Abstract
A rhesus macaque (Macaca mulatta) infected with simian-human immunodeficiency virus (SHIV) while undergoing AIDS research, required a comprehensive physical examination when it presented with slight peripheral edema, hypoalbuminemia, and proteinuria. Many of the clinical findings were consistent with nephrotic syndrome, which is an indication of glomerular disease, but the possibility of concurrent disease needed to be considered because lentiviral induced immune deficiency disease manifests multiple clinical syndromes. The animal was euthanized when its condition deteriorated despite supportive care that included colloidal fluid therapy. Histopathology confirmed membranoproliferative glomerulonephritis, the result of immune complex deposition most likely due to chronic SHIV infection. Clinical symptoms associated with this histopathology in SHIV-infected macaques have not previously been described. Here we offer suggestions for the medical management of this condition, which entails inhibition of the renin–angiotensin–aldosterone system and diet modifications.
Abbreviations: ACE, angiotensin-converting enzyme; SHIV, simian–human immunodeficiency virus
The development of the simian-immunodeficiency virus (SHIV) chimera for HIV-AIDS research was based on findings that SIV is genetically similar to HIV-2, and certain strains of SIV which are endemic and nonpathogenic in African old world primates—SIVagm from the African green monkey (Chlorocebus aethiops), and SIVsm from the sooty mangabey (Cercocebus atys)—can cause pathogenic disease when inoculated into Asian macaques.33 Investigators eventually isolated SIV strains from other species of nonhuman primates including the rhesus monkey (Macaca mulatta), SIVmac.33 Variants of SIVmac were later created which also had the ability to cause pathogenic disease in macaques.24,39,45,50
The first-generation SHIV chimera was generated in 1990, when investigators sought to combine SIVagm and HIV-1 to identify viral determinants for the narrow host range of HIV-1.47 Initially 2 types of chimeras were created—one virus carried an HIV-1 core with the SIV envelope, whereas the other had an SIV core and the HIV envelope. The construct with the SIV envelope did not replicate in monkey cells, whereas the one with the HIV envelope did.47 In time, researchers changed from the parental SIVagm strain to SIVmac, creating a construct with an SIV core and HIV-1 envelope that was infectious in vivo.45 Eventually highly pathogenic SHIVs were derived from nonpathogenic SHIVs through animal-to-animal passage.24,39,46 The pathogenic strains were able to induce a nearly complete depletion of circulating CD4+ T lymphocytes in macaques within weeks of infection, followed by an AIDS-like syndrome.24,39
Research to create a successful vaccine in man against HIV-AIDS is on going.2 The SHIV chimera in the macaque model for AIDS has been useful in vaccine development by serving as the challenge agent, with the level of infection assessed by monitoring plasma viral RNA load and virus-induced CD4+ T cell depletion.4 In regards to CD4+ T cell depletion, chronic disease as seen in humans is not duplicated in the highly pathogenic SHIV macaque model for HIV–AIDS. The course of the CD4 depletion in nonhuman primate is too rapid, resulting in death in 3 to 4 mo.22,24,39 However, lentivirus (SIV) infected macaques parallel many of the other clinical syndromes of AIDS patients, namely neuropathy, cardiomyopathy, enteropathy, and nephropathy,3,27,28,35,43,49 with SHIV-infected animals presumed to have the same capability.
Case Report
Case history.
In August 2005, a 9.7-kg, 7-y-old, singly housed, colony-bred, male rhesus monkey (Macaca mulatta) undergoing AIDS research presented with slightly edematous areas around the abdomen and below the rib cage and an erythrematous rash on the medial thighs and left axillary area. Later, edema in the scrotal area became apparent. The animal's simian retrovirus status was unknown, and there was no history of testing for Cercopithecine herpesvirus 1 (B virus). A measles vaccine (Attenuvax, Merck, Whitehouse Station, NJ) had been administered in July 1999, and the animal was inoculated in June 2002 with an SHIV (HIV envelope and SIV core DNA) mixture recovered from a late-stage infection.21,45 The study was approved by the institutional animal care and use committee, and the research was conducted in accordance with Public Health Service policies and federal regulations.
Husbandry.
The animal was housed in accordance with the Guide for the Care of and Use of Laboratory Animals37 under a 12:12-h light cycle at a room temperature of 17.8 to 28.9 °C and a relative humidity of 30% to 70%. Water was provided through an automatic water system, and the diet consisted of a high-fiber chow (Primate Diet 8788, Harlan Teklad, Madison, WI), fruits, and treats (Bio-serv, Frenchtown, NJ).
Case history and diagnostic approach.
Clinically significant findings from the initial hematology and serum chemistry were: anemia (PCV, 22.8%; RBC count, 3.51 × 106/μl), hypoalbuminemia (1.2 g/dl), and hypoproteinemia (4.4 g/dl; Table 1). The urinalysis performed on a pan-collected sample by using a chemistry strip (N-Multistix SG Reagent Strip, Bayer, Shawnee Mission, KS) revealed occult hematuria (3+) and proteinuria (3+). The urine specific gravity was 1.012 according to a handheld clinical refractometer (Atago, Tokyo, Japan; Table 3). Abnormalities included (but were not limited to): clinical peripheral edema, low serum total protein and albumin, anemia, proteinuria, and hematuria. Because the macaque was on an automatic watering system that provided continuous access to water, assessment of polyuria and polydipsia was difficult. Given the available findings, however, a tentative diagnosis of protein-losing nephropathy with cystitis was made.
Table 1.
Hematology
| PCV | RBC | Hemoglobin | WBC | CD4+ T cells/μl | |
| Date | 39.9–44.9% a | 6.6–7.2 × 106/μla | 12.9–14.3 g/dla | 8.9–14.7 × 103/μla | 1237b |
| 8/25/2005 | 28.4 | 4.32 | 9.8 | 12.5 | 614 |
| 9/29/2005 | 27.7 | 4.14 | 9.3 | 6.01 | 445 |
| 10/27/2005 | 30 | 4.54 | 10.1 | 9.1 | 523 |
| 11/23/2005 | 31.4 | 4.63 | 10.2 | 11.3 | 765 |
| 12/7/2005 | 27 | 4.24 | 9.2 | 10.5 | not available |
Normal ranges for healthy macaques.6
Baseline value for this animal.
Table 3.
Urinalysis
| Date | Specific gravity | Blood | Protein | Comments |
| 8/25/2005 | 1.012 | 3+ | 3+ | Collected from pan with plastic liner; sample clear yellow in color; refractometer used; cytology not performed |
| 9/23/2005 | 1.012 | 3+ | 2+ | Collected from pan with plastic liner; sample clear yellow in color; refractometer used; cytology not performed |
| 11/18/2005 | 1.010 | 3+ | 3+ | Collected from pan with plastic liner; sample clear yellow in color; refractometer used; cytology revealed myriad bacteria with plant debris and some desquamated epithelial cells, but red blood cells were not a prominent finding |
| 12/7/2005 | 1.005 | none | 3+ | Collected by cystocentesis; sample clear yellow in color; refractometer used; cytology revealed predominately epithelial cells with few neutrophils and lymphocytes; culture negative after 10 d |
| 12/15/2005 | 1.010 | none | 2+ | Collected by cystocentesis at necropsy; sample clear yellow in color; reagent strip used; cytology not performed; culture negative after 10 d |
Serial blood samples revealed anemia, azotemia, hypoalbuminema, and hypoproteinemia (Table 2). Proteinuria remained a consistent finding on multiple urinalyses, with cytology revealing epithelial cells as a predominate finding after centrifugation of urine samples at 55 × g for 4 min (Statspin Cytocentrifuge, Iris Sample Processing, Westwood, MA).
Table 2.
Serum chemistry
| BUN | Creatinine | Total protein | Albumin | Cholesterol | |
| Date | 17–23 mg/dla | 1.0–1.2 mg/dla | 7.3–8.3 g/dla | 4.1–4.9 g/dla | 133–177 mg/dla |
| 8/25/2005 | 43 | 1.5 | 4.4 | 1.2 | 137 |
| 9/29/2005 | 51 | 1.3 | 4.6 | 1.7 | 133 |
| 10/27/2005 | 60 | 1.7 | 4.2 | 1.3 | 187 |
| 11/23/2005 | 71 | 1.4 | 4.1 | 1.3 | 171 |
| 12/7/2005 | 67 | 1.5 | 3.1 | 0.9 | 156 |
Normal ranges for healthy macaques.6
We also explored other possibilities for the clinical findings (namely, intestinal malabsorption, hepatic impairment, and cardiovascular disease) to ensure that no other problems were present. The persistent anemia was surmised to be the combined result of SHIV infection and renal impairment. Our animal had no history of diarrhea, therefore protein loss through the intestinal tract was ruled out.13 Hypoproteinemia and hypoalbuminemia can be indications of impaired hepatic function. Protein production (of albumin, clotting factors, and α and β globulins), bile acid synthesis and excretion, and ammonia metabolism are measures of liver function.18,25,54 Our animal did not display neurologic dysfunction (ammonia metabolism), jaundice (bile synthesis and secretion) or signs of abnormal hemostasis (clotting factor production). The lack of clinical signs combined with unremarkable hepatocellular enzyme and total bilirubin values ruled out hepatic impairment as a concurrent condition (Table 2).
Some SIV infections result in cardiomyopathy that ultimately progresses to congestive heart failure with peripheral edema.43,44 Although this animal was inoculated with an SHIV chimera, we assumed that it had the same predisposition to develop cardiac disease as an SIV-infected animal. Radiography, electrocardiography, and an echocardiogram were performed under ketamine hydrochloride (Ketaset, Fort Dodge Animal Health, Madison, NJ) anesthesia. The cardiac silhouette on the radiographs was unremarkable, and electrocardiography revealed a normal rate and rhythm with no apparent conduction disturbances. The echocardiogram (Acuson Cypress portable ultrasound system, Siemens, Malvern, PA) obtained by using a 2.5-mHz transducer to generate images in M-mode and Doppler revealed an estimated ejection fraction of 70%, a right ventricle of normal size and function, no resting wall motion abnormalities, no evidence of valvular regurgitation, and no evidence of pericardial effusion.29,41 The results of the cardiac work-up ruled out congestive heart failure as a factor in the animal's condition.
Medical management.
From August to December 2005, the animal was placed on various treatment regimens, which included hydroxyethyl starch (10 to 20 ml/kg; Hextend, BioTime, Emeryville, CA) combined with lactated Ringer solution, vitamin B complex (0.1 ml/kg IM once daily; Fortified B Complex, Agri-Labs, St Joseph, MO), iron (1 ml/10 kg IM once daily; Iron Injectable, Vedco, St Joseph, MO), cefazolin sodium (25 mg/kg IM twice daily; Cefazolin, Eli Lilly, Indianapolis, IN), ketoprofen (2 mg/kg IM once daily; Ketofen, Merial, Duluth, GA), and furosemide (2 mg/kg IM once daily; Lasix, Sanofi-Aventis, Bridgewater, NJ). In addition, the animal experienced bouts of anorexia and weight loss (0.43 kg), which required the addition of extra fruit and supplements (Bio-serv) to the diet.
Cefazolin sodium was administered for 2 wk during the first month; ketoprofen also was administered during the first month but was discontinued after 3 d. Hydroxyl ethyl starch and furosemide were administered bimonthly for 3 d. Vitamin B complex injections were given for 1 wk every month; iron injections were given twice each month.
Some clinical improvement became evident between late October and late November 2005. The PCV increased to 30%, the animal gained weight, and the edema and skin erythrema disappeared (Table 1). However, the macaque's condition deteriorated in December 2005, when the animal became depressed and anorexic, with facial and scrotal edema and an erythrematous rash on the face. The animal developed diarrhea and as a result received erythromycin (25 mg/kg PO twice daily; Bio-serv), bismuth subsalicylate tablets (262 mg PO once or twice daily; Pepto-Bismol, Proctor and Gamble, Cincinnati, OH), and loperamide (0.5 mg PO once or twice daily; Imodium, Johnson and Johnson, New Brunswick, NJ). All medications were administered for 1 wk. The hematology and serum chemistry revealed a PCV of 27%, hemoglobin of 9.2 g/dl, total protein of 3.1 g/dl, albumin of 0.9 g/dl, and a urine specific gravity of 1.005 by refractometer (Tables 1 through 3). Physical examination revealed a turgid abdomen; the internal organs were difficult to assess. For humane reasons, the animal was euthanized with sodium pentobarbital (0.5 ml/kg IV; Beuthanasia-D, Schering-Plough, Kenilworth, NJ).
The previous value of 1.012 for the urine specific gravity of this animal was reviewed after euthanasia to assess whether it was an indication of impaired renal concentrating ability. A water deprivation test could have provided more insight into this aspect of renal function, but it was contraindicated in light of the animal's condition. A normal reference range for the urine specific gravity of rhesus monkeys has not been published, and comparison to those for dogs (1.015 to 1.040) and man (1.002 to 1.050)51 did not provide a clear answer. In an effort to determine a normal range for urine specific gravity in rhesus monkeys, samples collected from 20 healthy animals of mixed gender were measured and compared. The normal subjects ranged in age from 5 to 8 y and were housed under the same conditions as our macaque. Samples were collected from plastic-lined pans at 0900 and 1400.
The specific gravity of the morning samples ranged 1.001 to 1.015, and the afternoon sample values ranged from 1.000 to 1.009. The mean for morning and afternoon combined was 1.006 (95% confidence interval, 1.004 to 1.007). A paired Wilcoxon test indicated that the morning sample was significantly (P = 0.0236) more concentrated than the afternoon sample. In light of comparison to the colony values, we surmised that the value of 1.012 was probably a low-normal value for our animal and that the profound decrease to 1.005 before euthanasia was a true indication of loss of concentrating ability.
Necropsy findings.
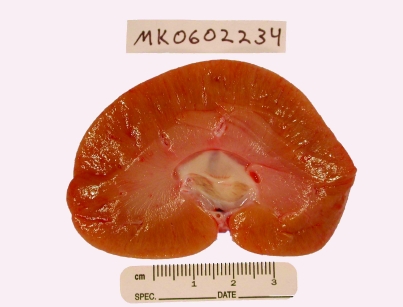
A complete necropsy was performed on the day of euthanasia. Grossly the animal was hydrated, well muscled with a small amount of body fat, and weighed 8.9 kg. Moderate subcutaneous edema was noted along the ventral mandible, abdomen, and scrotum. Approximately 150 ml of clear, colorless fluid with a total protein of 1.4 g/ dl as assessed by a total-solids meter (American Optical, Southbridge, MA), was present in the abdominal cavity. The heart appeared normal and weighed 44.9 g, with the left ventricle measuring 1.0 cm, septum 0.75 cm, and right ventricle 0.25 cm in thickness. Both kidneys were severely enlarged, pale, and tan. The right kidney weighed 64.9 g and measured 6.8 × 4.5 × 3.0 cm; the left kidney weighed 67.4 g and measured 7.0 × 4.0 × 2.8 cm (Figure 1). Normal kidneys in adult rhesus macaques measure approximately 3.5 × 2.5 × 1.5 cm.20 The ureters and urinary bladder appeared normal. Urinalysis of the small amount of urine in the bladder revealed a specific gravity of 1.010 with 2+ protein (N-Multistix SG reagent strip, Bayer; Table 3).
Figure 1.
Midsagittal section of right kidney. The kidney is severely enlarged, pale, and tan. Dimensions, 6.8 × 4.5 × 3.0 cm; weight, 64.9 g.
The lungs, liver, gall bladder, and adrenal glands appeared normal. The stomach, small intestine, cecum, and colon appeared grossly normal, with formed content present in the colon. Interlobular edema was noted in the pancreas and was associated with the ascites. The spleen was mildly enlarged uniformly. The reproductive system (namely, the testes and epididymis) appeared normal. All lymph nodes were moderately enlarged but otherwise appeared normal. The brain appeared grossly normal. Sections of all major organs were fixed in 10% formalin for histopathology, which revealed mild plasmacytic colitis, mild nonsuppurative pulmonary perivasculitis, mild lymphoid hyperplasia of lymph nodes and spleen, and mild hemosiderosis of the spleen and liver.
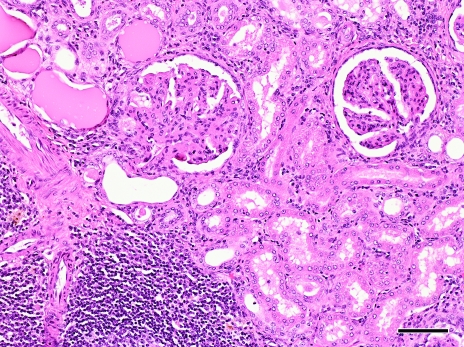
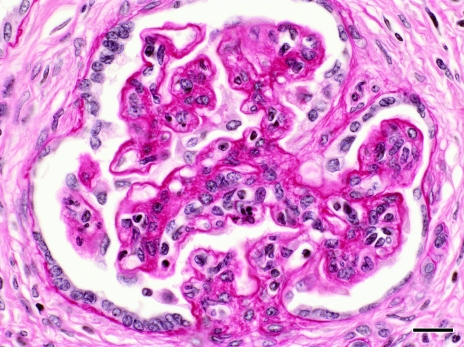
The kidneys demonstrated bilateral severe diffuse membranoproliferative glomerulonephritis characterized by the following glomerular changes: enlarged glomeruli with thickened capillary basement membranes (‘wire loops’), increased numbers of parietal and visceral epithelial cells, increased mesangial matrix and adhesions to the Bowman capsule. Glomeruli contained small numbers of necrotic cells and associated neutrophils. Prominent dilated tubules were also present and contained proteinaceous fluid. Moderate lymphoplasmacytic interstitial nephritis and multifocal lymphoid aggregates with associated tubular loss and tubular regeneration were present (Figure 2). Diffuse basement membrane thickening was apparent (Figure 3). Electron microscopy of the kidney revealed dense deposits consistent with immune deposition primarily in the basement membrane, with lesser amounts on the epithelial side of the basement membrane. These dense deposits formed humps, and the basement membrane was markedly thickened and wrinkled (Figure 4).
Figure 2.
Light microscopic section of kidney stained with hematoxylin and eosin, demonstrating glomerular adhesions to the Bowman capsule and hypercellularity of the glomerular tuft. Several dilated tubules containing proteinaceous fluid are present. Multifocal lymphocytic infiltrates are present in the interstitium. Magnification, ×100; bar, 100 μm.
Figure 3.
Periodic acid–Schiff-stained section of affected glomerulus, demonstrating thickening of basement membranes. Magnification, ×400; bar, 20 μm.
Figure 4.
Numerous dense deposits (arrows) are within basement membrane and the epithelial side of the basement membrane. The basement membrane is markedly thickened and wrinkled. Podocytes are fused. B, basement membrane; P, podocytes; N, nucleus. Bar, 1.0 μm.
Discussion
Many of the clinical findings in this case—hypoalbuminemia, proteinuria, and peripheral edema—were consistent with nephrotic syndrome, an indicator of protein-losing glomerular disease.16,53 Many glomerular diseases can result in proteinuria with hypoalbuminemia, including (but not limited to) minimal-change glomerulopathy, focal and segmental glomerulosclerosis, membranous glomerulonephritis, membranoproliferative glomerulonephritis, collagenofibrotic glomerulonephropathy, and IgA nephropathy.15 Acquired glomerular disease can be the result of damage sustained after immune complex formation or deposition or after amyloid deposition.53 In this case, the animal developed membranoproliferative glomerulonephritis that most likely was due to immune complex deposition as a result of chronic SHIV infection. Protein-losing renal disease with this histopathology in SHIV-infected macaques has not been previously described.1,7,8,16,17,49,50
Patients with AIDS can display various syndromes including HIV-induced cardiomyopathy, enteropathy, and nephropathy.3,30,35,42 In regard to nephropathy, the HIV-associated renal diseases in humans encompass a spectrum of nephropathies that can result in proteinuria and ultimately renal failure. The pathology of chronic renal failure has been grouped into 3 categories: focal and segmental glomerulosclerosis, thrombotic microangiopathies, and immune complex disease.55 One result of immune complex disease is membranoproliferative glomerulonephritis.5, 32,34 Some patients with membranoproliferative glomerulonephritis are asymptomatic. Others present with acute nephritic syndrome (hypertension, proteinuria, microscopic hematuria with red blood cell casts), nephrotic syndrome (proteinuria, edema, hypoalbuminemia with hypercholesterolemia), or a combination thereof.36,52
SIV-infected macaques develop SIV-induced syndromes that parallel the HIV induced syndromes.27,43,49 SHIV-infected animals are assumed to have the same potential. The nephropathy found in our macaque, membranoproliferative glomerulonephritis, had a similar presentation to that described in humans. Although we ruled out an enteropathy in this case, the pathology of gastrointestinal disease in SIV- infected animals is similar to that found in AIDS patients.27,28,30 Some AIDS patients however are hypoalbuminemic as a result of a concurrent protein losing enteropathy yet current literature does not describe a similar association in SIV infected animals.27,28,30
Medical management of human patients experiencing proteinuria as a result of glomerular disease entails the quantification of protein loss, blood pressure monitoring, inhibition of the renin–angiotensin–aldosterone system, and diet modifications.5,38,57 Companion animal medicine mirrors the same practices.11 Quantification of urine protein can be accomplished by measuring urine protein levels over a 24-h period or by determining the urine protein-to-creatinine ratio.57 Because 24-h measurement is tedious, the preferred method is to determine the protein-to-creatinine ratio.57 This ratio also is used routinely in companion animals to assess the severity of renal disease however more study is required to define normal values for the rhesus monkey.11
Inhibition of the renin–angiotensin–aldosterone system by using an angiotensin-converting enzyme (ACE) inhibitor combined with spironolactone reduces blood pressure within the glomeruli, resulting in a decrease in protein leakage.5,38 ACE inhibitors such as enalapril, captopril, benzepril, and ramipril can be used to prevent the formation of angiotensin II, which is a hypertensive agent. ACE inhibitors may be combined with angiotensin receptor blockers such as losartan to further reduce the blood pressure, however close monitoring is required to control the degree of hypotension.5,57 Spironolactone, a synthetic 17-lactone steroid, is the diuretic of choice for patients with glomerular proteinuria. Because it competes with aldosterone, the hormone that causes water retention, spironolactone is preferred over furosemide. Water retention is a natural mechanism for increasing blood pressure, and the competitive effect of spironolactone reduces fluid retention, resulting in blood pressure reduction.38 Spironolactone has the added benefit of reducing progressive proteinuria independent of blood pressure.14,57 The combination of spironolactone (a potassium-sparing diuretic) with an ACE inhibitor results in hyperkalemia, which requires monitoring and management.57
Managing our animal's condition with an ACE inhibitor and spironolactone had potential benefits. Suggested regimens for ACE inhibitors are captopril at 1 mg/kg PO daily or enalapril at an initial dose of 1 mg/kg IV daily for 8 wk followed by an additional 4-wk treatment at the same dose PO.19,40 The enalapril regimen comes from a study in cynomologus monkeys where the effects of bovine gamma globulin-induced immune complex glomerulonephritis were mitigated by a decrease in immune complex deposition through blood pressure reduction.19 An effective dose of spironolactone has not been described for macaques with glomerular disease, but the recommended dose for human patients in chronic renal failure is 12.5 to 50 mg daily.31
Dietary management for patients and companion animals experiencing glomerular disease entails the restriction of protein and salt. However, no dietary restrictions were placed in this case because the animal needed to eat and gain weight. The addition of ω3 polyunsaturated fatty acids (ω3 fatty acids) can be beneficial for patients and companion animals with glomerular disease.11,12 These fatty acids have been found to be renoprotective in dogs in renal failure and mitigate hypertension and lower cholesterol concentrations in human patients with nephrotic syndrome.11 The ω3 fatty acids cause immune modulation by serving as substrates for the cyclooxygenase and lipooxygenase pathways resulting in the production of less potent inflammatory mediators.12,48 Eicosapentaenoic acid and docosahexaenoic acid, found in fish oils, are more biologically potent than is linoleic acid.48 An effective dose has not been described for the nonhuman primate, but an effective dose in patients diagnosed with IgA nephropathy was 1.8 g eicosapentaenoic acid and 1.2 g docosahexaenoic acid; in 2 y a decreased progression of renal disease was noted.12
Other treatments for human patients experiencing glomerular disease with proteinuria are dialysis, renal transplantation, and immunosuppressive agents (for example, corticosteroids and cyclosporine).5,55 Dialysis and renal transplantation are not practical options for nonhuman primates, and corticosteroids and cyclosporine were contraindicated for the research in which our macaque was involved. Nonsteroidal antiinflammatory drugs have nephrotoxic potential and are used with caution in cases of renal impairment.9 Ketoprofen was acceptable for use in this animal because it was administered for a short duration (3 d) at a dose to minimize discomfort (2 mg/kg).
Our use of the diuretic furosemide and hydroxyethyl starch provided symptomatic treatment for the peripheral edema caused by hypoalbuminemia. Albumin, a natural colloid in blood plasma, is predominately responsible for exerting oncotic pressure within the capillary bed. To prevent peripheral edema, a balance must be maintained between hydrostatic and oncotic pressures.10,23 Hydroxyethyl starch, which is an artificial colloid, was used to raise the oncotic pressure and restore balance.10 Although the peripheral edema temporarily resolved with no adverse effects of hemorrhage or pruritis from the hydroxyethyl starch,26,56 the treatment did not reduce protein loss from the glomerulus.
Conclusion.
Findings of peripheral edema with hypoalbuminemia in a SHIV-infected macaque used for AIDS research required a careful review of many organ systems because of the clinical presentation and the different syndromes that this model can exhibit. To our knowledge, this is the first report of membranoproliferative glomerulonephritis in an SHIV-infected macaque with hypoalbuminemia and proteinuria consistent with nephrotic syndrome. The condition was presumed to have occurred as a result of immune complex deposition triggered by the chronic SHIV infection.
Protein loss from the glomeruli can be managed by inhibiting the renin- angiotensin-aldosterone system with spironolactone and an ACE inhibitor. This regimen had potential benefits for this case. More research is necessary to establish a normal reference range for the urine protein-to-creatinine ratio in rhesus macaques to aid in the management of protein-losing glomerular disease. Additional research is also necessary to determine whether dietary supplementation with Omega 3 fatty acids can reduce the effects of glomerulonephritis in rhesus monkeys.
Acknowledgments
We thank Malcolm Martin (Chief, Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, MD) and Tatsuhiko Igarashi (Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases) for their consent to publish. We also thank Jing Qin (Biostudies Branch, National Institute of Allergy and Infectious Diseases) for the statistical analysis and Richard Herbert and staff (National Institutes of Health Animal Center at Poolesville) for technical support.
References
- 1.Adachi K, Mori T, Ito T, Fujii E, Suzuki S, Kawai T, Suzuki M. 2005. Collagenofibrotic glomerulonephropathy in a cynomologus macaque (Macaca fascicularis). Vet Pathol 42:669–674 [DOI] [PubMed] [Google Scholar]
- 2.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. 2001. Control of a mucosal challenge and the prevention of AIDS by a multiprotein MVA/DNA vaccine. Science 292:69–74 [DOI] [PubMed] [Google Scholar]
- 3.Barbaro G, Fisher SD, Lipshultz SE. 2001. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis 1:115–123 [DOI] [PubMed] [Google Scholar]
- 4.Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, Bilska M, Craiu A, Zheng XX, Krivulka GR, Beaudry K, Lifton MA, Nickerson CE, Trigona WL, Punt K, Freed DC, Guan L, Dubey S, Casimiro D, Simon A, Davies ME, Chastain M, Strom TB, Gelman RS, Montefiori DC, Lewis MG, Emini EA, Shiver JW, Letvin NL. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486–492 [DOI] [PubMed] [Google Scholar]
- 5.Berggren R, Batuman V. 2005. HIV-associated renal disorders: recent insights into pathogenesis and treatment. Curr HIV/AIDS Rep 2:109–115 [DOI] [PubMed] [Google Scholar]
- 6.Bernacky BJ, Gibson SV, Keeling ME, Abee CR. 2002. Nonhuman primates. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed.San Diego: Academic Press; p 702–703 [Google Scholar]
- 7.Borda JT, Pauley DR, Mackey JJ, Alvarez X. 2004. Immunoglobulin A nephropathy with crecentic glomerulonephritis in a pigtailed macaque (Macaca nemestrina). Vet Pathol 41:44–49 [DOI] [PubMed] [Google Scholar]
- 8.Boyce JT, Giddens WE, Seifert R. 1981. Spontaneous mesangioproliferative glomerulonephritis in pigtailed macaques (Macaca nemestrina). Vet Pathol 18Suppl. 6:82–88 [DOI] [PubMed] [Google Scholar]
- 9.Cheng HF, Harris RC. 2005. Renal effects of non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors. Curr Pharm Des 11:1795–1804 [DOI] [PubMed] [Google Scholar]
- 10.DiBartola S. 2000. Section 4. Clinical procedures: fluid therapy. In: Bistner S, Ford R, Raffe M. Handbook of veterinary procedures and emergency treatment, 7th ed.Philadelphia: WB Saunders Company; p 585–597 [Google Scholar]
- 11.DiBartola S.2005. Section XVIII. Urinary system. In: Ettinger S, Feldman E. Textbook of veterinary internal medicine, 6th ed.Vol 2 St Louis: Elsevier-Saunders; p 1716–1730 [Google Scholar]
- 12.Donadio JV, Grande JP. 2004. The role of fish oil/omega-3 fatty acids in the treatment of IgA nephropathy. Semin Nephrol 24:225–243 [DOI] [PubMed] [Google Scholar]
- 13.eMedicine Protein losing enteropathy [Internet]. Omaha (NE): WebMD; [Google Scholar]
- 14.Epstein M. 2001. Aldosterone and the hypertensive kidney: its emerging role as a mediator of progressive renal dysfunction: a paradigm shift. J Hypertens 19:829–842 [DOI] [PubMed] [Google Scholar]
- 15.Falk RJ, Jennette JC, Nachman PH. 2003. Primary glomerular disease. In: Brenner BM. Brenner and Rector's the kidney. Philadelphia: Saunders; p 1293–1294 [Google Scholar]
- 16.Feldman DB, Bree MM. 1969. The nephrotic syndrome associated with glomerulonephritis in a rhesus monkey (Macca mulatta). J Am Vet Med Assoc 155:1249–1252 [PubMed] [Google Scholar]
- 17.Giddens WE, Boyce JT, Blakely GA, Morton WR. 1981. Renal diseases in the pigtailed macaque (Macaca nemestrina). Vet Pathol 18Suppl. 6:70–81 [DOI] [PubMed] [Google Scholar]
- 18.Gopal DV, Rosen HR. Abnormal findings on liver function tests: interpreting results to narrow the diagnosis and establish a prognosis. Post Graduate Medicine Online [Internet]. 2000[cited 2007 Nov 29]; 107:2. Available from: http://www.postgradmed.com/issues/2000/02_00/gopal.htm [DOI] [PubMed]
- 19.Herbert LA, Birmingham D, Mahan J. 1997. Effect of enalapril therapy on glomerular accumalation of immune complexes and mesangial matrix in experimental glomerulonephritis in a nonhuman primate. Am J Kidney Dis 30:243–252 [DOI] [PubMed] [Google Scholar]
- 20.Hill LR, Hess KR, Stephens LC, Price RE, Gray KN. 2001. Correlations of kidney weight and volume and selected skeletal parameters to sex in the adult rhesus monkey (Macaca mulatta). J Med Primatol 30:56–60 [DOI] [PubMed] [Google Scholar]
- 21.Igarashi T, Donau O, Imamichi H. 2003. Macrophage-trophic simian/human immunodeficiency trophic virus chimeras use CXCR4, not CCRS, for infections of peripheral macaque peripheral mononuclear blood cells and alveolar macrophages. J Virol 77:13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, Martin M. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc Natl Acad Sci USA 96:14049–14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakob SM. 2004. Prevention of acute renal failure—fluid repletion and colloids. Int J Artif Organs 27:1043–1048 [DOI] [PubMed] [Google Scholar]
- 24.Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol 70:3189–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson L. 2000. Section 5—Interpretation of laboratory tests: evaluation of liver function. In: Bistner S, Ford R, Raffe M. Handbook of veterinary procedures and emergency treatment, 7th ed.Philadelphia: WB Saunders Company; p 734–747 [Google Scholar]
- 26.Jungheinrich C, Scharpf R, Wargenau M. 2000. The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethl starch 130/0.4 (6%, 500 ml) in mid to severe renal impairment. Anesth Analg 95:544–551 [DOI] [PubMed] [Google Scholar]
- 27.Kaup F, Matz-Rensing K, Kuhn E. 1998. Gastrointestinal pathology in rhesus monkeys with experimental SIV infection. Pathobiology 66:159–164 [DOI] [PubMed] [Google Scholar]
- 28.Kewenig S, Schneider T, Hohloch K. 1999. Rapid mucosal CD4 T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116:1115–1123 [DOI] [PubMed] [Google Scholar]
- 29.Korcarz CE, Padrid P, Sanjeev S. 1997. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. J Med Primatol 26:287–298 [DOI] [PubMed] [Google Scholar]
- 30.Laine L, Garcia F, McGilligan K. 1993. Protein-losing enteropathy and hyypoalbuminemia in AIDS. AIDS 7:837–840 [DOI] [PubMed] [Google Scholar]
- 31.Lepenies J, Quinkler M. 2006. MR blockade in patients with chronic renal disease—not the more the merrier, but the earlier the better. Nephrol Dial Transplant 21:3343–3344 [DOI] [PubMed] [Google Scholar]
- 32.Lu Ting-chi. 2005. HIV-associated nephropathy. Mt Sinai J Med 72:193–197 [PubMed] [Google Scholar]
- 33.Mansfield K, King N. 1998. Viral diseases. In: Bennett BT, Abee CR, Henrickson R. Nonhuman primates in biomedical research: diseases. San Diego: Academic Press; p 39–43 [Google Scholar]
- 34.Matignon M, Lidove O, Dupuis E, Walker F, Abgrall S, Papo T. 2005. A lupus-like glomerulonephritis following acute HIV-1 seroconversion in an African American woman. Nephrol Dial Transplant 20:438–440 [DOI] [PubMed] [Google Scholar]
- 35.Moroni M, Antinori S. 2003. HIV and direct damage of organs: disease spectrum before and during the highly active antiretroviral therapy era. AIDS 17Suppl. 1:S51–S64 [PubMed] [Google Scholar]
- 36.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Glomerular disease primer [Internet]. Bethesda (MD): National Institutes of Health; [Google Scholar]
- 37.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 38.Praga M. 2005. Therapeutic measures in proteinuric nephropathy. Kidney Int Suppl 99:S137–S141 [DOI] [PubMed] [Google Scholar]
- 39.Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vitro passage in rhesus monkeys. J Virol 70:6922–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossoff IF. 2001. Primates. In: Carpenter JW, Mashima TY, Rupiper DJ. Exotic animal formulary, 2nd ed.Philadelphia: WB Saunders; p 383 [Google Scholar]
- 41.Sachdev V. 2006. Personal communication.
- 42.Schwartz EJ, Klotman PE. 1998. Pathogenesis of human immunodeficiency virus (HIV)-associated nephropathy. Semin Nephrol 18:436–445 [PubMed] [Google Scholar]
- 43.Shannon RP. 2001. SIV Cardiomyopathy in non-human primates. Trends Cardiovasc Med 11:242–246 [DOI] [PubMed] [Google Scholar]
- 44.Shannon RP, Simon MA, Mathier MA, Geng YJ. 2000. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation 101:185–193 [DOI] [PubMed] [Google Scholar]
- 45.Shibata R, Kawamura M, Sakai H, Hayami M, Ishimoto A, Adachi A. 1991. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J Virol 65:3514–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata R, Maldarell F, Siemon C. 1997. Infection and pathogenicity of chimeric simian human immunodeficiecy viruses in macaques: determinant of high virus loads and CD4 cell killing. J Infect Dis 176:362–373 [DOI] [PubMed] [Google Scholar]
- 47.Shibata R, Sakai H, Kiyomasu T, Ishimoto A, Hayami M, Adachi A. 1990. Generation and characterization of infectious chimeric clones between human immunodeficiency virus type 1 and simian immunodeficiency virus from an African green monkey. J Virol 64:5861–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simopoulos AP. 2002. Omega 3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505 [DOI] [PubMed] [Google Scholar]
- 49.Stephens EB, Tian C, Dalton S. 2000. Simian–human immunodeficiency virus-associated nephropathy in macaques. AIDS Res Hum Retroviruses 16:1295–1306 [DOI] [PubMed] [Google Scholar]
- 50.Stephens EB, Tian C, Li Z. 1998. Rhesus macaques infected with macrophage-trophic simian immunodeficiency virus (SIV mac R71/17E) exhibit focal segmental and global glomerulosclerosis. J Virol 72:8820–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swenson M. 1977. Chapter 37. The kidneys. In: Duke's physiology of domestic animals, 9th ed.London: Comstock Publishing Associates; p 490 [Google Scholar]
- 52.University of North Carolina at Chapel Hill (UNC) Nephropathology Laboratory Renal pathology tutorial MPGN type 1 [Internet]. Chapel Hill (NC): UNC; 2007[cited 2007 Oct 17]. Available from: http://www.uncnephropathology.org/ [Google Scholar]
- 53.Vaden SL. 2005. Section XVIII. Urinary system. In: Ettinger S, Feldman E. Textbook of veterinary internal medicine, 6th ed.Vol 2 St Louis: Elsevier-Saunders; p: 1786–1800 [Google Scholar]
- 54.Webster C. 2005. Section XV Liver and pancreatic diseases. In: Ettinger S, Feldman E. Textbook of veterinary internal medicine, 6th ed.Vol 2 St Louis: Elsevier-Saunders; p 1422–1434 [Google Scholar]
- 55.Weiner NJ, Goodman J, Kimmel P. 2003. The HIV-associated renal diseases: current insights into pathogenesis and treatment. Kidney Int 63:1618–1631 [DOI] [PubMed] [Google Scholar]
- 56.Wiedermann CJ. 2004. Hydroxyethyl starch—can safety problems be ignored? Wien Klin Wochenschr 116:583–94, 640-41 [DOI] [PubMed] [Google Scholar]
- 57.Wilmer WA, Rovin B, Hebert C. 2003. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol 14:3217–3232 [DOI] [PubMed] [Google Scholar]