Abstract
Objective
To assess prevalence of, and factors associated with tobacco smoking and dependence in HIV patients.
Design
A one-day cross-sectional national survey.
Methods
727 consecutive outpatients from a representative sample of 82 French units specialized in HIV-infected patient care were asked to complete a self-administered questionnaire, assessing smoking habits, dependence, cessation motivation, other substance abuse, socio-cultural characteristics, life with HIV and its treatment. Smoking prevalence and dependence were assessed and compared with a representative sample of the general French population; associated factors were determined.
Results
593 (82%) patients completed the questionnaire, 12% were active or ex-intravenous drug users, 37% were homosexual men; 43% were active smokers (31% in the French population). Fifty-six percent of smokers were classified as moderately or highly dependent, 14% of smokers were highly motivated and free of other substance abuse and of depressive symptoms. Smoking was independently associated with male sex (OR=2.38; 95% CI: 0.99–1.11), BMI (OR=1.08; 95% CI: 1.14-1.03), smoking environment (OR=4.75; 95% CI: 3.02–7.49), excessive alcohol consumption (OR=2.50; 95% CI: 1.20–5.23), illicit drug use (OR=2.43; 95% CI: 1.41–4.19), HIV status disclosure to family (OR=1.81; 95% CI: 1.16–2.85) and experience of rejection due to disclosure (OR=1.90; 95% CI: 1.14–3.17). Disclosure and drug substitutes’ usage were associated with high tobacco dependence.
Conclusions
Tobacco smoking is frequent and associated with other substance use and HIV disclosure. The rate of smokers who would be good candidates for a standard tobacco cessation program appears very low. Tobacco reduction or cessation strategies should be adapted to this population.
Keywords: Adaptation, Psychological, Adult, Africa South of the Sahara, Africa, Northern, Aged, Alcohol Drinking, epidemiology, Anti-Retroviral Agents, therapeutic use, Body Mass Index, Cross-Sectional Studies, Europe, Female, HIV Infections, drug therapy, epidemiology, psychology, Homosexuality, Male, statistics & numerical data, Humans, Male, Middle Aged, Motivation, Odds Ratio, Prejudice, Prevalence, Questionnaires, Risk Assessment, Risk Factors, Sex Factors, Smoking, epidemiology, prevention & control, psychology, Smoking Cessation, psychology, Substance-Related Disorders, epidemiology, Tobacco Use Disorder, epidemiology, psychology, therapy, Truth Disclosure
Keywords: Tobacco, HIV, dependence, motivation, associated factors
INTRODUCTION
Cardiovascular diseases and lung cancer are becoming competing causes of death in people living with HIV and AIDS on antiretroviral treatment [1, 2]. Recent evidence suggests that the prevalence of cigarette smoking among HIV-infected patients is elevated compared to the general population, smoking being a well-established risk factor for several diseases including pulmonary infectious diseases, lung cancer and cardiovascular diseases [2–7]. Prognosis of lung cancer is dramatically worse in the HIV-infected population as compared to the non-infected population [8, 9]. In the case of cardiovascular diseases, smoking’s negative effects add to the risks already posed by increased lipid level, increased insulin resistance and diabetes which are frequent in patients receiving antiretroviral treatments [10]. Given the association between smoking and adverse health outcomes, smoking cessation programs are clearly needed in HIV-infected patients [6].
However, the reasons for higher cigarette smoking prevalence in the HIV-infected patient population are unclear. Smoking characteristics in the HIV-infected population probably differ from those in the general population due to the constraints related to a chronic life-threatening disease and its therapy, related psychological and behavioural factors and, in some patients, other substance abuse. All these aspects probably likely impact smoking prevalence, the degree of tobacco dependence, motivation to stop smoking and smoking cessation strategies success rates.
To understand the basic mechanisms that regulate smoking and cessation in smokers living with HIV, we conducted a one-day national survey on tobacco use in a large representative population of HIV-infected outpatients to examine the relationship between tobacco use characteristics, cessation plans, and patient characteristics.
METHODS
Patients and Study Design
The survey was conducted on May 30th 2006 in a representative sample of French units specialized in the care of HIV-infected patients. One hundred and sixty-eight units were randomly chosen among all French units having declared at least 5 diagnosed cases of HIV each year during the 2000–2004 period (the declaration of diagnosed cases of HIV to the health ministry is mandatory in France). Among the 168 solicited units throughout France, 80 agreed to participate in the study.
All patients coming for their regular follow-up visit or for a day hospitalisation were asked to participate in this survey. Written informed consent was obtained for all patients. The local ethics committee of Paris Hôtel-Dieu approved the study.
Data Collected
We collected the following data for each patient through a practitioner (provider ??) questionnaire (whether patients agreed to respond to the self-administered questionnaire or not): sex, age, geographic origin, risk factors for HIV infection, worst stage of disease in patient as described by Center for Disease Control and Prevention, CD4 cell count and HIV-RNA level at the time of the consultation as well as the then current prescription of antiretroviral therapy. For patients who refused to participate in the study, additional questions answered by the practitioner provided the following information: smoking status, geographic origin, cardiovascular risk factors, and professional status (active worker, retired or unemployed). Patients who participated in the study filled out an anonymous self-administered multiple-choice questionnaire, which asked for demographic data, socio-economic status, HIV infection treatment and history, substance abuse, and symptoms of anxiety and depression. Self-reported symptoms related to antiretroviral drugs were assessed using two approaches. First, patients were asked if they had ever experienced an adverse drug reaction related to antiretroviral therapy. Secondly, information about self-reported symptoms was obtained using a 13-item scale. This scale corresponds to the French version of the symptom index, validated by Justice et al. [11]. This scale was previously adapted and used in a French cohort of patients treated by antiretroviral therapy [12]. Patients were asked if they had experienced at least one of the following symptoms during the previous 4 weeks: diarrhoea, nausea, stomach pain, headache, change in taste, itching skin, muscle pain, heartburn, sore mouth, vomiting, fever, kidney stones or fatigue. Patients were categorized as having a subjective perception of antiretroviral adverse events if they reported at least one symptom.
Tobacco, alcohol, drugs and psychoactive substance use
The following tobacco use characteristics were monitored: prevalence, contact with other smokers, tobacco dependence using the Fagerström Test for Nicotine Dependence (no or low dependence (< 5) versus moderate or strong dependence (≥5)) [13], motivation to stop smoking (using Q mat scale; high and intermediate motivation versus insufficient motivation) as well as use of other substances (alcohol, cocaine …), antidepressants or anti-anxiety medications in the previous six months [14]. Patients were asked if they have consumed rarely, frequently or every day one of any substances on a predefined list (Figure 1) during the preceding six months. Patients were classified as an active illicit drug user if they declared having consumed at least one illicit drug during the preceding six months. Reasons for smoking were also assessed. Alcohol consumption was categorized using the CAGE-DETA scale (0–4) [15]. Patients were considered to have excessive consumption if their total score was above 2. According to the World Health Organisation recommendations, regular tobacco smokers are defined as consumers of one or more cigarettes a day for at least one year [16]. Individuals were considered as former smokers if they had a history of regular smoking and declared having quit smoking for at least 1 month and had not smoked any cigarettes during the month preceding the survey. In order to ensure confidentiality, each questionnaire was distributed in a sealed envelope identifiable only by a unique identification number for each patient. Pack years of smoking were determined based on the average daily amount of cigarettes reported by the patients over the entire time of use. For active smokers, the involvement of their health provider in providing tobacco education and cessation program referral was assessed. Patients’ perception of the risk of developing tobacco related health problems was assessed through a visual scale (0 (none) to 10 (high)).
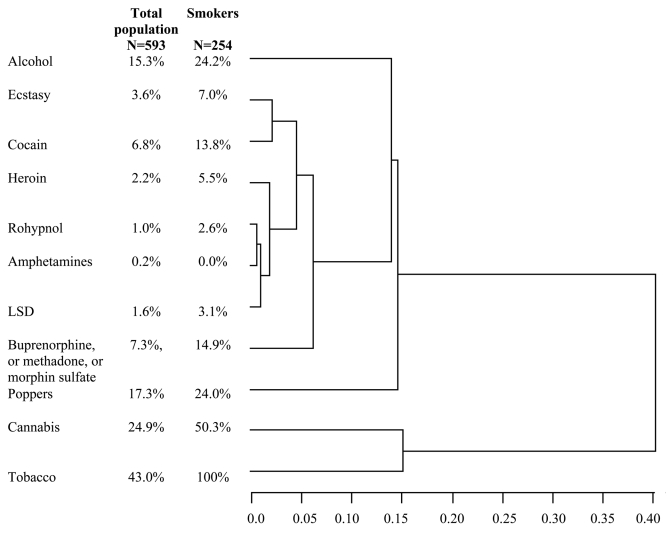
Figure 1.
Dendrogram showing the results of Ward’s cluster analysis: hierarchical relationships among substance abuse in the study population.
Note: Results are expressed by name of substance consumed, % of consumers among the study population (n=593), and among the smokers (n=254); LSD: lysergic acid diethylamide. The semi-partial R-square is an indicator of cluster isolation: high semi-partial r-square values indicate a high level of cluster independence.
Anxiety, depression and experience of disclosure
Anxiety and depression were assessed using the hospital anxiety and depression (HAD) scale which includes 14 questions (7 relating to anxiety and 7 to depression) concerning individuals’ experience over the previous week [17]. Patients were asked whether they had experienced rejection due to disclosure of HIV seropositive status.
Comparison to the French HIV infected population and the general French population
The main characteristics (sex, age, HIV risk factors, CD4 cell count) between the population of the study and the French database on HIV infection (using the DMI-2 software) which reports information on 43, 244 HIV-infected patients were compared to confirm the representativity of our studied population.
Cigarette smoking prevalence, tobacco dependence using Fagerström scale, and alcohol consumption were compared to the 2005 French National health survey (Baromètre santé INPES; available at http://www.inpes.sante.fr/index.asp?page=Barometres/Baro2005_1R/ouvrage/presentation.asp) in the general population using a representative sample of 28,221 individuals aged 18–75 years.
Statistical Analysis
The quantitative variables were described as mean ± standard deviations while qualitative variables were described as percentages.
To assess comparisons between our sample and reference populations data (the French HIV-infected population and the general French population), we used the one-sample t test when comparing quantitative variables and the one-way chi-square test when comparing qualitative variables.
Factors associated with each of the 3 following outcomes were investigated in our HIV population: smoking status (current/former/non), tobacco dependence (yes/no) and high smoking cessation motivation (yes/no). For each of these outcomes, a bivariate and a multivariate analysis were performed. In bivariate analysis, a one-way ANOVA was used to compare quantitative variables. The chi-square test was used to compare categorical variables. Variables that were related to outcomes in bivariate analysis (p<0.20) were kept in the multivariate analysis. Multivariate analysis of associated factors was performed using a polytomous nominal logistic regression or standard logistic regression, when appropriate. Based on an a priori significance level of 0.05, stepwise logistic regression (mixed methods) was performed was used to select the significant terms of the model. Odds ratios with 95% confidence interval (CI) were estimated. All statistical analyses were performed with SAS software, version 9.1 (SAS institute).
A hierarchical cluster analysis (Ward method with a square Euclidean distance measure) was used to identify clusters of correlated variables among alcohol and drugs. All the variables included in the cluster analysis are binary, so that each of them contributes equally to the formation of clusters. A tree diagram illustrating the hierarchical relationships among variables was generated.
RESULTS
Studied population
Out of the 727 patients who were requested to fill in the questionnaire, 125 declined to participate. Of those who accepted, 593 (82%) responded clearly to the question on smoking status while 9 did not respond to the question on smoking status. Among the 125 patients who refused, 75 were male (22 homosexual and 53 heterosexual). Based on the provider completed survey, there was no difference in epidemiological data, CD4 cell count, plasmatic HIV-RNA value, CDC stage or percentage of HAART-receiving patients and rate of active smokers between the 593 patients who accepted and others (n=134). Among the 134, unemployed individuals were more frequent (22% vs 15%, p=0.0495), as were patients who had acquired HIV through IV drug addiction (23% vs 12%, p<0.0001).
Comparison to the general French HIV-infected population
As compared to the French Hospital Database on HIV, the present study population included older patients (mean age 45.1±9.8 versus 42.1±10.0, p<0.0001) and less frequently, patients who acquired HIV through IV drug addiction (12% vs 15%; p=0.0758). Mean CD4 cell count (479.0±265.7 vs 466.7±274.4, p=0.2656), sex ratio (M/F) (2.3 versus 2.3, p=0.7456) and percentage of patients with CDC stage C HIV disease (25% vs 24%, p=0.3164) were not statistically different between the two populations.
Characteristics of the studied population and tobacco use
Among the 593 patients who participated in the study, 70% (415) were men; patients infected through intravenous drug use accounted for 12% (71/593), 37% (219/593) were men who had sex with men, 25% (148/593) of the patients were at CDC stage C, 80% (474/593) of the patients were receiving antiretrovirals, and 57% (338/593) had undetectable viral load. Thirty-nine percent of patients had at least one cardiovascular risk factor (other than tobacco).
Forty-three (255/593) percent of patients reported that they were active smokers, 17% (100/593) being former smokers and 40% (237/593) never having smoked, 53% (314/593) of patients declared being in a smoking environment. The rate of smoking was highly variable according to sex, age strata, geographic origin and HIV risk factors (Table 1). Mean age of regular tobacco consumption was 18 years old; mean cumulative consumption was 25 ± 4 pack-years. Among smokers, 52% reported having been questioned about tobacco by their practitioner, and only 10% were referred to smoking cessation program.
Table 1.
Rate of active smoking according to demographic data and HIV risk factors in the 593 HIV-infected patients who participated in the study.
| Rate of active smokers (%) | |
|---|---|
| Total population | 43 |
| Sex | |
| Male | 48 |
| Female | 31 |
| Age | |
| 18–24 | 17 |
| 25–34 | 41 |
| 35–44 | 50 |
| 45–54 | 45 |
| 55–64 | 18 |
| 65–75 | 10 |
| Geographic origin | |
| Europe | 52 |
| North Africa | 43 |
| Sub-Saharan Africa | 25 |
| Other | 19 |
| HIV risk factors | |
| Homosexuality | 49 |
| IV drug addiction | 82 |
| Other | 30 |
Figure 1 shows the rate of patients with illicit consumption whether they were active smokers or not. Forty-one percent of patients reported taking tranquilisers, 20% taking antidepressants, 15% having excessive alcohol consumption (CAGE-DETA), 38% having at least one illicit consumption in the previous 6 months, 25% reported having taken cannabis during the previous 6 months.
Among smokers, 38% reported having experienced rejection due to disclosure of HIV status as compared to 20% both in former-smokers and in non-smokers (p<0.0001) (Table 2).
Table 2.
Bivariate and multivariate analyses of factors associated with smoking, former smoking and non-smoking status in the 593 HIV-infected patients who participated in the study.
| Bivariate analysis | Multipartite analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active smokers versus non-smokers |
Former smokers versus non-smokers |
|||||||||
| Variables | Active smokers N=254 | Former smokers N=103 | Non smokers N=236 | p | OR | 95% CI | p | OR | 95% CI | p |
| Age (mean ± SD; years) | 43.1±7.6 | 47.1±10.0 | 46.1±11.3 | 0.0002 | 0.98 | 0.96–1.01 | 0.1124 | 1.02 | 0.99–1.05 | 0.1017 |
| Sex (%male) (female versus male) | 79% | 72% | 60% | <0.0001 | 2.38 | 1.43–4.00 | 0.0008 | 1.56 | 0.89–2.70 | 0.1187 |
| Body Mass Index (kg/m2) mean ± SD | 22.4+53 | 23.3±3.4 | 24.4±3.8 | <0.0001 | 1.08 | 1.14-1.03 | 0.0007 | 1.05 | 0.99–1.11 | 0.0819 |
| Geographic origin | ||||||||||
| Europe | 81% | 72% | 49% | |||||||
| North Africa | 4% | 5% | 3% | |||||||
| Sub-Saharan Africa | 9% | 11% | 25% | <0.0001 | ||||||
| Other | 6% | 12% | 23% | |||||||
| Smoking environment (yes vs no) | 74% | 47% | 33% | <0.0001 | 4.75 | 3.02–7.49 | <0.0001 | 1.69 | 1.01–2.82 | 0.0463 |
| IV drug addiction as HIV risk factor (yes vs no) | 23% | 10% | 1% | <0.0001 | 24.46 | 4.75–126.14 | 0.0001 | 14.60 | 2.61–81.48 | 0.0022 |
| Disclosure of HIV status (yes vs no) | ||||||||||
| Disclosure to family | 77% | 63% | 56% | <0.0001 | 1.81 | 1.16–2.85 | 0.0097 | 1.08 | 0.66–1.77 | 0.7622 |
| Disclosure to friends | 80% | 70% | 55% | <0.0001 | ||||||
| Experience of rejection due to disclosure | 38% | 20% | 20% | <0.0001 | 1.90 | 1.14–3.17 | 0.0134 | 1.08 | 0.59–2.00 | 0.8051 |
| Depression* (0–21) mean ± SD | 5.4±3.6 | 5.0±4.1 | 4.6±3.9 | 0.0921 | ||||||
| Anxiety* (0–21) mean ± SD | 9.2±4.0 | 8.0±4.2 | 7.8±4.4 | 0.0011 | ||||||
| CD4 cell count (/mm3) | ||||||||||
| <200 | 12% | 15% | 9% | |||||||
| 200–500 | 41% | 46% | 53% | 0.0583 | ||||||
| >500 | 47% | 39% | 38% | |||||||
| HIV therapy | ||||||||||
| Perception of antiretroviral toxicity | 74% | 78% | 64% | 0.0287 | ||||||
| Subjective perception of adverse antiretroviral effects** | 95% | 89% | 91% | 0.1254 | ||||||
| Excessive alcohol consumption (yes vs no)*** | 25% | 14% | 6% | <0.0001 | 2.50 | 1.20–5.23 | 0.0145 | 2.03 | 0.86–4.76 | 0.1057 |
| Illicit drug consumption (yes vs no)**** | 39% | 17% | 14% | <0.0001 | 2.43 | 1.41–4.19 | 0.0014 | 1.04 | 0.53–2.05 | 0.8996 |
| Social plans (yes versus no) | ||||||||||
| Plans to change job | 46% | 33% | 44% | 0.1092 | ||||||
| To move house | 68% | 52% | 58% | 0.0113 | ||||||
| To have children | 18% | 17% | 26% | 0.0867 | ||||||
| To practice sports | 68% | 75% | 61% | 0.0415 | 0.90 | 0.57–1.42 | 0.6621 | 1.81 | 1.07–3.08 | 0.0282 |
| To take part in a cultural activity | 68% | 64% | 52% | 0.0021 | ||||||
| To make new friends | 83% | 73% | 69% | 0.0026 | ||||||
| To take better care of oneself | 96% | 99% | 98% | 0.1639 | ||||||
Note:
Hospital Anxiety and Depression (HAD) scale
Experience of at least one of a predefined list of symptoms during the previous 4 weeks
Defined as DETA-CAGE above 2
At least one illicit substance consumptium (excepte cannabis) during the previous 6 months.
OR: Odds ratio; CI 95% confidence interval
Factors associated with active smoking and with former smoking status
Cluster analysis identified 5 groups of illicit consumptions: Tobacco, cannabis, poppers, alcohol and others (including ectasy, cocain, heroin, rohypnol, amphetamines, LSD, morphin or substitute drugs). Cannabis was the group most closely related to tobacco consumption (Figure 1). As cannabis consumption was co-linear with tobacco consumption, we did not include this variable in the multivariate analysis. Characteristics (other than cannabis consumption) associated with active smoking in bivariate analysis are shown in Table 2. Compared to non-smokers, multivariate analysis revealed active smoking to be associated with male sex, living with smoking environment, lower body mass index, excessive alcohol consumption, illicit consumption, disclosure of HIV status to family, reported experience of disclosure rejection, and IV drug addiction as a HIV risk factor. (Table 2).
Factors associated with former smoking status as compared to non-smoking status were a smoking environment, IV drug addiction as a HIV risk factor and plans to practice a sport.
Factors associated with tobacco dependence
High and intermediate tobacco dependence assessed through the Fagerström scale was noted in 56% of patients. Table 3 shows variables associated with high and intermediate tobacco dependence in bivariate analyses. In multivariate analysis, disclosure of HIV status to family, and having taken substitution drugs or morphine during the 6 previous months were associated with intermediate and high tobacco dependence, whereas practicing sports was associated with lower dependence.
Table 3.
Bivariate and multivariate analyses of factors associated with dependence on tobacco in the 210 out of the 254 HIV-infected active smokers for whom the Fagerstrom test was assessable.
| Variables | Bivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Tobacco Dependency |
p | OR |
95% CI |
p |
||
| Strong or moderate (Fagerström test ≥5) |
Mild or none (Fagerström test <5) |
|||||
| N=119(57%) | N=91 (43%) | |||||
| Body surface area (m2) mean ± SD | ||||||
| HIV risk factor | 1.78±0.18 | 1.82±0.19 | 0.1190 | |||
| IV drug addiction | 31% | 18% | 0.0376 | |||
| Homosexuality | 34% | 51% | ||||
| Other | 35% | 31% | ||||
| Disclosure of HIV status | ||||||
| Disclosure to family (yes vs no) | 86% | 67% | 0.0022 | 2.04 | 1.04–3.77 | 0.0308 |
| Number of individuals aware of HIV status > 5 | 74% | 62% | 0.1629 | |||
| Depression* (0–21) mean + SD | 5.9+3.8 | 4.9+4.4 | 0.0671 | |||
| HIV therapy toxicity | ||||||
| Perception of antiretroviral toxicity (yes vs no) | 59% | 93% | 0.1199 | |||
| Negative side effects** | 98% | 92% | 0.0802 | |||
| Social activities or plans | ||||||
| To practice sport | 43% | 63% | 0.0069 | 0.45 | 0.25–0.79 | 0.0062 |
| To have children | 17% | 10% | 0.1870 | |||
| To take part in a cultural activity | 65% | 77% | 0.0778 | |||
| To travel | 82% | 89% | 0.1484 | |||
| To take better care of oneself | 95% | 99% | 0.1139 | |||
| Other addictions | ||||||
| Anti-anxiety, sleeping pills | 39% | 18% | 0.0012 | |||
| Morning alcohol consumption to feel good | 8% | 0% | 0.0189 | |||
| Heroin consumption during one’s life | 42% | 28% | 0.0514 | |||
| Substitute drugs, morphine consumption during one’s life | 31% | 17% | 0.0473 | |||
| Substitute drugs, morphine consumption during the previous 6 months | 23% | 7% | 0.0056 | 3.33 | 1.22–9.60 | 0.0227 |
Note:
Dependence assessed through Fagerström test
Hospital Anxiety and Depression (HAD) scale
Experience of at least one of a predefined list of symptoms during the previous 4 weeks
OR: Odds ratio; CI 95% confidence interval
Factors associated with high smoking cessation motivation
Intermediate to very strong motivation to quit smoking was reported in 37% of patients. Motivation to quit smoking was independently associated with higher body surface area (OR=13.36, 95% CI =2.72–65.22), and with patients’ perception of the risk of developing tobacco related health problems (OR=1.24, 95% CI =1.09–1.41). Among smokers, the rate of patients who were highly motivated, had no other illicit drug use, and no depressive symptoms (total score on HAD depression subscale between 0 and 7) was 14%.
Comparison to the French general population
The active smoking rate was significantly lower in the general French population than in the studied HIV-infected population (31% vs 43%)(p<0.0001). Intermediate and high tobacco dependence was 25% less frequent in the general French population as compared to the studied HIV-infected population (p<0.0001). Concerning other substance abuse, excessive alcohol consumption was reported by 14% of the smokers in the general French population as compared to 24% of the HIV-infected smokers (p<0.0001). Furthermore, 11% of the smokers in the general population declared cannabis consumption in the 6 previous months as compared to 50% of the HIV-infected smokers (p<0.0001).
DISCUSSION
Active smoking appears frequent in the HIV-infected population; however it is highly variable according to sex, HIV risk factors, and geographic origin. Smoking rate and characteristics are both associated with factors commonly found in the general population such as male sex or smoking environment, but also with factors specific to the HIV population such as disclosure of HIV status to family and reported experience of disclosure rejection. High rates of illicit substance use and tobacco dependence appear specific to this population.
This study confirms the significantly higher prevalence of active tobacco smoking (43 %) as compared to the French general population (31%). This rate is difficult to compare with the results reported in other studies of HIV-infected patients (50 to >70%), as the rate of active smokers among HIV-infected individuals appears to be highly variable according to sex, HIV-risk factors or geographic origin [4–6, 10, 18] and is thus dependent on the repartition of these characteristics in the studied population. For example, it ranges in our study from 9% in women originating from sub-Saharan Africa to 81% in patients who acquired HIV through IV drug addiction.
This high prevalence of tobacco consumption may be due to difficulties linked with an increased need for tobacco consumption or reduced motivation to stop tobacco consumption encountered by HIV-infected smokers. Several factors reported in our study could explain this situation. The HIV disease itself or its treatment, affecting the quality of life, may act as a predisposing factor. Of note is that the perception of antiretrovirals’ adverse effects was more frequently reported by active smokers as compared to non-smokers in bivariate analysis. Family and friends’ perception of HIV status may also have an impact. In fact, we found that disclosure of HIV status to family was associated with active smoking. This association is difficult to interpret; on the one hand, a behavioral factor may explain both the propensity to smoke and also to talk about HIV disease without any direct relationship between these behaviors; on the other hand, we can hypothesize that the stress induced by HIV status disclosure may exacerbate the tendency to smoke and/or to continue smoking. The identification of the perceived experience of rejection due to disclosure as independently associated with smoking status supports the hypothesis that the difficulties related to HIV status disclosure constitute a risk factor for tobacco consumption in this patient population. Of note is that HIV status disclosure was also independently associated with high dependence on tobacco.
On the contrary, we did not find any differences in social status between smokers and non-smokers. In fact, precarious social conditions have been found to be associated with smoking status in HIV-infected patients [4]. This shows that smoking concerns the whole socio-economic spectrum. At the same time, the high rate of refusal to participate in this study among unemployed individuals may have had an impact on the results.
In addition, the identification of smoking environment and IV drug addiction as HIV risk factors as being associated with both active smoker status and former smoker status suggests that these characteristics, while more frequent in active smokers, are not incompatible with quitting smoking.
The rates of illicit consumptions (alcohol, cannabis…etc.) and also of excessive alcohol consumption were considerably higher in the HIV-infected smokers (whether or not patients acquired HIV through IV drug addiction) as compared to the general population. Moreover, these two characteristics were independently associated with active smoking status whereas they were not with former smoking status. This argues for a global approach to substance abuse treatment in the HIV-infected smoking population.
The rate of smokers moderately to highly dependent on tobacco according to the Fagerström scale was significantly higher in the HIV-infected population as compared to the general population. This level of dependency usually necessitates medical intervention to help quit smoking, which in the case of prescribing medication could interfere with antiretroviral treatments. This medical intervention is all the more essential in patients receiving substitutes for IV drugs, a characteristic associated with nicotine dependency in the smokers of this study.
The rate of 37% of patients motivated to quit smoking seems comparable to those reported by others (20–40%) using other means to estimate motivation [6, 18, 19]. This motivation in the current study population appears to be related to the patients’ perception of the risk of developing tobacco-related health problems. Given the perception that death from AIDS is the inevitable outcome of a HIV infection diagnosis, many patients may have believed that quitting smoking was not going to improve their health. The health risks of smoking should be clearly communicated by the practitioner in charge of each patient. However, the rate of active smokers whose practitioner evoked tobacco consumption or who referred them to a tobacco specialist appears very low. The well-known “brief counseling method to quit smoking which consists of the 5 A’s”: Ask (about smoking), Advise (the patient to quit smoking), Assess (determine willingness to make a quit attempt), Assist (the patient to quit), Arrange (follow-up…) seems rarely used by physicians within this study [20].. Physicians in charge of HIV-infected patients should encourage smokers to consult practitioners specialized in smoking cessation. Less than 15% of HIV-infected smokers in the present study had profiles suggesting a capacity to easily stop smoking: high motivation, no other illicit drug use, and no depressive symptoms, and to whom a standard tobacco cessation program could be proposed. In the large majority of cases, multidisciplinary intervention is needed using behavioral support, together with medication and the need to take into account depressive symptoms and anxiety in some.
We acknowledge potential limitations to our study. First, the comparison of the characteristics of this study population with those of the population who refused to fill out the questionnaire shows that they were not comparable for all characteristics such as the proportions of IV drug addiction and of unemployed individuals, which were more frequent in the population of patients who refused to fill out the questionnaire. As the selected sample was randomly chosen among French hospitals, we assume that the studied population was as close to representative as possible of the whole population of HIV-infected outpatients monitored in France. We cannot exclude the possibility that the decision by certain French hospitals and certain HIV-infected patients to not participate in the study may have introduced a bias in the results. In fact, the characteristics of the HIV-infected patients of the present study (a younger population and fewer drug addicts) are not completely comparable to those of HIV patients monitored in French hospitals.
Second, we only included outpatients and the studied population could thus be considered as not totally representative of HIV-infected individuals. However, our survey was conducted to evaluate the possibility of implementing a cessation program to specifically target outpatients. Finally, as we performed a cross-sectional study, it is neither possible to study the dynamic process of tobacco consumption nor to establish a causal relationship between tobacco consumption and the identified associated factors.
In light of these results, it stands to reason that tobacco consumption has specific characteristics in the HIV-population and is closely related to life with HIV, and how the patients’ circle of family and friends copes with his or her HIV status. Patients must be informed about the risk of continuing to smoke. Physicians must be aware of their central role in motivating this population of smokers to consider quitting. Efficacy of strategies combining cessation medication and a global behavioral approach should be assessed in this population using clinical trials.
Acknowledgments
We are grateful to Pr Joel Menard for his support in conducting this study, Mr JC. Désenclos and Ms C. Larsen from the “Institut de Veille Sanitaire” who helped us determine the representative sample of French units, Mrs Ph. Guilbert and A. Gautier A. who gave us the opportunity to compare our results with the “Baromètre santé 2005” INPES, Ms D. Costagliola for allowing us to compare the characteristics of our patient population to those of patients included in the “French Database on HIV”. Finally, to S. Irali and G. Tsimba for organizing the survey.
Appendix
EVIT Study group
Steering committee: principal investigator: X. Duval; other members: G. Baron, PM. Carrieri, D. Garelik, F. Lert, P. Perreti-Watel. P. Ravaud, B. Spire, G. Tsimba, V. Villes.
Investigators
R. Vromet, P. Granet Brunello (Digne les Bains), J.M. Bressieux, L. Rezzouk (Troyes), T. Allègre (Aix en Provence), R. Verdon, Ph. Féret (Caen), M. Bonnefoy (Angoulême), J.F. Abino (Ajaccio), E. Duhamel, C. Daniel (Saint Brieuc), Ph. Lataste (Périgueux), C. Drobacheff-Thiebaut, D. Devred, A. Foltzer (Besançon) P. Perfezou (Quimper), J. Jourdan, I. Rovanet, A. Anaud, C. Barbuat, F. Del Bucchia (Nîmes), P. Massip, B. Marchou, L. Cuzin (Toulouse), B. Kitschke (Sète), J.M. Plassart (Grenoble), F. Lucht, V. Ronat (Saint-Etienne), S. Sire (Cahors), Y. Poinsignon (Vannes), F. Truchetet, Ph. Muller (Thionville), Y. Mouton, S. Pavel, Y. Yasdanpanah, S. Vandamme (Tourcoing), A. Vermersch, C. Fontier (Valenciennes), F. Cordier (Creil), B. Schubert, Dr Michel, Beck Halna (Mulhouse), P. Yéni, G. Fraqueiro, (Paris Bichat), D. Salmon, T. Tahi (Paris Cochin) G. Huchon, A. Compagnucci (Paris Hôtel Dieu), J.F. Bergman, P. Sellier (Paris Lariboisière), B. Dupont, O. Lortholary (Paris Necker), S. Herson, A. Simon, M. Iguertsira (Paris Pitié Salpétrière), P.M. Girard, J.L. Lagneau (Paris Saint Antoine), D. Séréni, C. Lascoux-Combe (Paris Saint Louis), J.M. Décamps, M. Lapine (Niort), Ph. Perre (La Roche sur Yon), R. Bakir (Auxerre), J.P. Faller (Belfort), L. Raffenne (Paris Institut Montsouris) P. Gilquin (Paris Fondation Saint Joseph), E. Rouveix, C. Dupont (Boulogne Billancourt Ambroise Paré), P. Galanaud, Dr Chassaing, A.M. Delavallée (Clamart Antoine Béclère), Ph. Vinceneux, A.M. Simonpoli (Colombes Louis Mourier), S. Végni (Nanterre Max Fourestier), C. Perronne, P. De Truchis, H. Berthe (Garches Raymond Poincaré), O. Bouchaud, R. Guerrero (Bobigny Avicenne), C. Goujard, M. Mole (Kremlin Bicêtre), C. Jung, A. Sobel (Créteil Henri Mondor), Ph. Genet (Argenteuil), M.T. Goerger-Sow, I. Lamaury (Pointe à Pitre), A. Cabie (Fort de France) P. Poubeau (Saint Pierre de la Réunion), O. Danne, L. Blum (Pontoise) P. Chavanet, C. Braconnier (Dijon), J.A. Gastaud, I. Poizot-Martin, E. Peyronnet (Marseille Sainte Marguerite), J.G. Fuzibet, E. Rosenthal (Nice), F. Raffi, B. Bonnet (Nantes), T. May, L. Boyer (Vandoeuvre), C. Michelet, M.C. Delmont (Rennes), G. Le Moal (Poitiers), T. Debord, P. Imbert (Saint Mandé Bégin), J.M. Lang (Strasbourg), C. Gaud (Saint Denis de La Réunion), S. Paucod, F. Bissuel (Saint Martin), C. Leport, J.L. Ecobichon (Paris Bichat), P. Choutet, P. Nau (Tours), D. Vittecoq, O. Derraji (Villejuif), J. Moreau (Marseille Hop. Nord), F. Bricaire, C. Duvivier, H. Ait Mohand, N. Bentaleb (Paris Pitié Salpétrière), C. Jacomet, L. Cormerais (Clermont Ferrand).
Financial support: Grant SIDACTION, ensemble contre le SIDA.
Other supports: Association pédagogique nationale pour l’enseignement de la thérapeutique (APNET), Collège des universitaires de maladies infectieuses et tropicales (CMIT), Société nationale française de médecine interne (SNFMI), Société de pathologies infectieuses de langue française (SPILF).
Footnotes
The authors have no commercial or other associations that might pose a conflict of interest
References
- 1.Lewden C, Raffi F, Chene G, Sobel A, Leport C. Mortality in a cohort of HIV-infected adults started on a protease inhibitor-containing therapy: standardization to the general population. J Acquir Immune Defic Syndr. 2001;26:480–482. doi: 10.1097/00126334-200104150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 3.Collins RL, Kanouse DE, Gifford AL, et al. Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol. 2001;20:351–360. [PubMed] [Google Scholar]
- 4.Gritz ER, Vidrine DJ, Lazev AB, Amick BC, 3rd, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res. 2004;6:71–77. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- 5.Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS. 2002;16:39–42. doi: 10.1089/108729102753429389. [DOI] [PubMed] [Google Scholar]
- 6.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 7.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 8.Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–299. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 9.Kirk GD, Merlo C, P OD, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. Aids. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 11.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 12.Duran S, Spire B, Raffi F, et al. Self-reported symptoms after initiation of a protease inhibitor in HIV-infected patients and their impact on adherence to HAART. HIV Clin Trials. 2001;2:38–45. doi: 10.1310/R8M7-EQ0M-CNPW-39FC. [DOI] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Aubin H, Lagrue G, Legeron P, et al. Questionnaire de motivation à l’arret du tabac, Q-mat; construction et validation. Alccologie addictologie. 2004;26:311–316. [Google Scholar]
- 15.Beresford TP, Blow FC, Hill E, Singer K, Lucey MR. Comparison of CAGE questionnaire and computer-assisted laboratory profiles in screening for covert alcoholism. Lancet. 1990;336:482–485. doi: 10.1016/0140-6736(90)92022-a. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for controlling and monitoring the tobacco epidemic. Geneva: WHO; 1998: [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Benard A, Bonnet F, Tessier JF, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS. 2007;21:458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- 19.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7:511–522. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- 20.A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. Jama. 2000;283:3244–3254. [PubMed] [Google Scholar]