Abstract
Purpose of review
To describe recent advances in the processing of gastrointestinal hormones, and the consequences for human disease of mutations in the enzymes involved.
Recent findings
Although gastrointestinal prohormones were long regarded as devoid of biological activity, recent data indicates that the prohormones for both gastrin and gastrin-releasing peptide are bioactive, through different receptors from the mature hormones. Mutations in the family of prohormone convertases responsible for the initial steps in the processing of gastrointestinal hormones are associated with several different pathophysiological conditions in humans.
Summary
Human mutational studies, when taken together with the phenotypes observed in mice deficient in the prohormone convertases, emphasize the crucial importance of the processing enzymes in mammalian biology. Although the phenotypes may often be ascribed to defective production of a mature hormone or growth factor, the recognition that the precursors are independently bioactive suggests that the increased precursor concentrations may also contribute to the symptoms. The observation that the precursors often act through different receptors from the mature hormones may permit the development of precursor-selective antagonists for therapeutic use.
Keywords: Carboxypeptidase, cholecystokinin, gastrin, gastrin-releasing peptide, progastrin, prohormone convertase
Introduction
Gastrointestinal peptides are synthesised as preprohormones in enteroendocrine cells, and post-translational processing of the precursors is required to make mature peptides prior to secretion. Although the prohormones formed after removal of the signal peptide were thought to be devoid of activity, recent data suggests that a variety of gastrointestinal prohormones may be biologically active. Examples include the precursors of gastrin, gastrin-releasing peptide (GRP), secretin and vasoactive intestinal peptide. Cancer cells often lack components of the “processing machinery” that produces mature peptides and thus the incompletely processed forms predominate. The crucial importance of the processing enzymes has been recently emphasised by the demonstration that their mutation is responsible for several different pathophysiological conditions in humans. This review discusses new advances in our knowledge of the relationship of peptide processing to biological activity and to human disease, with particular reference to the gastrointestinal hormones gastrin and GRP.
Post-translational processing - the generation of biologically active fragments of prohormones
Post-translational processing begins with the cleavage of the signal peptide to form a prohormone. The prohormones then undergo proteolytic cleavages, which are catalysed by two types of proteolytic enzymes: endopeptidases and exopeptidases. Endopeptidases (prohormone convertases, PCs) usually cleave after double basic amino acid residues (e.g. Lys-Lys), although their specificity is influenced by the adjoining sequence [1*]. The family of mammalian PCs now contains nine members, called PC1/3, PC2, furin, PC4, PC5/6, PACE4, PC7, SKI-1 and PCSK9 [2*], at least three of which are expressed in the gastrointestinal tract [3]. Exopeptidases such as carboxypeptidase B or E then remove basic residues from the new carboxyl terminus formed after endopeptidase cleavage. Recently, the importance of the tissue specificity of endopeptidase expression for generating various cell-specific prohormone precursors has been demonstrated by the finding that cholecystokinin (CCK) is processed by different PCs in the brain and the intestine [4*]. Using PC1/3 and PC2 null mice, Rehfeld and coworkers demonstrated that PC1/3 governs CCK processing in intestinal endocrine cells, while PC2 is involved in the processing of CCK in neuronal cells [4*].
Following cleavage, peptides are amidated at the C-terminus by the enzyme peptidylglycine α-amidating monooxygenase (PAM) [5]. Peptide amidation is often required to achieve full biological activity, as the presence of an amide group can significantly increase the affinity of the peptide for its receptor as in the case of GRP [6]. Although C-terminally amidated peptides were thought to be the only biologically active forms, there is an increasing number of examples where a glycine-extended intermediate is also bioactive, for example gastrin-gly [7], secretin-gly [8], vasoactive intestinal peptide-gly [9], GRP-gly [10], glucagon-like peptide-1-gly [11] and substance P-gly [12].
Progastrin
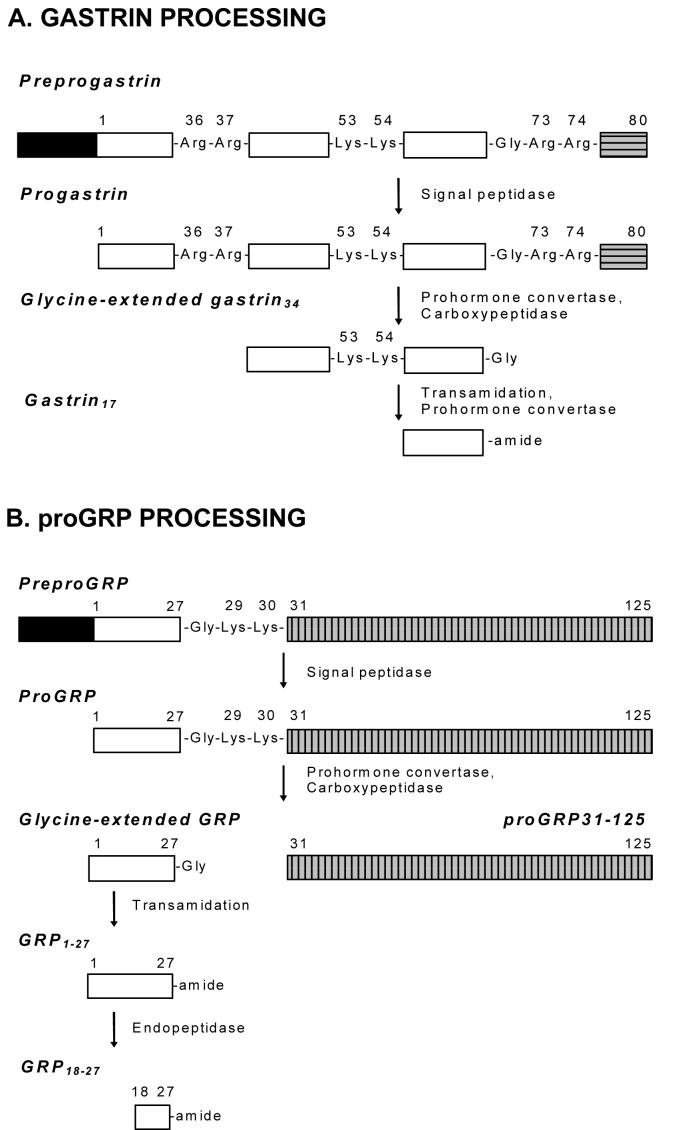
There are three dibasic cleavage sites within the progastrin molecule (Figure 1). Cleavage following Arg36-Arg37 generates the N-terminus of gastrin34, cleavage following Arg73-Arg74 releases the C-terminal flanking peptide (CTFP), and cleavage following Lys53-Lys54 yields the N-terminus of gastrin17. The PCs responsible for cleavage at each site within the immature Golgi vesicles have only recently been determined using transgenic mice. Using PC1/3 null mice, Rehfeld and coworkers demonstrated that processing begins with PC1/3, which is solely responsible for cleavage after Arg36-Arg37. Subsequently, PC1/3 cleaves after Arg73-Arg74, while later on PC2 partially cleaves after Lys53-Lys54 to produce gastrin17 [13]. Furthermore, PC5/6 was found not to play a significant role in the maturation of progastrin within G cells.
Figure 1. Processing of progastrin and proGRP.
Preprogastrin (A, 101 amino acids) and preproGRP (B, 148 amino acids) are converted to progastrin (80 amino acids) and proGRP (125 amino acids), respectively, by removal of their signal peptides (black boxes). The sequential action of prohormone convertases and carboxypeptidase E then converts the prohormones to glycine-extended forms. The extent of conversion of glycine-extended to amidated forms by the amidation enzyme PAM is dependent on the tissue. Both the prohormones and amidated forms are independently active via different receptors. Fragments from the C-terminal region of progastrin (CTFP, horizontally hatched gray box) and proGRP (proGRP31-125, vertically hatched gray box) are also biologically active, and activity is again mediated by different receptors from the corresponding amidated hormones.
Although amidated gastrin17 and gastrin34 were initially the only recognised biologically active peptides derived from progastrin, interest in the past decade has focussed on non-amidated forms of the hormone. Recombinant progastrin has been produced in bacteria by two groups, and shown to stimulate proliferation and migration of a variety of gastrointestinal cell lines [14,15]. Glycine-extended gastrin17 is also biologically active in cell lines [16], albeit via a different receptor from amidated gastrins, and activity requires the presence of ferric ions [17]. The in vitro data is in agreement with experiments in transgenic animals, in which over-expression of progastrin or glycine-extended gastrin17 resulted in hyperproliferation in the colonic mucosa [18,19]. Infusion of gastrin-gly replicates the colonic hyperproliferation observed in transgenic mice [20]. In contrast over-expression of amidated gastrin17 resulted in hyperproliferation in the gastric mucosa [18]. Importantly the non-amidated forms are the major type of gastrin present in colorectal cancers [21].
The C-terminal flanking peptide (CTFP) of progastrin is also biologically active [22]. Initially CTFP was found in similar concentrations to amidated gastrin17 in the circulation [23]. A newly developed antiserum was used to demonstrate that CTFP was the predominant progastrin-derived peptide in the antrum (4-fold higher than amidated gastrins) and circulation (60-fold higher than amidated gastrins), and was released after meal stimulation [22]. The observation that CTFP concentrations were unaltered in patients with multiple endocrine neoplasia type 1 or pernicious anemia, even though plasma amidated gastrins were elevated, demonstrated that the two peptides were differentially secreted after cleavage from progastrin. The CTFP was also shown to stimulate proliferation and migration, and activation of the mitogen-activated protein kinase pathway, in several gastrointestinal cell lines [22].
In the future gastrin research should also focus on N-terminal fragments of progastrin. Such fragments, including gastrin1-35, gastrin6-35, and gastrin20-35, have been detected in both normal antral mucosa [24] and gastrinoma extracts [25-27]. Although the biological activity of the N-terminal progastrin fragments has not yet been examined, residues 1-35 are not required for cellular sorting to the secretory pathway and subsequent regulated secretion of gastrins [28]. The observation of an intriguingly high concentration of gastrin1-35 in the circulation compared to amidated gastrin17 [29] suggests that the biology of N-terminal progastrin fragments should be examined as well as their metabolism.
Gastrin-releasing peptide
Gastrin-releasing peptide (GRP) belongs to the bombesin-like peptide family, which consists of at least 13 closely related peptides. Although the endopeptidase that processes the 125 amino acid prohormone (proGRP) after Lys29-Lys30 (Figure 1) has not been identified, processing has been postulated to be mediated by either PC1/3 or PC2 [30]. Following endopeptidase cleavage, a carboxypeptidase B-like activity removes the two C-terminal basic residues giving rise to the glycine-extended form, GRP1-28, which is then amidated to GRP1-27 amide by the amidation enzyme PAM. Finally cleavage after Arg17 produces GRP18-27 (GRPamide).
proGRP itself is now known to be biologically active as a growth factor for several cell lines of divergent origin [31]. Comparison of proGRP sequences between species indicated that blocks of homology in addition to GRP18-28 were also present in the C-terminal region of proGRP [32]. Recombinant peptides including proGRP31-125 and proGRP42-98 were then expressed in bacteria, purified, and shown to stimulate proliferation in several cell lines independently of the GRP receptor [33,34]. Using a newly developed antiserum directed to the N terminus of human proGRP, proGRP immunoreactivity was detected in all of the endometrial, prostate, and colon cancer cell lines tested and in nine of 10 resected colorectal carcinomas [31]. The presence of additional cleavage sites within the C-terminal extension peptide (proGRP31-125) raises the possibility of multiple peptides being generated from the processing of proGRP by endopeptidases.
Processing enzymes in human disease
The crucial importance of peptide processing enzymes has recently been emphasised through the study of knock-out mice [35,36*], and by the demonstration of mutations in PCs in several human conditions (Table 1). The potential pathologies include Alzheimer’s disease, obesity, diabetes, lipid disorders, infection and tumorigenesis [2,35].
Table 1. Processing Enzymes and Disease.
| Enzyme | Gene | Associated Condition | Likely Target | Reference |
|---|---|---|---|---|
| PC 1/3 | PCSK1 | Obesity | Not identified | [37] [38*] |
| Furin | PCSK3 | Defective immune tolerance | TGFβ | [39*] |
| PC 5/6 | PCSK5 | VACTERL | GDF11 | [40*] |
| PC 9 | PCSK9 | Decreased LDL cholesterol; Protection against heart disease | LDL receptor | [41] |
| Carboxy-peptidase E | CPE | Obesity | Not identified | [42] |
PC1/3
Mutations in the PCSK1 gene that encodes prohormone convertase1/3 have now been described in 3 patients with the common symptoms of hyperphagia, obesity and persistent diarrhoea [37,43,44]. A recent study of single nucleotide polymorphisms in a large group of Europeans further revealed that the PCSK1 mutations Asn221Asp and Gln665Glu-Ser690Thr were consistently associated with obesity in children and adults [38*]. Surprisingly none of the mutations appeared to affect maturation or secretion of the enzyme, and only the Asn221Asp mutation reduced enzyme activity (by 10%). In mouse models, although mutation of the adjacent residue Asn222Asp also results in increased body fat content and mature onset obesity [45], global deletion of the PCSK1 gene did not cause obesity when animals were fed a diet containing normal concentrations of fat [46,47].
Furin
Furin has recently been found to mediate the release of soluble hemojuvelin from its membrane-bound precursor [48,49]. The importance of hemojuvelin in iron homeostasis is demonstrated by the fact that mutations in the hemojuvelin gene result in reduced production of the iron regulatory peptide hepcidin and hence to juvenile hemochromatosis [50]. Furin is also directly involved in the processing of hepcidin from its precursor [51*]. The connection between furin and iron may have important implications in the development of Alzheimer’s disease as iron attenuates the production of furin which in turn impairs the ability of α-secretase to produce the neuroprotective form of the amyloid protein precursor [52]. Although absence of furin in mice as a result of PCSK3 null mutation results in embryonic lethality [53], conditional deletion of the PCSK3 gene from mouse T cells resulted in reduced Transforming Growth Factor β1 (TGFβ1) production and impaired regulatory and effector T cell function. In particular Treg cells were less protective in a T cell transfer colitis model [39*].
PC5/6
A mutation in the mouse PCSK5 gene has recently been identified as the cause of a phenotype that includes cardiac, tracheoesophageal, anorectal and anteroposterior patterning defects, and hindlimb and pulmonary hypoplasia [40*]. Mutations in the human PCSK5 gene were identified in patients with the multisystemic features of the VACTERL association (OMIM 192350: Vertebral anomalies, Anal atresia, Cardiac malformations, Tracheo-Esophageal fistula, Renal anomalies and Limb anomalies) and caudal regression syndrome [40*]. The phenotype was ascribed to a failure of the mutant PC5/6 to cleave and activate Growth Differentiation Factor 11 (GDF11), which is a member of the TGFβ family responsible for anterior-posterior patterning in the embryo [54*].
PC9
A considerable body of work over the last decade indicates that mutations in the PCSK9 gene result in low circulating concentrations of Low Density Lipoprotein (LDL) cholesterol, which in turn protect against coronary heart disease (see [41,55] for reviews). Conversely gain of function mutations reduce the concentration of the hepatic LDL receptor, with a consequent increase of plasma cholesterol and increased susceptibility to coronary heart disease [41,55]. The recent determination of the structure of the complex between PC9 and the Epidermal Growth Factor (EGF)-A domain of the LDL receptor opens the way for the development of selective inhibitors of the PC9/LDL receptor interaction [56]. Targetting PCSK9 gene products with siRNA also shows promise as a therapy for hypercholesterolemia [57].
Carboxypeptidase E
Mice with a mutation in the gene encoding carboxypeptidase E develop hyperglycemia and obesity [42]. While the phenotype has been ascribed to defective prohormone processing of proinsulin in particular, recent work also indicates an unexpected structural role for carboxypeptidase E. Thus interaction of the cytoplasmic tail of carboxypeptidase E with dynactin and the microtubule motor kinesin may assist in transport of vesicles containing peptide hormones to the cell membrane [58,59].
Processing enzymes in cancer
Abnormal expression and processing of PCs has been demonstrated in both animal models and human cancers [60]. Recently, overexpression of furin was shown to increase cancer cell motility and invasiveness [61*]. These observations led to the identification of a small-molecule furin inhibitor, named B3, which could potentially be used as a novel cancer therapy [61*]. Moreover, some hormones have also been shown to upregulate PC expression and thus contribute to cancer progression. For example thyroid hormone was found to promote cell invasion by increasing the expression of furin in human hepatoma cell lines [62]. Finally, since the amidation enzyme PAM is essential for the production of α-amidated peptides, the expression of PAM has been used as a marker of bioactive peptides in a number of cancers including prostate [63], glioblastoma [64] and lung [65].
As mentioned above, PCs are involved in the cleavage of peptide precursors to produce growth factors, which may be directly involved in cancer development and metastasis by regulating cell growth and survival in an autocrine or paracrine manner [66,67]. PCs are also involved in activation of growth factor receptors such as the receptor for insulin-like growth factor-1 [68]. Hence selective inhibition of PCs has great potential as a cancer therapy [61*,69**]. On the other hand the possibility must be kept in mind that the continuous production of bioactive precursors acting via independent receptors may also contribute to tumor development in cells and tissues in which prohormone processing is faulty because of lack of the full suite of processing enzymes.
Conclusions
The phenotypes of PC-deficient mice and the association between PC mutation and human disease independently confirm the crucial role played by PCs in processing of gastrointestinal prohormones. Alterations in the prohormone processing machinery in cancer cells could potentially lead to an excess of hormone precursor forms which may then contribute to cancer progression. The fact that the bioactivity of many prohormones is mediated by receptors distinct from those utilised by the corresponding mature hormones may permit the use of prohormone-selective antagonists in conditions such as obesity, neural degeneration and cancer.
Acknowledgments
This work was supported in part by grants from the National Health and Medical Research Council of Australia (400062, 454322) and the National Institutes of Health (5RO1GM065926-06).
Abbreviations
- CCK
cholecystokinin
- CTFP
C-terminal flanking peptide of progastrin
- EGF
epidermal growth factor
- GDF11
growth differentiation factor 11
- GRP
gastrin-releasing peptide
- LDL
low density lipoprotein
- PAM
peptidylglycine α-amidating monooxygenase
- PC
prohormone convertase
- TGFβ
transforming growth factor β
- VACTERL
vertebral anomalies, anal atresia, cardiac malformations, tracheo-esophageal fistula, renal anomalies and limb anomalies
References
- *1.Remacle AG, Shiryaev SA, Oh ES, et al. Substrate cleavage analysis of furin and related proprotein convertases. A comparative study. J Biol Chem. 2008;283:20897–20906. doi: 10.1074/jbc.M803762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article presents a systematic analysis of the substrate specificity of PC family members.
- *2.Seidah NG, Mayer G, Zaid A, et al. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- A comprehensive review.
- 3.Gagnon J, Mayne J, Mbikay M, et al. Expression of PCSK1 (PC1/3), PCSK2 (PC2) and PCSK3 (furin) in mouse small intestine. Regul Pept. 2008 doi: 10.1016/j.regpep.2008.07.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- *4.Rehfeld JF, Bundgaard JR, Hannibal J, et al. The cell-specific pattern of cholecystokinin peptides in endocrine cells versus neurons is governed by the expression of prohormone convertases 1/3, 2, and 5/6. Endocrinology. 2008;149:1600–1608. doi: 10.1210/en.2007-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article demonstrates that prohormone processing differs between different cell types.
- 5.Eipper BA, Milgram SL, Husten EJ, et al. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993;2:489–497. doi: 10.1002/pro.5560020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel O, Dumesny C, Giraud AS, et al. Stimulation of proliferation and migration of a colorectal cancer cell line by amidated and glycine-extended gastrin-releasing peptide via the same receptor. Biochem Pharmacol. 2004;68:2129–2142. doi: 10.1016/j.bcp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- 8.Solomon TE, Varga G, Zeng N, et al. Different actions of secretin and Gly-extended secretin predict secretin receptor subtypes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G88–94. doi: 10.1152/ajpgi.2001.280.1.G88. [DOI] [PubMed] [Google Scholar]
- 9.Fahrenkrug J. Glycine-extended processing intermediate of proVIP: a new form of VIP in the rat. Biochem Biophys Res Commun. 1991;178:173–177. doi: 10.1016/0006-291x(91)91795-e. [DOI] [PubMed] [Google Scholar]
- 10.Oiry C, Pannequin J, Bernad N, et al. A synthetic glycine-extended bombesin analogue interacts with the GRP/bombesin receptor. Eur J Pharmacol. 2000;403:17–25. doi: 10.1016/s0014-2999(00)00576-8. [DOI] [PubMed] [Google Scholar]
- 11.Wettergren A, Pridal L, Wojdemann M, Holst JJ. Amidated and non-amidated glucagon-like peptide-1 (GLP-1): non-pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regul Pept. 1998;77:83–87. doi: 10.1016/s0167-0115(98)00044-5. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Mohamed GA, Huang J, Oldham CD, et al. Vascular and endothelial actions of inhibitors of substance P amidation. J Cardiovasc Pharmacol. 2000;35:871–880. doi: 10.1097/00005344-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Rehfeld JF, Zhu X, Norrbom C, et al. Prohormone convertases 1/3 and 2 together orchestrate the site-specific cleavages of progastrin to release gastrin-34 and gastrin-17. Biochem J. 2008;415:35–43. doi: 10.1042/BJ20080881. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin GS, Hollande F, Yang Z, et al. Biologically active recombinant human progastrin(6-80) contains a tightly bound calcium ion. J Biol Chem. 2001;276:7791–7796. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Lu X, Cobb S, et al. Progastrin1-80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284:G328–339. doi: 10.1152/ajpgi.00351.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hollande F, Imdahl A, Mantamadiotis T, et al. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 17.Pannequin J, Barnham KJ, Hollande F, et al. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602–48609. doi: 10.1074/jbc.M208440200. [DOI] [PubMed] [Google Scholar]
- 18.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh TJ, Dockray GJ, Varro A, et al. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–313. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- 21.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Smith KA, Patel O, Lachal S, et al. Production, secretion, and biological activity of the C-terminal flanking peptide of human progastrin. Gastroenterology. 2006;131:1463–1474. doi: 10.1053/j.gastro.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Pauwels S, Desmond H, Dimaline R, Dockray GJ. Identification of progastrin in gastrinomas, antrum, and duodenum by a novel radioimmunoassay. J Clin Invest. 1986;77:376–381. doi: 10.1172/JCI112315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehfeld JF, Johnsen AH. Identification of gastrin component I as gastrin-71. The largest possible bioactive progastrin product. Eur J Biochem. 1994;223:765–773. doi: 10.1111/j.1432-1033.1994.tb19051.x. [DOI] [PubMed] [Google Scholar]
- 25.Reeve JR, Jr., Walsh JH, Tompkins RK, et al. Amino terminal fragments of human progastrin from gastrinoma. Biochem Biophys Res Commun. 1984;123:404–409. doi: 10.1016/0006-291x(84)90428-5. [DOI] [PubMed] [Google Scholar]
- 26.Desmond H, Pauwels S, Varro A, et al. Isolation and characterization of the intact gastrin precursor from a gastrinoma. FEBS Lett. 1987;210:185–188. doi: 10.1016/0014-5793(87)81334-0. [DOI] [PubMed] [Google Scholar]
- 27.Huebner VD, Jiang RL, Lee TD, et al. Purification and structural characterization of progastrin-derived peptides from a human gastrinoma. J Biol Chem. 1991;266:12223–12227. [PubMed] [Google Scholar]
- 28.Bundgaard JR, Birkedal H, Rehfeld JF. Progastrin is directed to the regulated secretory pathway by synergistically acting basic and acidic motifs. J Biol Chem. 2004;279:5488–5493. doi: 10.1074/jbc.M310547200. [DOI] [PubMed] [Google Scholar]
- 29.Goetze JP, Hansen CP, Rehfeld JF. Antral content, secretion and peripheral metabolism of N-terminal progastrin fragments. Regul Pept. 2006;133:47–53. doi: 10.1016/j.regpep.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Rounseville MP, Davis TP. Prohormone convertase and autocrine growth factor mRNAs are coexpressed in small cell lung carcinoma. J Mol Endocrinol. 2000;25:121–128. doi: 10.1677/jme.0.0250121. [DOI] [PubMed] [Google Scholar]
- 31.Dumesny C, Patel O, Lachal S, et al. Synthesis, expression and biological activity of the prohormone for gastrin releasing peptide (ProGRP) Endocrinology. 2006;147:502–509. doi: 10.1210/en.2005-0574. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin GS, Patel O, Shulkes A. Phylogenetic analysis of the sequences of gastrin-releasing peptide and its receptors: biological implications. Regul Pept. 2007;143:1–14. doi: 10.1016/j.regpep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Patel O, Dumesny C, Shulkes A, Baldwin GS. Recombinant C-terminal fragments of the gastrin-releasing peptide precursor are bioactive. Cancer Lett. 2007;254:87–93. doi: 10.1016/j.canlet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Patel O, Dumesny C, Shulkes A, Baldwin GS. C-terminal fragments of the gastrin-releasing peptide precursor stimulate cell proliferation via a novel receptor. Endocrinology. 2007;148:1330–1339. doi: 10.1210/en.2006-0466. [DOI] [PubMed] [Google Scholar]
- 35.Scamuffa N, Calvo F, Chretien M, et al. Proprotein convertases: lessons from knockouts. Faseb J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- *36.Creemers JW, Khatib AM. Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front Biosci. 2008;13:4960–4971. doi: 10.2741/3055. [DOI] [PubMed] [Google Scholar]
- This review presents an up-to-date comparison of mouse models of PC deficiency.
- 37.Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- *38.Benzinou M, Creemers JW, Choquet H, et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40:943–945. doi: 10.1038/ng.177. [DOI] [PubMed] [Google Scholar]
- This article strengthens the link between Pcsk1 mutation and obesity.
- *39.Pesu M, Watford WT, Wei L, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article describes the phenotype of the first tissue-specific furin-deficient mouse.
- *40.Szumska D, Pieles G, Essalmani R, et al. VACTERL/caudal regression/Currarino syndrome-like malformations in mice with mutation in the proprotein convertase Pcsk5. Genes Dev. 2008;22:1465–1477. doi: 10.1101/gad.479408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article is the first report of developmental abnormalities in Pcsk5-deficient mice.
- 41.Costet P, Krempf M, Cariou B. PCSK9 and LDL cholesterol: unravelling the target to design the bullet. Trends Biochem Sci. 2008;33:426–434. doi: 10.1016/j.tibs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 43.Jackson RS, Creemers JW, Farooqi IS, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112:1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farooqi IS, Volders K, Stanhope R, et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab. 2007;92:3369–3373. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15:1884–1893. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- 46.Mbikay M, Croissandeau G, Sirois F, et al. A targeted deletion/insertion in the mouse Pcsk1 locus is associated with homozygous embryo preimplantation lethality, mutant allele preferential transmission and heterozygous female susceptibility to dietary fat. Dev Biol. 2007;306:584–598. doi: 10.1016/j.ydbio.2007.03.523. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, Zhou A, Dey A, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 49.Kuninger D, Kuns-Hashimoto R, Nili M, Rotwein P. Pro-protein convertases control the maturation and processing of the iron-regulatory protein, RGMc/hemojuvelin. BMC Biochem. 2008;9:9. doi: 10.1186/1471-2091-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson GJ, Frazer DM. Iron metabolism meets signal transduction. Nat Genet. 2006;38:503–504. doi: 10.1038/ng0506-503. [DOI] [PubMed] [Google Scholar]
- *51.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40:132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article provides the first evidence for the involvement of PCs in iron homeostasis.
- 52.Silvestri L, Camaschella C. A potential pathogenetic role of iron in Alzheimer’s Disease. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00356.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roebroek AJ, Umans L, Pauli IG, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- *54.Essalmani R, Zaid A, Marcinkiewicz J, et al. In vivo functions of the proprotein convertase PC5/6 during mouse development: Gdf11 is a likely substrate. Proc Natl Acad Sci U S A. 2008;105:5750–5755. doi: 10.1073/pnas.0709428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article identifies the differentiation factor, deficiency of which is responsible for developmental abnormalities in Pcsk5-deficient mice.
- 55.Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res. 2008;49:1595–1599. doi: 10.1194/jlr.CX00001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon HJ, Lagace TA, McNutt MC, et al. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci U S A. 2008;105:1820–1825. doi: 10.1073/pnas.0712064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park JJ, Cawley NX, Loh YP. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol. 2008;22:989–1005. doi: 10.1210/me.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JJ, Cawley NX, Loh YP. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci. 2008;39:63–73. doi: 10.1016/j.mcn.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzimas GN, Chevet E, Jenna S, et al. Abnormal expression and processing of the proprotein convertases PC1 and PC2 in human colorectal liver metastases. BMC Cancer. 2005;5:149. doi: 10.1186/1471-2407-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Coppola JM, Bhojani MS, Ross BD, Rehemtulla A. A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia. 2008;10:363–370. doi: 10.1593/neo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article demonstrates that a furin inhibitor can reduce the invasiveness of human fibrosarcoma cells by interfering with the processing of a matrix metalloproteinase.
- 62.Chen RN, Huang YH, Lin YC, et al. Thyroid hormone promotes cell invasion through activation of furin expression in human hepatoma cell lines. Endocrinology. 2008;149:3817–3831. doi: 10.1210/en.2007-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocchi P, Boudouresque F, Zamora AJ, et al. Expression of adrenomedullin and peptide amidation activity in human prostate cancer and in human prostate cancer cell lines. Cancer Res. 2001;61:1196–1206. [PubMed] [Google Scholar]
- 64.Ouafik L, Sauze S, Boudouresque F, et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160:1279–1292. doi: 10.1016/S0002-9440(10)62555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwai N, Martinez A, Miller MJ, et al. Autocrine growth loops dependent on peptidyl alpha-amidating enzyme as targets for novel tumor cell growth inhibitors. Lung Cancer. 1999;23:209–222. doi: 10.1016/s0169-5002(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 66.Khatib AM, Bassi D, Siegfried G, et al. Endo/exo-proteolysis in neoplastic progression and metastasis. J Mol Med. 2005;83:856–864. doi: 10.1007/s00109-005-0692-y. [DOI] [PubMed] [Google Scholar]
- 67.Muller EJ, Caldelari R, Posthaus H. Role of subtilisin-like convertases in cadherin processing or the conundrum to stall cadherin function by convertase inhibitors in cancer therapy. J Mol Histol. 2004;35:263–275. doi: 10.1023/b:hijo.0000032358.51866.a2. [DOI] [PubMed] [Google Scholar]
- 68.Stawowy P, Kallisch H, Kilimnik A, et al. Proprotein convertases regulate insulin-like growth factor 1-induced membrane-type 1 matrix metalloproteinase in VSMCs via endoproteolytic activation of the insulin-like growth factor-1 receptor. Biochem Biophys Res Commun. 2004;321:531–538. doi: 10.1016/j.bbrc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- **69.Scamuffa N, Siegfried G, Bontemps Y, et al. Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells. J Clin Invest. 2008;118:352–363. doi: 10.1172/JCI32040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This article reports that stable expression of the general PC inhibitor α1-antitrypsin Portland reduces the ability of colorectal cancer cells to form liver metastases in mice. This observation may lead to the development of novel therapies for colorectal cancer in humans.