Abstract
There is a need to further our understanding of the neurophysiological effects of chiropractic spinal manipulation on brain activity as it pertains to both musculoskeletal and non-musculoskeletal complaints. This paper aims to provide a basic overview of the most commonly utilised techniques in the neurosciences for functional imaging the brain (positron emission tomography, single-photon emission computerised tomography, functional magnetic resonance imaging, electroencephalography, and magnetoencephalography), and discuss their applicability in future chiropractic research. Functional neuroimaging modalities are used in a wide range of different research and clinical settings, and are powerful tools in the investigation of neuronal activity in the human brain. There are many potential applications for functional neuroimaging in future chiropractic research, but there are some feasibility issues, mainly pertaining to access and funding. We strongly encourage the use of functional neuroimaging in future investigations of the effects of chiropractic spinal manipulation on brain function.
Keywords: imaging, neuroimaging, brain, chiropractic
Abstract
Il faut pousser plus loin notre compréhension des conséquences neurophysiologiques de la manipulation rachidienne comme traitement chiropratique, notamment sur l’activité du cerveau en ce qui a trait aux symptômes au niveau musculo-squelettique ou non musculo-squelettique. La présente étude présente un aperçu élémentaire des techniques couramment utilisées en science neurologique quant à l’imagerie fonctionnelle du cerveau (la tomographie par émission de positons, la tomographie par émission de simple photon, l’imagerie par résonnance magnétique, l’électroencéphalographie et la magnétoencéphalographie) et commenter leur applicabilité dans les recherches futures en chiropratique. Les modalités de la neuroimagerie fonctionnelle sont utilisées dans une vaste gamme de recherches différentes et de conditions cliniques et s’avèrent de puissants outils dans les recherches sur l’activité neuronale du cerveau humain. Il existe plusieurs applications possibles de la neuroimagerie fonctionnelle dans les recherches futures en chiropratique mais il reste certaines questions de faisabilité, notamment celles reliées à l’accès et au financement. Nous encourageons avec ferveur le recours à la neuroimagerie fonctionnelle dans les recherches sur les effets des conséquences neurophysiologiques de la manipulation rachidienne sur le fonctionnement du cerveau.
Introduction
Chiropractic research to date has mostly been concerned with various aspects of low back pain, neck pain, and headaches.1 However, a number of chiropractic patients are receiving chiropractic care and reporting improvements for other non-musculoskeletal complaints.2,3 Indeed, some of the complaints are conditions and disorders that are closely associated with the function of the brain, including such as autistic spectrum disorder (ASD), depression and attention deficit hyperactivity disorder (ADHD). Although there is anecdotal evidence that suggest chiropractic spinal manipulation (SM) can be beneficial for some of these patients, the nature of SM effects on these conditions and complaints are by and large poorly investigated.4 Unfortunately, undisciplined rhetoric from some practitioners promulgating unsupported claims of benefit from SM has occurred based on these preliminary and limited studies. Such speculation is unwarranted and casts chiropractic in a poor light with other disciplines.
Chiropractic SM has been shown to induce a barrage of mechanoreceptive afferent input, originating mainly from paraspinal muscle spindle receptors and Golgi tendon organs.5 Such afferent input has been shown to modulate local spinal cord neurophysiology affecting both pain processing and the motor control system. In a study by Terrett and Vernon6 it was demonstrated that SM caused a reduction in pain sensitivity. An increase in paraspinal electromyography (EMG) activity was found in asymptomatic subjects following cervical, thoracic, lumbar and sacroiliac SM.7 Murphy, Dawson and Slack8 demonstrated a decrease in the H-reflex from the tibial nerve following sacroiliac SM in asymptomatic subjects. Attenuation of motorneuron activity following both cervical and lumbar spine SM has also been demonstrated.9,10 Furthermore, modulation of local spinal cord neurophysiology can cause a change to the afferent arm of the somatovis-ceral reflex, however it is likely that supraspinal influences play a major role in this effect.11
Limited evidence suggests chiropractic SM can induce changes in the central nervous system (CNS) above the level of the spinal cord.12,13 Indeed, a few case studies have used objective neurophysiological measurement tools, such as electroencephalography (EEG), to evaluate changes in CNS neural activity following SM.14–16 In a recent pseudo-randomised study using EEG technology (somatosensory evoked potentials[SEP]), Haavik-Taylor and Murphy17 demonstrated that chiropractic SM can indeed modulate sensorimotor integration in the CNS. However, little other objective neurophysiological evidence is available.4
This manuscript aims to: (i) provide a basic overview of the most commonly utilised techniques used for imaging in neurosciences, namely functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single-photon emission computerised tomography (SPECT), electroencephalography (EEG), and magnetoencephalography (MEG); (ii) discussing how functional neuroimaging might serve the profession in investigations of the supraspinal effects of chiropractic SM; and (iii) discussing the feasibility of utilising functional neuroimaging in future chiropractic research. The different modalities will be presented in terms of necessary equipment and facilities, the degree of invasiveness, spatial resolution, and temporal resolution.
The electronic database MEDLINE was searched from 1997 to present for literature concerning functional neuroimaging techniques. Although many different technologies are available, some of which with several applications, this paper is limited to the neuroimaging techniques mentioned above and their utilisation in investigating brain activity.
Discussion
In the human brain, elevated neural activity increases the local neuronal metabolic demand, and regional synaptic activity correlates with neuronal glucose utilisation and subsequent changes in cerebral blood perfusion.18 However, the traditional view that glucose consumption directly reflects neural activity has recently changed.19 Kasischke et al20 have shown that neurons preferentially metabolise lactate rather than glucose, and that astrocytes, a type of glial cell in the brain, respond to neuronal activity by consuming more glucose as well as producing more lactate. Consequently, imaging based on glucose consumption primarily reflects astrocytic activity rather than neuronal activity. However, the activation of both cell types is correlated in the majority of cases.21 Hence, an indirect measure of the underlying neural activity can be obtained by measuring the haemodynamic response.
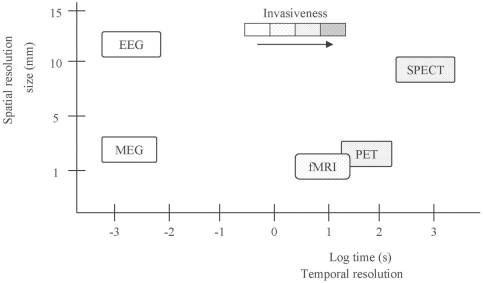
Below is a brief overview of the most common functional neuroimaging modalities. Table 1 summarises the characteristics of the various modalities, and Figure 1 is a graphical representation of the differences in spatial and temporal resolution and degree of invasiveness across the various neuroimaging techniques.
Table 1.
Comparison of functional neuroimaging techniques
| PET | SPECT | fMRI | EEG | MEG | |
|---|---|---|---|---|---|
| Measure of neuronal activity | indirect | indirect | indirect | direct | direct |
| – biological process measured | haemodynamic response | haemodynamic response | haemodynamic response | neuroelectrical potentials | neuromagnetic field |
| Invasiveness | invasive | invasive | non-invasive | non-invasive | non-invasive |
| – confined space | yes | yes | yes | no | yes |
| – radiation | yes (0.5–2.0 mSv) | yes (3.5–12.0 mSv) | none | none | none |
| Cost of equipmenta | $8,000,000 | $350,000 | $2,000,000 | $100,000 | $2,000,000 |
| Operating costsb | $1,500 | $1,000 | $800 | $150 | $600 |
| Temporal resolution | poor (1–2 min) | poor (5–9 min) | reasonable (4–5 s) | excellent (<1 ms) | excellent (<1 ms) |
| Spatial resolution | good/excellent (4 mm) | good (6 mm) | excellent (2 mm) | reasonable/good (10 mm) | good/excellent (5 mm) |
Approximate costs, there may be considerable variation among manufacturers and device specifications.
Typical commissioned operating costs.
Figure 1.
Comparison of spatial and temporal resolutions.
The area occupied by the boxes represents the spatial and temporal resolutions of the respective functional neuroimaging technique. The shading of the boxes represents the degree of invasiveness of each modality, darker shading indicates higher invasiveness.
Functional brain imaging techniques
Positron emission tomography (PET)
PET typically measure regional cerebral blood flow (rCBF) or regional cerebral metabolic rate of glucose (rCMTG), using radioactively labelled molecules (tracer molecules), which are usually injected intravenously or continuously inhaled by the patient or research participant.19,22,23 See Figure 2 for a schematic diagram of a PET scanner. Positron-emitting radioisotopes of carbon, nitrogen, oxygen, and fluorine are commonly used in these tracer molecules, and a variety of data may be obtained with these different tracer molecules.24 A particle accelerator (cyclotron) is required to produce the radioisotopes used in PET imaging, and it has to be in close proximity to the PET scanner due to the short radioactive half-life of the radioisotopes.24 The cyclotron contributes significantly towards the higher acquisition cost of a PET system compared to the other modalities. Furthermore, the comparatively high operating cost of PET can be attributed to the production of expensive tracers.
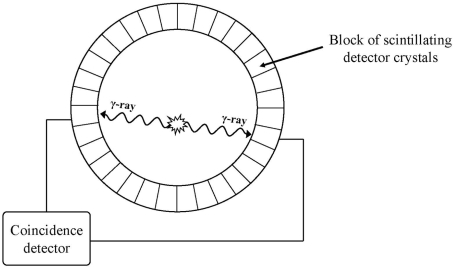
Figure 2.
Schematic diagram of a single ring detector of a PET camera.
When an emitted positron comes in contact with an electron, they annihilate each other and produce two photons (-rays) travelling in opposite directions. The PET camera employs paired gamma detectors linked in a coincidence circuit that only records decay events when photons trigger both detectors “simultaneously.” Coincidence detection can be localised to the line connecting a pair of isolated detectors.
Temporal resolution refers to how closely the measured activity corresponds to the timing of the actual neuronal activity.22 The temporal resolution with PET is poor compared to both fMRI, EEG and MEG, and is limited by both the technique and the metabolism of the tracer molecule.22 Spatial resolution refers to how accurately the measured activity is localised within the brain.22 The spatial resolution of PET is good, but it depends on several different factors pertaining to the characteristics of various components of the PET camera, in particular the size and material of the scintillating crystal used in the coincidence detectors, and the number of detector rings.25 Figure 3a depicts a modern PET system.
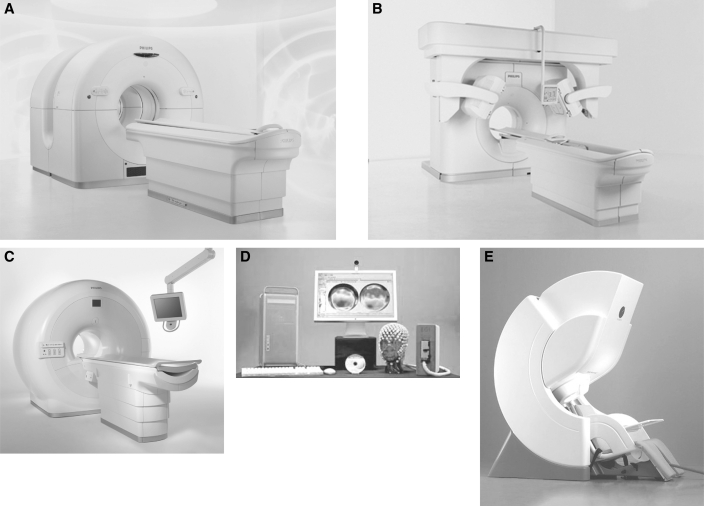
Figure 3.
Pictures of functional neuroimaging equipment.
(A) Example of a PET system: Philips Gemini GLX PET/CT (Courtesy of Koninklijke Philips Electronics NV); (B) Example of a SPECT system: Philips Precedence SPECT/CT (Courtesy of Koninklijke Philips Electronics NV); (C) Example of an MRI scanner: Philips Achieva 3.0T X-series MRI (Courtesy of Koninklijke Philips Electronics NV); (D) Example of a EEG system: EGI Geodesic EEG System 300 (Courtesy of Electrical Geodesics Inc); (E) Example of a MEG system: Elektra Neuromag (Courtesy of Elektra AB)
An individual typically absorbs 0.5 to 2 mSv (milli-Sieverts) of radiation per PET study.23 This is comparable to diagnostic imaging, for example an anterior to posterior (AP) lumbar spine x-ray or a whole head CT scan, which have effective doses of typically 0.7 mSV and 2 mSv, respectively.26 The reader is referred to Wintermark et al23 for a more thorough discussion of PET.
Single-photon emission computerised tomography (SPECT)
SPECT detects neuronal activity indirectly by measuring changes in rCBF.19 Similar to PET, SPECT generates tomographic images of the three dimensional distribution of a radioisotope.23 However, the radioisotopes utilised in SPECT have more advantageous radioactive half-lives and decay to directly emit gamma rays.18 Nevertheless, the typical effective radiation dose in SPECT is considerably higher compared to PET.23 Repeated scanning with both PET and SPECT is usually restricted because the participants are exposed to radiation, thus limiting task repetitions or multiple tasks in the same session.22
SPECT cameras are either rotating cameras, comparable to x-ray computed tomography (CT) systems (except that the emitting source is within the patient and the type of radiation is gamma rays), or detector rings similar to those in PET. Figure 3b depicts a modern SPECT system. SPECT systems using multiple detector-rings are designed to maximise the number of detectors recording activity from the brain, and can achieve a reasonably good spatial resolution.23 Less expensive detector cameras and no need for a cyclotron are responsible for making the acquisition of a SPECT system more affordable than a PET system. SPECT is dependent on the haemodynamic response and sufficient detection of photon emissions, and has therefore relatively poor temporal resolution. The spatial resolution of SPECT is fairly good, albeit not quite that of PET. The reader is referred to Wintermark et al23 for a more in-depth presentation of SPECT.
Functional magnetic resonance imaging (fMRI)
Functional MRI relies on detecting small changes in the signals used to produce magnetic resonance images.27 There is no need to introduce radioisotopes in fMRI as the iron in the blood haemoglobin serves as an inherent intravascular contrast agent.18 This is possible due to the fact that deoxygenated haemoglobin has greater magnetic susceptibility as compared with tissue and oxygenated haemoglobin. The balance of oxygenated and deoxygenated haemoglobin in the blood is blood oxygenation level dependent (BOLD), and changes in blood oxygenation occur as a consequence of neuronal activity.28 The main limitation of fMRI arise from the vascular origin of the signal changes, hence, a temporal resolution of no better than 2 to 5 seconds can be expected with BOLD-fMRI.24
Conventional MRI scanners utilise a large superconductive magnet capable of generating the strong magnetic field (1.5 T or above) necessary in MRI procedures.24 Figure 3c depicts a modern MRI system. As the signal intensity increases with the magnetic field strength, many fMRI researchers are turning to scanners of 3 T or greater to improve spatial resolution.24 Newer techniques and stronger magnets are expected to enable researchers to identify structures in the submillimeter range.29 Thus, fMRI is considered to have the best spatial resolution among the functional neuroimaging techniques.18,22 The operating costs of fMRI are more reasonable than the nuclear imaging techniques (PET and SPECT). The reader is referred to a review by Hennig et al30 for more information regarding methodological aspects and clinical applications of fMRI.
In regards to the exposure to strong magnetic fields in fMRI, a few things are worth noting. Although no known health risks are associated with MRI scanning, induction of flow potentials around the heart, induction of ectopic heart beats and an increased likelihood of re-entrant arrhythmia, have been observed.33 Furthermore, induction of vertigo and nausea have been observed during movement in static fields greater than 2 T.33 However, movement is detrimental to the image quality in MRI, and every effort is made to prevent movement to occur during MRI procedures. The main concern with MRI scanning is the potential for movement or heating of ferromagnetic objects in the body. This issue is resolved by excluding participants with inserted pacemakers, surgical clips, or any other ferromagnetic objects.22 Subjects are exposed to loud acoustic noise (100–130 decibel, 1–4 kHz) with mechanical vibration during the typically 2 to 30 minutes it takes to acquire the necessary data.24,28,31 Earplugs that can provide about 20–30 dB noise attenuation at these frequencies are therefore commonly used in both functional and conventional MRI procedures.32
Electroencephalography (EEG)
EEG records changes in the electrical potentials generated by large populations of synchronously active neurons.34 Although electrical activity can be measured directly from the cortical surface (electrocortiography) or by using depth probes (electrography), this paper will only refer to EEG recorded from the scalp surface. From its neuronal source, current has to penetrate through skull, skin, and other layers, before being detected by electrodes on the scalp.35,36 The spatial orientation of neuronal populations, relative to the scalp surface, varies from radial in the gyri to tangential in the sulci. EEG is sensitive to both tangential and radial components of a current source, but seems to be dominated by radial sources.34 See Figure 4 for an illustration of radial and tangential current sources in the cerebral cortex.
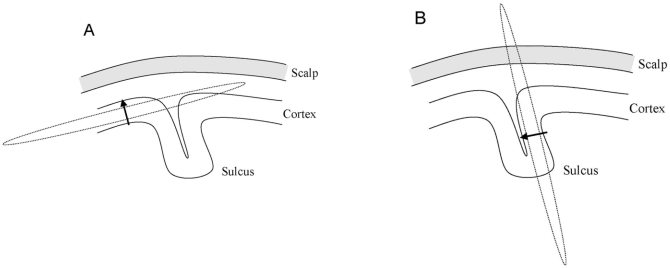
Figure 4.
Radial and tangential sources and their associated magnetic fields.
The electrical current source is indicated by an arrow, and its associated magnetic field is indicated by a dashed line. (A) A radial current source located in the gyrus of the cerebral cortex (radial source) has its associated magnetic field oriented tangential to the scalp surface. (B) The magnetic field associated with a current source located in the sulcus (tangential source) is perpendicular to the scalp surface. Only tangential current sources generate neuromagnetic fields detectable by MEG.
EEG systems are relatively novel and consist of electrodes with conductive media, amplifiers with filters, analogue to digital (A/D) converters, and computer equipment for recording and signal processing.36 Thus, EEG equipment can be acquired for only a fraction of the cost of the other technologies. Figure 3d depicts the components of a modern EEG system. Skin preparation of the scalp and the use of a conductive medium are usually required to obtain appropriate conductance and lowering of contact impedance at the electrode-skin interface.36 This is generally well tolerated, but it may cause irritation, pain, bleeding, and infection, especially when repeated EEG measurements are made from the same electrode points.36
EEG has an excellent temporal resolution of only a fraction of a millisecond enabling brain activity to be recorded in real-time.37 Furthermore, with the use of modern signal analysis methods the spatial resolution of EEG, which often is considered as its biggest limitation, might in fact approach that of conventional fMRI.39 The reader is referred to a recent review by Michel et al39 for a discussion on various methods of signal analysis in EEG source imaging.
Magnetoencephalography (MEG)
MEG is a sophisticated non-invasive functional neuroimaging technique that measures the external magnetic field generated by the neural activity of the brain. Changes in the neuromagnetic field are a direct and instantaneous reflection of neural events in the brain. In order to generate a neuromagnetic field detectable by MEG sensors, large neuronal populations have to be aligned tangentially to the scalp surface and be synchronously active.40 See Figure 4 for an illustration of radial and tangential current sources and their associated neuromagnetic fields. Thus, EEG and MEG provide a complimentary detection of the electromagnetic activity of neuronal networks in the brain.
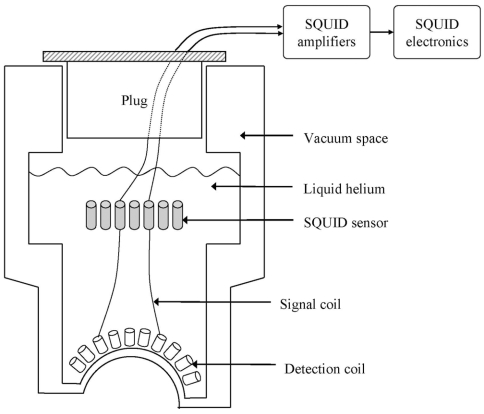
The neuromagnetic field (approximately 10 to 103 fT) is several orders of magnitude weaker than the ambient magnetic noise (approximately 108 fT ), which includes the earth magnetic field.34 Hence, both sufficient magnetic shielding against external magnetic fields and exquisitely sensitive equipment are required to measure the neuromagnetic field. Enclosing the MEG system within a magnetically shielded room is the most straightforward method for reduction of environmental noise.41 Different enclosure approaches are available, including; shielding by eddy currents using high-conductivity metal, shielded mu-metal rooms, and superconducting shields.41 The equipment sensitivity is provided by a device known as SQUID (Superconductiong QUantum Interference Device), which is immersed in liquid helium, along with other components and contained within a vacuum-insulated container (dewar).40 See Figure 5 for a schematic of an MEG dewar. Figure 3e depicts a modern MEG system.
Figure 5.
Schematic diagram of a MEG dewar with SQUIDs.
Detection coils are placed tangential to the scalp surface. The detection coil “senses” the neuromagnetic field and produce currents that are “transmitted” to the SQUID by signal coils. The SQUID then “converts” the currents in the signal coils to voltages that can be amplified and recorded by conventional electronic devices.
One of the most important features of MEG is that the technology provides measurements with an excellent temporal resolution, allowing the recording of neuronal activity in real-time.37 The spatial resolution of MEG is reasonably good.34 Source localisation is less complicated with MEG than with EEG because the tissues are transparent to the neuromagnetic field, whereas knowledge of the tissue conductivity distribution is usually required in EEG.41 Thus, MEG has traditionally been able to offer better source localisation than EEG. Although the operational costs of MEG are typically less expensive than fMRI, they are both significantly more expensive than EEG. The reader is referred to Ioannides37 for a more thorough discussion of MEG as a research tool.
Current use of neuroimaging techniques
Functional neuroimaging has many applications in the clinical setting, in particular investigating cerebrovascular disorders (PET and SPECT),23 pre-surgical localisation of cortical areas (PET, SPECT, MEG, EEG and fMRI),23,30,42–43 and detecting the location of epileptic discharges (MEG, EEG, PET and SPECT).23,35,44 To a lesser degree, PET is also used in the localisation of brain tumours, and the investigation of dementia and movement disorders.23 SPECT is used to confirm brain death by assessing cerebral perfusion, and is also used for investigations in a variety of psychiatric disorders such as, depression, post-traumatic stress disorder, schizophrenia, and dementia.23 Indeed, an expanding body of clinical evidence supports the value of using non-invasive functional neuroimaging (MEG and EEG) with cases of attention deficit hyperactivity disorder, autism, dyslexia, and epilepsy.45
In the research setting, functional neuroimaging has traditionally been used in studies of brain activation, and for this purpose fMRI has to a large extent replaced PET.23,30 The main reasons being fMRI: (i) does not involve exposing participants to ionising radiation; (ii) offers superior spatial resolution; (iii) has faster data acquisition time; and (iv) has a lower operating cost per investigation. Nevertheless, PET (and SPECT) may still be particularly helpful in the study of specific neurotransmitter-receptor interactions due to the many tracers that can be utilised with these technologies.46
EEG, MEG and fMRI are used extensively by clinical researchers in both basic and cognitive neuroscience, as well as in contemporary psychophysiology.27,47–49 The neural correlates of several psychological and psychiatric disorders have been investigated, including, but not limited to; ADHD,50 ASD,51 and depression.52 In addition to basic brain activation studies, functional neuroimaging has a history of investigating the effects of pharmaceuticals on psychiatric disorders.53 However, more recently there have also been investigations into the effects of psychotherapies in these disorders.53 An increasing number of neuroimaging investigations involve topics important to rehabilitation, such as investigations of the neural correlates of recovery after stroke and determining the effects of treatment interventions.22,54 Similar investigations have been conducted in patients with Parkinson’s disease, multiple sclerosis and Alzheimer’s disease.54 Furthermore, the effects of acupuncture on brain activity have also recently been investigated with neuroimaging techniques.38 Neuroimaging research in fibromyalgia and other chronic pain conditions without a known nociceptive source have provided objective evidence of abnormal central regulation of pain.55 Nakabeppu et al56 observed decreased rCBF in the thalamus in patients with chronic pain using SPECT. Flor et al57 found extensive reorganisation of the primary somatosensory cortex in patients with chronic low back pain, using MEG. Further investigations are warranted to elucidate the mechanism(s) by which SM benefit patients with these conditions.
Potential applications in chiropractic research
There is a need to investigate the effects of SM on brain activity and further our understanding of the neurophysiological effects of chiropractic SM as it pertains to both musculoskeletal and non-musculoskeletal complaints. Increased knowledge of how the brain responds to SM will also further the understanding and the development of chiropractic, and will therefore have an impact on both chiropractors and their patients. Expanding evidence of central neural changes with SM from basic science research may also eventually extend the scope of practice as an increasing number of health professionals may encourage and refer patients to chiropractors. Functional neuroimaging modalities appear to be appropriate tools to obtain the valuable information about how brain function is altered with chiropractic care.
Investigating the effects of dysfunctional spinal motion segments on brain function may seem like a natural place to start. However, the design of appropriate experiments may be ethically challenging with human subjects, and perhaps animal studies may be more appropriate initially. Bakkum et al58 demonstrated in a study on cats that chronic vertebral hypomobility of lumbar segments (surgically induced) resulted in plastic changes in the spinal cord. This is for obvious reasons not feasible with healthy human subjects. However, it may be possible to evaluate a cohort of patients scheduled for spinal fusion before and after surgery using functional neuroimaging. Alternatively, possible changes in brain activity may be detected in animal studies using a similar experimental set-up used by the beforehand mentioned study by Bakkum et al.58
In humans, documenting normative data from basic science studies whether SM can change brain function would be a priority before investigations on various clinical entities are undertaken. Different parameters of brain function are of interest and should be investigated with regards to magnitude, time-scale, distribution, and location of altered brain activity.
Various aspects of sensorimotor integration are of particular interest in relation to musculoskeletal conditions. Indeed, recent studies by Haavik-Taylor and Murphy17,59 using EEG have shown changes in sensorimotor integration following cervical SM. Whereas these initial studies have used electrical nerve stimulation to evoke electrical potentials detectable with EEG, future investigations could perhaps use passive or active movement to evoke electrical potentials and magnetic fields, which could be recorded by EEG and MEG, respectively. Future studies should look beyond cervical SM and include investigations of changes in sensorimotor integration in relation to lumbopelvic SM. Furthermore, potential differences across various types of SM could also be investigated. In addition, improvements in reaction times following SM has previously been identified,12–13 and further elucidation of changes in sensorimotor integration may be of interest from both a sports chiropractic and athlete performance enhancement point of view, as well as in the context of chiropractic care and fall prevention strategies for the geriatric population. Furthermore, the effect of SM on sensorimotor integration may also be investigated in relation to neurodevelopmental disorders (e.g. ADHD, ASD and cerebral palsy), which commonly display motor impairments. Functional neuroimaging may help explain some of the improvements in patients with these conditions receiving chiropractic care.
Another parameter worth investigating is central pain processing, and in particular the potential modulation of central pain processing in patients with chronic pain syndromes. Functional neuroimaging modalities could be utilised to provide objective neurophysiological evidence of the efficacy of chiropractic SM by measuring potential changes in the abnormal brain function already identified in these patients. These outcomes could then be coupled with validated paper based disability and pain inventories. Furthermore, functional neuroimaging may be used to identify the best treatment approaches, which patients respond to SM, and which combinations of treatment have synergistic therapeutic effects.
A range of other parameters are of interest with regards to non-musculoskeletal complaints. Neural correlates of various brain states, including attention deficits and depression, have been identified with functional neuroimaging. Chiropractic researchers could potentially adapt the methodology and research design of these studies to investigate the effect of chiropractic intervention in specific cohorts of subjects. For example neuroimaging research in psychiatry has implicated hypoactivation of the left prefrontal cortex in depression.60 Thus, the effect of chiropractic intervention for patients with depression could potentially be evaluated by functional neuroimaging of left prefrontal cortex activation. A similar approach is possible with ADHD which is associated with prefrontal brain dysfunction related to processes of response inhibition.61 In the advent of positive findings it would be appropriate to set up clinical trials where objective neurophysiological evidence of efficacy could be correlated with validated paper based clinical assessment tools. Depression, ADHD, and ASD are just a few disorders that may be candidates for investigating chiropractic interventions using functional neuroimaging.
Determining which technology is better is complicated as all functional neuroimaging techniques have advantages and disadvantages, but some modalities are generally better suited to answer certain types of research questions. Generally, the functional neuroimaging techniques relying on the haemodynamic response (PET, SPECT and fMRI) would to be better suited for answering questions concerning where, and neuroelectrical techniques (EEG and MEG) are preferred to answer questions concerning when. However, technological advances continue to improve the functional neuroimaging techniques. fMRI appears to have replaced PET (and SPECT) to a large extent with its superior spatial and temporal resolutions and simpler operating (no use for radiopharmceuticals). MEG and EEG, in addition to providing superior temporal resolution, are becoming increasingly better suited for source localisation. Whilst EEG and MEG are complimentary techniques, MEG appears to be superior in some aspects, including spatial resolution if cost is not a factor.
Feasibility
Cost and access
Cost and accessibility appear to be major obstacles for utilisation of functional neuroimaging in chiropractic research. The cost of acquiring most functional neuroimaging equipment is very high, which may explain the relatively limited number of these systems in existence. In addition, these modalities typically require specialised staff to operate the sophisticated equipment. The limited number of functional neuroimaging systems are therefore typically located in either medical institutions (e.g. hospitals) or research facilities dedicated to particular research interests in specific fields (e.g. cognitive neuroscience, psychiatry and linguistics). Thus, chiropractic researchers are likely to compete for laboratory time at facilities which typically have other research priorities. It would therefore be pertinent for chiropractic researchers to establish research partnerships with these institutions in order to gain access to functional neuroimaging equipment and conduct their experiments. Furthermore, it is generally expensive to conduct research using most functional neuroimaging modalities. Unfortunately there is very limited funding available for chiropractic research, thus making it difficult for chiropractic researchers to conduct experiments using functional neuroimaging.
In comparison to the other neuroimaging modalities, EEG equipment is relatively inexpensive to acquire and operate. Chiropractic researches could potentially set up their own dedicated EEG research facilities and avoid competing for laboratory time to conduct their experiments. With appropriate training it would be possible for the chiropractic researcher to both operate the equipment and analyse the recorded data. Thus, the operating costs would be limited to relatively inexpensive basic consumables (conductive gel and electrodes), which would amount to far less then the operating costs of the other modalities.
Technical considerations
It is important to avoid any head movement whenever investigating brain activity using functional neuroimaging. In addition, many of the functional neuroimaging systems feature confined spaces in order to obtain the data or protect the investigators, or both. For these reasons, it is not feasible to measure brain activity concurrent with chiropractic SM. Admittedly, it would be interesting to demonstrate the neural pathways and circuitries that are activated during SM. However, the elicited activity of certain somatosensory neural circuitries during SM is arguably of less importance than the sustained changes in brain function that might occur in response to SM. These changes are best investigated by comparing brain activity before and after SM using various stimulus presentations depending on which aspect of brain function is of interest. A pre-post SM intervention design is readily achievable and comparable to functional neuroimaging studies in other fields. Depending on the brain region or function of interest, an array of different stimuli may be presented during data acquisition, including, but not limited to: somatosensory, auditory, visual, motor, or cognitive tasks (e.g. solving mathematical problems).
Most MEG and EEG somatosensory studies utilise evoked response paradigms, which looks at the transient response to brief sensory stimuli.38 Responses that occur at the same time after each stimulus (time-locked) become visible against background noise after averaging trials. These time-locked, averaged responses are called somatosensory evoked fields (SEFs) when recorded with MEG and somatosensory evoked potentials (SEPs) for EEG studies. Quantitative spectral analysis is another analysis method that is often used to quantify EEG and MEG signals on the basis of the amount of oscillations present (oscillatory power).38 Cortical oscillations are a widespread feature of normal brain activity and occur at a variety of different frequencies.62 Interestingly, the evoked response paradigm is increasingly being substituted by event-related changes in cortical oscillatory power.37,63 In the evoked response paradigm, a stimulus initiates a sequence of activations that tends to propagate from one area to the next. Any background rhythms are just uninteresting noise that must be eliminated. However, because the cortex is always active, any stimulus-evoked effect must be seen as a perturbation of ongoing activity in several areas. In order to understand the influence of the stimulus on the activity of one area, the interaction between the effect of the stimulus and the local background activity must be taken into account. In addition, other indirect stimulus-evoked effects mediated by other areas should also be taken into account. Oscillatory power changes do not just occur in response to a brief event, but can exist over quite long periods of time during a ”state change”.64
Due to the enclosed nature of MEG and the brain perfusion imaging techniques, participants may become anxious or claustrophobic.22 However, if participants do not suffer anxiety or claustrophobic disorders, it has been shown that participants feel safe when inside the scanner, and they would recommend participating in neuroimaging research to their family and friends.65
Conclusions
Functional neuroimaging techniques are powerful tools in the investigation of neuronal activity in the human brain. The chiropractic research community is encouraged to utilise the potential of functional neuroimaging to discern how chiropractic SM may best stimulate positive neuroplastic change in combination with clinical recovery. The use of functional neuroimaging techniques, in particular fMRI, MEG and EEG, in future chiropractic research is strongly recommended.
References
- 1.Haas M, Bronfort G, Evans RL. Chiropractic clinical research: progress and recommendations. J Manipulative Physiol Ther. 2006;29(9):695–706. doi: 10.1016/j.jmpt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Hawk C, Long CR, Boulanger KT. Prevalence of nonmusculoskeletal complaints in chiropractic practice: report from a practice based research program. J Manipulative Physiol Ther. 2001;24(3):157–69. [PubMed] [Google Scholar]
- 3.Leboeuf-Yde C, Pedersen EN, Bryner P, Cosman D, Hayek R, Meeker WC, Shaik J, Terrazas O, Tucker J, Walsh M. Self-reported nonmusculoskeletal responses to chiropractic intervention: a multination survey. J Manipulative Physiol Ther. 2005;28(5):294–302. doi: 10.1016/j.jmpt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Lystad RP, Pollard H. Neurophysiological evidence of changes in brain function following chiropractic spinal manipulative therapy: a systematic review. J Manipulative Physiol Ther. 2008 accepted. [Google Scholar]
- 5.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2(5):357–71. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 6.Terrett ACJ, Vernon H. Manipulation and pain tolerance: a controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. Am J Phys Med. 1984;63(5):217–25. [PubMed] [Google Scholar]
- 7.Herzog W, Scheele D, Conway PJ. Electromyographic responses of back and limb muscles associated with spinal manipulative therapy. Spine. 1999;24(2):146–52. doi: 10.1097/00007632-199901150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Murphy BA, Dawson NJ, Slack JR. Sacroiliac joint manipulation decreases the H-reflex. Electromyogr Clin Neurophysiol. 1995;35(2):87–94. [PubMed] [Google Scholar]
- 9.Dishman JD, Bulbulian R. Spinal reflex attenuation associated with spinal manipulation. Spine. 2000;25(19):2519–25. doi: 10.1097/00007632-200010010-00015. [DOI] [PubMed] [Google Scholar]
- 10.Dishman JD, Burke J. Spinal reflex excitability changes after cervical and lumbar spinal manipulation: a comparative study. Spine J. 2003;3(3):204–12. doi: 10.1016/s1529-9430(02)00587-9. [DOI] [PubMed] [Google Scholar]
- 11.Pollard H. The somatovisceral reflex: how important for the ”type O” condition? Chiropr J Aust. 2004;34(3):93–102. [Google Scholar]
- 12.Kelly DD, Murphy BA, Backhouse DP. Use of a mental rotation reaction-time paradigm to measure the effects of upper cervical adjustments on cortical processing: a pilot study. J Manipulative Physiol Ther. 2000;23(4):246–51. doi: 10.1067/mmt.2000.106099. [DOI] [PubMed] [Google Scholar]
- 13.Smith DL, Dainoff M, Smith J. The effect of chiropractic adjustments on movement time: a pilot study using Fitts Law. J Manipulative Physiol Ther. 2006;29(4):257–66. doi: 10.1016/j.jmpt.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Hospers LA. EEG and CEEG studies before and after upper cervical or SOT category II adjustment in children after head trauma, in epilepsy and in “hyperactivity”. Proceedings of the National Conference on Chiropractic and Pediatrics, Sherman College of Straight Chiropractic. 1992:84–139. [Google Scholar]
- 15.Baumbick R, Hondorp C, Miller B, Penney B. Quantitative electroencephalogram (QEEG) responses to cervical spinal manipulation. Logan College of Chiropractic; Chesterfield, MO, United States: 1996. [Google Scholar]
- 16.Barwell R, Long A, Byers A, Schisler C. The effect of the chiropractic adjustment on the brain wave pattern as measured by QEEG. Summarizing an additional 100 (approximately) cases over a three year period. International Research and Philosophy Symposium; Sherman College of Straight Chiropractic. 2004. [Google Scholar]
- 17.Haavik-Taylor H, Murphy B. Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study. Clin Neurophysiol. 2007;118(2):391–402. doi: 10.1016/j.clinph.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Otte A, Halsband U. Brain imaging tools in neurosciences. J Physiol Paris. 2006;99(4–6):281–92. doi: 10.1016/j.jphysparis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Sestini S. The neural basis of functional neuroimaging signal with positron and single-photon emission tomography. Cell Mol Life Sci. 2007;64(14):1778–84. doi: 10.1007/s00018-007-7056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305(5680):99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 21.Pellerin L, Magistretti PJ. Let there be (NADH) light. Science. 2004;305(5680):50–3. doi: 10.1126/science.1100428. [DOI] [PubMed] [Google Scholar]
- 22.Kimberly TJ, Lewis SM. Understanding neuroimaging. Phys Ther. 2007;87(6):670–83. doi: 10.2522/ptj.20060149. [DOI] [PubMed] [Google Scholar]
- 23.Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36(9):e83. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- 24.Turner R, Jones T. Techniques for imaging neuroscience. Br Med Bull. 2003;65(1):3–20. doi: 10.1093/bmb/65.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Moses WW, Virador PRG, Derenzo SE, Huesman RH, Budinger TF. Design of a high-resolution, high-sensitivity PET camera for human brains and small animals. IEEE Trans Nucl Sci. 1997;44(4):1487–91. [Google Scholar]
- 26.Wall BF, Hart D. Revised radiation doses for typical x-ray examinations. Br J Radiol. 1997;70(833):437–9. doi: 10.1259/bjr.70.833.9227222. [DOI] [PubMed] [Google Scholar]
- 27.Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112(1):4–9. doi: 10.1172/JCI19010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaro E, Jr, Barker GJ. Study design in fMRI: Basic principles. Brain Cogn. 2006;60(3):220–32. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Ugurbil KM, Toth L, Kim DS. How accurate is magnetic resonance imaging of brain function? Trends Neurosci. 2003;26(2):108–14. doi: 10.1016/S0166-2236(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 30.Hennig J, Speck O, Koch MA. Functional magnetic resonance imaging: A review of methodological aspects and clinical applications. J Magn Reson Imaging. 2003;18(1):1–15. doi: 10.1002/jmri.10330. [DOI] [PubMed] [Google Scholar]
- 31.More SR, Lim TC, Li M, Holland CK, Boyce SE, Lee JH. Acoustic noise characteristics of a 4 Telsa MRI scanner. J Magn Reson Imaging. 2006;23(3):388–97. doi: 10.1002/jmri.20526. [DOI] [PubMed] [Google Scholar]
- 32.Fuchino Y, Sato H, Maki A, Yamamoto Y, Katura T, Obata A, Koizumi H, Yoroa T. Effect of fMRI acoustic noise on sensorimotor activation examined using optical topography. NeuroImage. 2006;32(2):771–7. doi: 10.1016/j.neuroimage.2006.04.197. [DOI] [PubMed] [Google Scholar]
- 33.Static fields: environmental health criteria monograph n0.232. World Health Organization; Geneva, Switzerland: World Health Organization; 2006. http://www.who.int/peh-emf/publications/reports/ehcstatic/en/ [Google Scholar]
- 34.Baumgartner C. Controversies in clinical neurophysiology. MEG is superior to EEG in the localization of interictal epileptiform activity: Con. Clin Neurophysiol. 2004;115(5):1010–20. doi: 10.1016/j.clinph.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Barkley GL. Controversies in neurophysiology. MEG is superior to EEG in localization of interictal epileptiform activity: Pro. Clin Neurophysiol. 2004;115(5):1001–9. doi: 10.1016/j.clinph.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Teplan M. Fundamentals of EEG measurement. Measurement Science Review. 2002;2(2):1–11. [Google Scholar]
- 37.Ioannides AA. Magnetoencephalography as a research tool in neuroscience: state of the art. Neuroscientist. 2006;12(6):524–44. doi: 10.1177/1073858406293696. [DOI] [PubMed] [Google Scholar]
- 38.Dhond RP, Kettner N, Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med. 2007;13(6):603–16. doi: 10.1089/acm.2007.7040. [DOI] [PubMed] [Google Scholar]
- 39.Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115(10):2195–222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Lu ZL, Kaufman L. Magnetic source imaging of the human brain. Mahwah, NJ, United States: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 41.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25(2):249–71. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 42.Rampp S, Stefan H. Magnetoencephalography in presurgical epilepsy diagnosis. Expert Rev Med Devices. 2007;4(3):335–47. doi: 10.1586/17434440.4.3.335. [DOI] [PubMed] [Google Scholar]
- 43.Sarco DP, Burke JF, Madsen JR. Electroencephalography in epilepsy surgery planning. Childs Nerv Syst. 2006;22(8):760–5. doi: 10.1007/s00381-006-0128-1. [DOI] [PubMed] [Google Scholar]
- 44.Cappell J, Schevon C, Emerson RG. Magnetoencephalography in epilepsy: tailoring interpretation and making inferences. Curr Neurol Neurosci Rep. 2006;6(4):327–31. doi: 10.1007/s11910-006-0026-7. [DOI] [PubMed] [Google Scholar]
- 45.Etchepareborda M, Mulas F, Gandia R, Abad-Mas L, Moreno F, Diaz-Lucero A. Techniques for the functional evaluation of neurodevelopmental disorders. Rev Neurol. 2006;42(Suppl 2):S71–81. [PubMed] [Google Scholar]
- 46.Berns GS. Functional neuroimaging. Life Sci. 1999;65(24):2531–40. doi: 10.1016/s0024-3205(99)00297-0. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM. Overview of fMRI analysis. Br J Radiol. 2004;77(Special Issue):S167–75. doi: 10.1259/bjr/33553595. [DOI] [PubMed] [Google Scholar]
- 48.Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25(2):199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coutin-Churchman P, Anez Y, Uzcategui M, Alvarez L, Vergara F, Mendez L, Fleitas R. Quantitative spectral analysis of EEG in psychiatry revisited: drawing signs out of numbers in a clinical setting. Clin Neurophysiol. 2003;114(12):2294–306. doi: 10.1016/s1388-2457(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 50.Dockstader C, Gaetz W, Cheyne D, Wang F, Castellanos FX, Tannock R. MEG event-related desynchronization and synchronization deficits during basic somatosensory processing in individuals with ADHD. Behav Brain Funct. 2008;4:8. doi: 10.1186/1744-9081-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the ’new psychophysiology’. Int J Psychophysiol. 2007;63(2):164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez A, Rodriguez-Palancas A, Lopez-Ibor M, Zuluaga P, Turrero A, Maestu F, Amo C, Lopez-Ibor JJ, Jr, Ortiz T. Increased occipital delta dipole density in major depressive disorder determined by magnetoencephalography. J Psychiatry Neurosci. 2005;30(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 53.Roffman JL, Marci CD, Glick DM, Dougherty DD, Rauch SL. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med. 2005;35(10):1385–98. doi: 10.1017/S0033291705005064. [DOI] [PubMed] [Google Scholar]
- 54.Boyd LA, Vidoni ED, Daly JJ. Answering the call: the influence of neuroimaging and electrophysiological evidence on rehabilitation. Phys Ther. 2007;87(6):684–703. doi: 10.2522/ptj.20060164. [DOI] [PubMed] [Google Scholar]
- 55.Cook DB, Stegner AJ, McLoughlin MJ. Imaging pain of fibromyalgia. Curr Pain Headache Rep. 2007;11(3):190–200. doi: 10.1007/s11916-007-0190-8. [DOI] [PubMed] [Google Scholar]
- 56.Nakabeppu Y, Nakajo M, Gushiken T, Tsuchimochi S, Tani A, Kanmura Y. Decreased perfusion of the bilateral thalami in patients with chronic pain detected by Tc-99m-ECD SPECT with statistical parametric mapping. Ann Nucl Med. 2001;15(5):459–63. doi: 10.1007/BF02988354. [DOI] [PubMed] [Google Scholar]
- 57.Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224(1):5–8. doi: 10.1016/s0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- 58.Bakkum BW, Henderson CNR, Hong SP, Cramer GD. Preliminary morphological evidence that vertebral hypomobility induces synaptic plasticity in the spinal cord. J Manipulative Physiol Ther. 2007;30(5):336–42. doi: 10.1016/j.jmpt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Haavik Taylor H, Murphy B. Altered central integration of dual somatosensory input following cervical spine manipulation. J Manipulative Physiol Ther. 2008 doi: 10.1016/j.jmpt.2010.01.005. in press. [DOI] [PubMed] [Google Scholar]
- 60.Flor-Henry P, Lind JC, Koles ZJ. A source-imaging (low resolution electromagnetic tomography) study of the EEGs from unmedicated males with depression. Psychiatry Res. 2004;130(2):191–207. doi: 10.1016/j.pscychresns.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Fallgatter AJ, Ehlis AC, Rosler M, Strik WK, Blocher D, Herrmann MJ. Diminished prefrontal brain function in adults with psychopathology in childhood related to attention deficit hyperactivity disorder. Psychiatry Res. 2005;138(2):157–169. doi: 10.1016/j.pscychresns.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Kilner JM, Salenius S, Baker SN, Jackson A, Hari R, Lemon RN. Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. NeuroImage. 2003;18(1):67–73. doi: 10.1006/nimg.2002.1322. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi M, Kato A, Fujita N, Hirata M, Tanaka H, Kihara T, Ninomiya H, Hirabuki N, Nakamura H, Robinson SE, Cheyne D, Yoshimine T. Movement-related desynchronization of the cerebral cortex studied with spatially filtered magnetoencephalography. NeuroImage. 2000;12(3):298–306. doi: 10.1006/nimg.2000.0611. [DOI] [PubMed] [Google Scholar]
- 64.Oshino S, Kato A, Wakayama A, Taniguchi M, Hirata M, Yoshimine T. Magnetoencephalographic analysis of cortical oscillatory activity in patients with brain tumors: Synthetic aperture magnetometry (SAM) functional imaging of delta band activity. NeuroImage. 2007;34(3):957–64. doi: 10.1016/j.neuroimage.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 65.Cooke R, Peel E, Shaw RL, Senior C. The neuroimaging research process from the participants’ perspective. Int J Psychophysiol. 2007;63(2):152–8. doi: 10.1016/j.ijpsycho.2006.03.014. [DOI] [PubMed] [Google Scholar]