22 | 77.22–227.56 |
| RR (ms) | 29 | 354.83 | 55.42 | 16 | 0.11 | 184.90–524.76 | 29 | 377.75 | 60.56 | 16 | –0.21 | 192.05–563.44 |
| EPSS (cm) | 118 | 0.01 | 0.07 | 592 | 0.19 | −0.18–0.21 | 119 | 0.02 | 0.15 | 602 | 0.03 | −0.36–0.41 |
| LA (cm) | 107 | 0.89 | 0.15 | 16 | 0.07 | 0.51–1.28 | 108 | 0.99 | 0.16 | 16 | 0.31 | 0.55–1.42 |
| LA:AO | 107 | 1.14c | 0.19 | 17 | –0.30 | 0.62–1.66 | 108 | 1.18 | 0.18 | 16 | –0.20a | 0.69–1.66 |
| IVSd (cm) | 107 | 0.37b | 0.06 | 16 | –0.07 | 0.21–0.53 | 108 | 0.40 | 0.07 | 17 | 0.09 | 0.22–0.58 |
| LVFWd (cm) | 107 | 0.36 | 0.05 | 14 | –0.16 | 0.22–0.50 | 108 | 0.39 | 0.07 | 17 | 0.37 | 0.21–0.57 |
| LVIDd (cm) | 118 | 1.35c | 0.16 | 12 | 0.29a | 0.92–1.77 | 119 | 1.50 | 0.17 | 12 | 0.32a | 1.05–1.96 |
| LVIDs (cm) | 118 | 0.81 | 0.14 | 18 | 0.28a | 0.43–1.19 | 119 | 0.92 | 0.15 | 16 | 0.34a | 0.52–1.31 |
| FS (%) | 118 | 39.93 | 7.88 | 20 | –0.08 | 19.05–60.82 | 119 | 39.15 | 6.05 | 16 | –0.15 | 23.13–55.17 |
r, Pearson coefficient of correlation between indicated parameter and body weight.
Correlation between the indicated parameter and body weight is statistically significant (P < 0.05).
Value for female monkeys differs significantly (P < 0.05; 1-way ANCOVA of body-weight-adjusted means) from that for males.
Value for female monkeys differs significantly (P < 0.01; 1-way ANCOVA of body-weight-adjusted means) from that for males.
Table 2 is a summary of the cardiac parameters, separately by gender and sedation administered. The tiletamine hydrochloride plus zolazepam–atropine and ketamine sedation methods each were used at different facilities, which also differed in the equipment used for echocardiography. Therefore, differences between sedation methods might also be attributed to differences in equipment. Sedation method means were compared by ANCOVA, after adjustment of parameters for possible body weight differences between the animals at the 2 facilities. For male monkeys, significant (P < 0.01) differences between sedation methods emerged for AO, LVET, R-to-R interval (RR), IVSd, and LVFWd. For female monkeys, we noted significant differences for AO (P < 0.05) and IVSd (P < 0.01). The mean values for the 2 sedation methods were generally within 12% of each other, and no single method consistently produced higher or lower values than did the other. Although the statistical analysis was performed by using adjusted means, arithmetic means are presented in Table 2.
Table 2.
Cardiac parameters of cynomolgus monkeys by sedation type
| Female |
Male |
|||||||||||||||
| Telazol–atropine |
Ketamine |
Telazol–atropine |
Ketamine |
|||||||||||||
| N | Mean | SD | CV (%) | N | Mean | SD | CV (%) | N | Mean | SD | CV (%) | N | Mean | SD | CV (%) | |
| BW (kg) | 64 | 2.35 | 0.35 | 15 | 54 | 2.82 | 0.34 | 12 | 65 | 3.24 | 0.49 | 15 | 54 | 3.80 | 0.80 | 21 |
| AO (cm) | 53 | 0.74 | 0.10 | 13 | 54 | 0.84a | 0.09 | 10 | 54 | 0.79 | 0.07 | 9 | 54 | 0.89b | 0.10 | 11 |
| AVmax (m/s) | nd | nd | nd | nd | 54 | 0.82 | 0.12 | 14 | nd | nd | nd | nd | 54 | 0.79 | 0.14 | 18 |
| LVET (s) | 64 | 0.19 | 0.03 | 14 | 54 | 0.20 | 0.03 | 15 | 65 | 0.19 | 0.02 | 12 | 54 | 0.21b | 0.03 | 14 |
| LVPEP (s) | 64 | 0.03 | 0.01 | 35 | nd | nd | nd | nd | 65 | 0.03 | 0.01 | 37 | nd | nd | nd | nd |
| LVPEP/ET | 64 | 0.14 | 0.04 | 31 | nd | nd | nd | nd | 65 | 0.15 | 0.04 | 30 | nd | nd | nd | nd |
| HR (bpm) | 63 | 156.33 | 30.31 | 19 | 54 | 167.42 | 24.60 | 15 | 65 | 157.01 | 29.35 | 19 | 54 | 146.82 | 26.40 | 18 |
| RR (ms) | 11 | 336.92 | 66.51 | 20 | 18 | 365.78 | 46.06 | 13 | 11 | 332.43 | 40.42 | 12 | 18 | 405.44b | 54.21 | 13 |
| EPSS (cm) | 64 | 0.00 | 0.02 | 453 | 54 | 0.02 | 0.11 | 476 | 65 | 0.00 | 0.01 | 462 | 54 | 0.05 | 0.21 | 425 |
| LA (cm) | 53 | 0.88 | 0.15 | 17 | 54 | 0.90 | 0.14 | 16 | 54 | 0.97 | 0.17 | 17 | 54 | 1.01 | 0.16 | 16 |
| LA:AO | 53 | 1.20 | 0.19 | 16 | 54 | 1.08 | 0.18 | 17 | 54 | 1.22 | 0.19 | 15 | 54 | 1.13 | 0.17 | 15 |
| IVSd (cm) | 53 | 0.39 | 0.06 | 15 | 54 | 0.35b | 0.06 | 16 | 54 | 0.42 | 0.06 | 14 | 54 | 0.38b | 0.07 | 18 |
| LVFWd (cm) | 53 | 0.38 | 0.05 | 13 | 54 | 0.35 | 0.05 | 15 | 54 | 0.40 | 0.06 | 14 | 54 | 0.38b | 0.08 | 20 |
| LVIDd (cm) | 64 | 1.32 | 0.13 | 10 | 54 | 1.38 | 0.18 | 13 | 65 | 1.48 | 0.17 | 11 | 54 | 1.53 | 0.18 | 12 |
| LVIDs (cm) | 64 | 0.78 | 0.11 | 14 | 54 | 0.84 | 0.17 | 20 | 65 | 0.89 | 0.13 | 14 | 54 | 0.95 | 0.17 | 18 |
| FS (%) | 64 | 40.43 | 8.13 | 20 | 54 | 39.35 | 7.62 | 19 | 65 | 39.67 | 5.57 | 14 | 54 | 38.53 | 6.58 | 17 |
Value significantly (P < 0.05; 1-way ANCOVA of body-weight adjusted means) different than that for Telazol–atropine.
Value significantly (P < 0.01; 1-way ANCOVA of body-weight adjusted means) different than that for Telazol–atropine.
Discussion
We used a large number of healthy subjects (118 of each gender) to develop gender-specific echocardiographic reference ranges for cynomolgus monkeys. The large sample sizes for most parameters improved the description of the statistical distribution for each parameter, the mean for each parameter, and clarified the variability inherent in the data. Interpretation of the significance of sedation-associated differences should be approached with caution, because these differences might also be attributed to the clinical setting, personnel, and echocardiographic equipment used. A previous study3 demonstrated variation in cardiac histology in monkeys from different geographic origins (Philippines, Mauritius, Vietnam), but they noted no significant variation in relative heart weight. Given this data, we did not expect echocardiographic assessment of heart size to differ among animals on the basis of origin. In the cited study,3 46 of the 90 monkeys had focal inflammatory cell infiltration of the myocardium, with occasional eosinophilic granulocyte infiltrates in the epicardial adipose tissue. Additional occasional findings (1 individual each) were: minimal focal fibrosis, focal myocardial mucin deposition, ectopic epithelial structures, and blood-filled valvular cysts.3 However, these changes would not be evident by echocardiography.
A limitation of the current study is that the subjects were evaluated at only 1 time point, and therefore longitudinal data are not available. In addition, the ages of the animals were often unknown however, all monkeys were older than 2 y of age and our data were corrected to adjust for body weight. Therefore, despite these study limitations, the echocardiographic reference ranges we derived from these normal, adult cynomolgus monkeys likely will prove useful for future biomedical research using this species, especially when noninvasive serial evaluation of cardiac structure and function are indicated. In particular, these reference values may enable investigators to reduce the numbers of animal needed and thus also reduce experimental costs.
References
- 1.American Association for Laboratory Animal Science 2007. AALAS position statement on the humane care and use of laboratory animals. Comp Med 57:518. [PubMed] [Google Scholar]
- 2.Dhindsa DS, Ochsner AJ. 1981. Hemodynamic and metabolic effects of chronic carbon monoxide exposure in cynomolgus monkeys (Macaca fascicularis). J Med Primatol 10:255–262 [DOI] [PubMed] [Google Scholar]
- 3.Drevon-Gaillot E, Perron-Lepage M, Clement C, Burnett R. 2006. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol 58:77–88 [DOI] [PubMed] [Google Scholar]
- 4.Hendrickx AG, Peterson P, Hartmann D, Hummler H. 2000. Vitamin A teratogenicity and risk assessment in the macaque retinoid model. Reprod Toxicol 14:311–323 [DOI] [PubMed] [Google Scholar]
- 5.Hulsebos LH, Oliver NB, Goldenthal EI. 1998. Echocardiographic measurements in normal cynomolgus monkeys [abstract]. Lab Anim Sci 48:403 [Google Scholar]
- 6.Keenan CM, Vidal JD. 2006. Standard morphologic evaluation of the heart in the laboratory dog and monkey. Toxicol Pathol 34:67–74 [DOI] [PubMed] [Google Scholar]
- 7.Korcarz CE, Padrid PA, Shroff SG, Weinert L, Lang RM. 1997. Doppler echocardiographic refernce values for healthy rhesus monkeys under ketamine hydrochloride sedation. J Med Primatol 26:287–298 [DOI] [PubMed] [Google Scholar]
- 8.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 9.Picard MH. 1994. M-mode echocardiography: principles and examination techniques. In: Weyman AE. Principles and practice of echocardiography, 2nd ed.Philadelphia (PA): Lea and Febiger; p 282–300 [Google Scholar]
- 10.Tsusaki H, Yonamine H, Tamai A, Shimomoto M, Iwao H, Nagata R, Kito G. 2005. Evaluation of cardiac function in primates using real-time three-dimensional echocardiography as applications to safety assessment. J Pharmacol Toxicol Methods 52:182–187 [DOI] [PubMed] [Google Scholar]
- 11.Vardeman SB. 1992. What about the other intervals? Am Stat 46:193–197 [Google Scholar]