Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by aberrant immune responses against intracellularly derived self antigens. Treatment for SLE relies on the use of aggressive immunosuppressants and steroids that are nonspecific and can cause serious adverse effects. The observation that a systemic immune tolerance to self antigens or generation of regulatory T cells may follow mucosal (nasal or oral) exposure to self proteins or monoclonal antibody against CD3 respectively suggests that induction of mucosal tolerance offers the basis of a side effect free therapy that could re-establish the ability to distinguish self from non-self and restore peripheral tolerance in individuals susceptible to developing autoimmune diseases. Here I review studies on mucosal tolerance in autoimmune diseases and discuss the therapeutic potential of inducing tolerance for the treatment of SLE.
Introduction
The primary and most worrying problem with all existing treatments for autoimmune diseases is specificity. The nonspecific nature of the treatments compromises normal immune surveillance and hampers protective immunity. As a result patients are often vulnerable to opportunistic infectious agents that can cause serious complications. This problem associated with treatment is most challenging in systemic lupus erythematosus (SLE). Lupus, as it is sometimes referred to, is a chronic autoimmune syndrome that is characterized by a destruction of tissues and organs such as joints, kidneys, heart, lungs, brain, skin, and blood vessels by the very immune system that is designed to protect them. Disease pathogenesis is a result of a cognate interaction between T and B cells that recognize intracellularly derived self antigens 1. Autoreactive T and B cells mediate inflammation and/or direct tissue damage by secreting inflammatory cytokines and anti-nuclear autoantibodies respectively 2. The fact that many vital organs may be targeted in lupus has led to the use of powerful immune suppressive or modulating drugs in disease treatment. Thus there is a real sense of urgency for development of new therapies that can be given over long periods without causing global immune malfunction to treat lupus.
The observation that a systemic immune hyporesponsiveness or tolerance to a protein may follow mucosal (nasal or oral) exposure to the protein has led to a surge of excitement in the immunology community devoted to finding an effective treatment for autoimmune diseases. As induction of mucosal tolerance to self-antigens associated with autoimmune diseases could re-establish the ability to distinguish self from non-self in individuals susceptible to developing autoimmune diseases, it offers the basis of a side effect free therapy that could potentially replace current nonspecific immunosuppressive drugs 3. Virtually all manifestations of specific immune responsiveness tested can be suppressed by different regimens of mucosal antigen administration. This includes in vivo responses such as formation of Ig of different isotypes, 4,5 delayed hypersensitivity reactions 6,7, and changes in the rate of antigen clearance from the circulation 8, as well as in vitro assays such as specific plaque forming cells 9,10, lymphocyte proliferation 10–13, and cytokine production except for IL-10 and TGF-β 14–21.
The immunological mechanisms of mucosal tolerance
Three independent mechanisms behind mucosal tolerance have been put forward: firstly, ignorance of the antigen by the immune system (anergy); secondly, deletion of T cells that respond to the inhaled or ingested antigen; thirdly, generation of regulatory T cells that control and/or down modulate the inflammatory response against the antigen. Since identification of these mechanisms, evidence has been accumulating that suggest the three forms of tolerance are not mutually exclusive and there are considerable overlaps. One finding among others that could link these apparently distinct mechanisms is the secretion of the regulatory cytokine TGF-β that can be induced by treating T cells with anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibody, despite the fact that CTLA-4 was first described as being involved in the induction of ‘anergy’ in vivo 22,23. Other studies also describe them as anergic 24–26. The primary factor that determines which form of tolerance develops following mucosal administration of antigen is the dose of antigen given. Low doses of antigen favors the generation of regulatory T cell-driven tolerance whereas high doses of antigen favor deletion or anergy-driven tolerance 7,27.
Regulatory T cells and mucosal tolerance-associated cytokine network
Regulatory T cells were first described in the 1970s when they were considered to be mainly CD8+ and were referred to as suppressor T cells. More recently there has been a surge of research activity aimed at elucidating the phenotype(s) and function(s) of regulatory T cells in various areas of immunology. The naturally occurring, thymus derived regulatory T cells that are positive for CD4 and CD25 surface expression have generated the highest level of interest amongst immunologists. CD4+CD25+ regulatory T cells play a major role in the maintenance of self-tolerance and the control of various autoimmune diseases 28,29. They are also involved in the regulation of T cell homeostasis 30,31 and in the modulation of immune responses to allergens 32, cancer cells 33,34, and pathogens 35,36. Initially reported by Nishizuka 37,38 and further explored by Sakaguchi 39–41 in studies on animals thymectomized as neonates showed that autoimmune pathology, characterized by gastritis, oophoritis and orquitis arise as a result of the ablation of a subpopulation of thymic T cells. These cells are able to restore immunoregulatory function in disease mice upon adoptive transfer. The expression of a high affinity receptor for IL-2 on the cell surface (CD25) is required for the function of these regulatory T cells, although it is not clear whether IL-2 acts as a peripheral differentiation factor or an expansion factor 42,43 or it is directly involved in their function 44,45. Recently other markers such as glucocorticoid-induced tumor necrosis factor (TNF) receptor 46,47 and the nuclear transcription factor foxhead/winged helix 3 (foxp3) have been found in CD4+CD25+ regulatory T cells 48–50. Conversion of naïve T cells to CD4+CD25+ regulatory T cells can be achieved by stimulation via the T cell receptor and ligand-activated transcription factor aryl hydrocarbon receptor (AHR) which directly interacts with target sequences on the foxp3 gene and upregulates its expression 51. CD4+CD25+ regulatory T cells are anergic, have suppressive properties that work in a cell contact dependent fashion. Further studies have demonstrated that foxp3 physically interacts with AML1 (acute myeloid leukaemia 1)/Runx1 (Runt-related transcription factor 1), a transcription factor crucially required for normal haematopoiesis including thymic T-cell development, activates IL-2 and IFN-γ gene expression in conventional CD4+ T cells through binding to their respective promoters, and this interaction suppresses IL-2 and IFN-γ production, upregulates regulatory T cell-associated molecules and results in suppressive activity 52. A number of studies in lupus prone animals 53,54 and SLE patients 55–58 have demonstrated a significant reduction in number and function of these naturally occurring foxp3+CD4+CD25+ regulatory T cells and adoptive transfer of ex vivo expanded regulatory T cells reduced the incidence of glomerulonephritis and prolonged survival in mice with established lupus 59. Induction of nasal tolerance using a histone peptide expressing a dominant T cell epitope in histone H4 protein of the nucleosome 60 was shown to have a positive effect on the number of the CD4+CD25+ regulatory T cells 53. Furthermore, suppression of disease was demonstrated following injection of the peptide in mice and disease protection was associated with an upregulation of CD4+CD25+ regulatory T cells that produced high levels of TGF-β and exerted suppression in a partially cell contact dependent fashion 61,62. Upregulation of regulatory T cell activity and TGF-β production leading to suppression of autoantibody production and disease development in lupus prone mice can also be achieved by injection of consensus peptides based on V(H) regions 63,64 or CDR1 regions of anti-dsDNA autoantibody 65,66.
As an important part of maintenance of peripheral tolerance other types of regulatory T cells (Th3, Tr1 and CD4+CD25−LAP+ cells) can be triggered by nasal or oral administration of antigen. These cells mediate their suppressive function by secreting anti-inflammatory cytokines IL-10 and/or TGF-β. They are referred to as ‘acquired’ regulatory T cells as opposed to the naturally occurring or ‘innate’ thymus derived CD4+CD25+ regulatory T cells 67. The relationship between the two classes of regulatory T cells is largely unknown however, in studies of colitis induced in mice by adoptive transfer of CD45RBhigh T cells it was shown that disease could be suppressed by CD4+CD25+CD45RBlow T cells with regulatory properties that resembled both the adoptive and innate population in that suppression was mediated via secretion of IL-10 and TGF-β 68,69. Moreover, TGF-β has been shown to contribute to the development and expansion of CD4+CD25+ regulatory T cells 70–72.
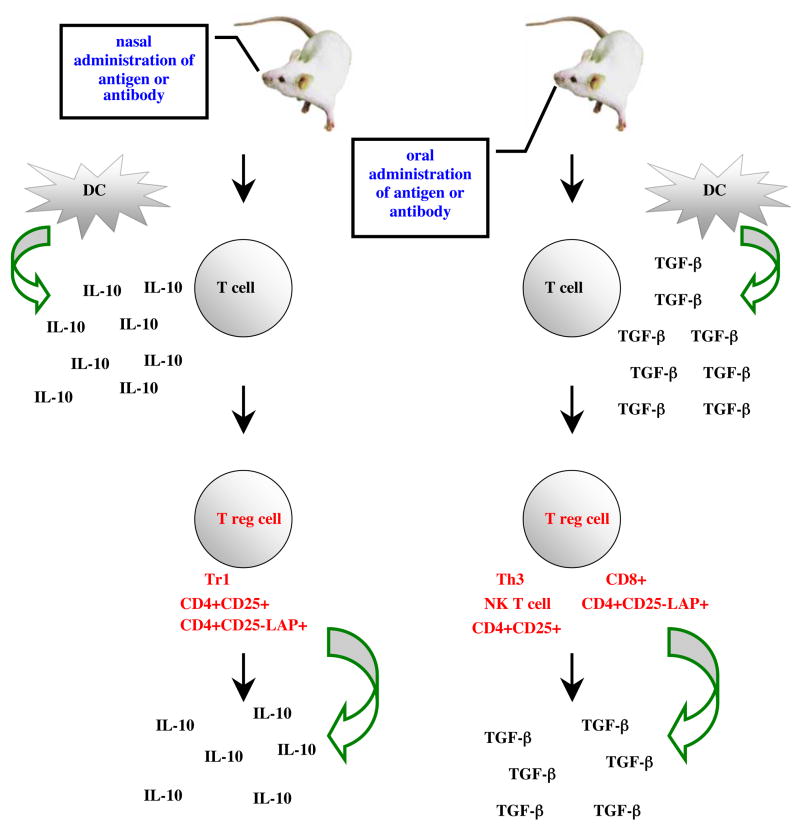
Initially the regulatory activity of CD4+ T cells was associated with an upregulation of Th2 type cytokines IL-10 and IL-4 and a suppression of Th1 type cytokines IL-2 and IFN-γ 14,73–78. Suppression of Th1 type immune responses is still a widely used indication of induction of mucosal tolerance 79–81 and Th2 regulatory T cells generated by mucosal (nasal or oral) administration of antigen have been shown to suppress experimental allergic encephalomyelitis, EAE 82 and diabetes 83 in mice. IL-10 is a Th2 cytokine with potent anti-inflammatory properties. A variety of cell types produce IL-10 but it is produced at exceptionally high levels by CD4+ regulatory T cells (Tr1 cells) 69. Production of high levels of IL-10 by regulatory T cells is more often seen with nasal tolerance rather then oral tolerance induction. In fact neutralization of IL-10 does not abrogate oral tolerance induction nor block established tolerance in vivo 84. Our recent studies on mucosal administration of anti-CD3 monoclonal antibody in mice with EAE and mice that develop lupus spontaneously demonstrated that delivery of the antibody orally induced CD4+CD25−LAP+ regulatory T cells that suppressed EAE and lupus (Wu et al unpublished) in a TGF-β dependent fashion without the need for IL-10 21. However, nasal administration of anti-CD3 suppressed lupus before and after disease onset by inducing CD4+CD25−LAP+ regulatory T cells that suppressed the function of CD4+ICOS+CXCR5+ follicular helper T cells thereby inhibiting helper T and B cell interaction leading to downregulation of plasma cell formation and autoantibody production. In vitro suppression was IL-10 dependent and both IL-10 and TGF-β are required for in vivo suppression by the LAP+ regulatory T cells (Wu et al in press). In an elegant study by Akbari et al it was shown that dendritic cells (DCs) are at the heart of immunological tolerance and pulmonary DCs isolated after nasal administration of allergen produced large quantities of IL-10 which was required for nasal tolerance induction 85. On the contrary, DCs isolated from mesenteric lymph nodes of the gut following antigen feeding express increasing amounts of TGF-β and enhanced production of TGF-β by CD4+ cells, a phenotype consistent with Th3 regulatory T cells 86. Thus it appears that the mucosal immune system has a unique immunological milieu that is based on two tolerance inducing cytokines, IL-10 and TGF-β and the milieu acts, in part, via the DCs to induce different phenotypes of regulatory T cells (Diagram 1).
Diagram 1.
Regulatory T cells and cytokines associated with mucosal tolerance induction.
As mucosal tolerance has been usually defined in terms of Th1 responses, anything that suppress Th1 and/or enhance regulatory T cell induction and Th2 responses would enhance mucosal tolerance. Th3 cells appear to use IL-4 and TGF-β for growth and differentiation. It has been shown that oral administration of IL-4 and antigen enhanced oral tolerance induction to the antigen 87. In the arthritis model, administration of TGF-β intraperitoneally enhanced the induction of oral tolerance to collagen II even after the onset of disease 88. Large doses of IFN-γ given intraperitoneally abrogate oral tolerance induction 89 and anti-IL-12 enhanced oral tolerance and upregulated TGF-β production 90. In addition, subcutaneous administration of IL-12 prevents the induction of oral tolerance 91. Oral IFN-γ and IFN-t synergize with the induction of oral tolerance in mice fed low doses of myelin basic protein (MBP) 92–94. Nasal administration of cytokines also enhances tolerance induction and regulatory T cell differentiation. Nasal but not subcutaneous administration of IL-10 suppressed clinical signs of EAE in Lewis rats and prevented the development and relapse of protracted-relapsing EAE in rats 95. Co-administration of IL-10 and antigen reduced proliferative responses and IFN-γ production, increased IL-10 production by T cells and enhanced protection from EAE compared to antigen alone 96. Nasal administration of minute amounts of IFN-γ and acetylcholine receptor (AChR) reversed tolerance to AchR and inhibited protection from autoimmune myasthenia gravis (EAMG) by AchR administration alone in rats 97. Suppression of IFN-γ production in response to systemic challenge is almost a universal finding in mucosally induced tolerance. However, some reports have shown that induction of tolerance is preceded by priming of antigen-specific IFN-γ producing cells 74,90,98–101. Furthermore, studies that support a regulatory role of T cells bearing γδ TCRs in mucosal tolerance 102–104 have shown that suppression of IgE antibody production following nasal administration of proteins appears to be mediated by IFN-γ 105. In addition, there are reports that oral tolerance cannot be induced in IFN-γ-deficient mice 106 or in adoptively transferred TCR transgenic T cells on the IFN-γ-deficient background 107. Studies in children with peanut allergies showed that tolerance to peanuts was associated with a Th1-skewed response to peanuts and Th2 responses were only observed in allergic children. Peanut reactive T cell clones from orally tolerized children produce high levels of IFN-γ and TNF-α, suggesting that oral tolerance to Th2 type of inflammatory responses, such as in allergic reactions, may be accomplished by immune deviation toward Th1 responses 108. Thus the role of Th1 cytokines, particularly IFN-γ, in the induction and mechanisms of mucosal tolerance needs to be further investigated.
Several factors were determinant in the growing interest on the role of CD4+ T cells in mucosal tolerance. Namely, removal of CD4+ T cells at the time of oral administration of Ovalbumin (OVA), prevented the induction of tolerance to a subsequent challenge with OVA 109,110 and CD4 T cell deficient animals failed to become tolerized to contact sensitizing agents 111. Apart from this growing interest in CD4+ T cells however, the original reports on oral tolerance have suggested that CD8+ T cells might be the suppressor cells involved in its induction 6,9,76,112,113. Later studies showed that feeding of Lewis rats with MBP induced TGF-β producing CD8+ regulatory T cells that suppressed EAE upon adoptive transfer 114. Further evidence that CD8+ T cells may participate in oral tolerance comes from studies showing that a population of CD8+ regulatory T cells that produce IL-4 or IL-10 can be primed by antigen feeding, even when CD8+ CTLs are tolerized 103,115. One of the questions regarding the relationship between CD8+ regulatory T cells and mucosal tolerance is how such cells could recognize mucosally administered exogenous antigen. A conceivable mechanism is ‘cross-presentation’. Several reports have demonstrated that soluble molecules presented by APCs, specifically DCs, can leak into the major histocompatibility complex (MHC) class I pathway and be presented to CD8+ T cells 116,117. Alternatively, priming of CD8+ T cells with regulatory properties in the gut can occur via presentation of fed antigens by mucosal DCs in the context of a MHC Ib molecule Qa-1 that is able to recognize self antigens and bacterial heat shock proteins 118,119. The role of CD8+ T cells has also been examined using genetically engineered CD8 deficient mice 120–122 and in mice treated with anti-CD8 antibodies 109,110,123. In all the studies, oral tolerance was induced normally suggesting that there is no absolute requirement for CD8+ T cell in the induction or maintenance of systemic tolerance. Nevertheless, whether these cells contribute to individual aspects of the tolerant state or play discrete roles in different tissues, such as the mucosa itself, remains to be elucidated. In addition to CD8+ regulatory T cells, there have been three reports that suggest NK T cells can transfer tolerance following feeding of haptenized colonic proteins 124,125 or allo-antigens 126. However, other workers have shown normal oral tolerance in mice lacking NK T cells due to a genetic deficiency in Jα 281 component of the invariant TCR found on most of these cells 127. Taken together, these results indicate that as well as CD4+ T cells, other T cells may have a role in the regulatory events triggered by oral tolerance, but they do not seem to be essential for them. Thus induction of mucosal tolerance can trigger different types of regulatory T cells (Diagram 1), suggesting that the mucosal route is a robust way to induce or restore peripheral tolerance and may benefit individuals with autoimmune diseases.
Therapeutic applications of mucosal tolerance in autoimmune diseases and its potential in the treatment of SLE
Several human trails of oral tolerization have been carried out in patients with multiple sclerosis (MS), rheumatoid arthritis (RA), diabetes and uveitis. In all studies no systemic toxicity or exacerbation of disease was observed, although clinical efficacy resulting in an approved drug has yet to be achieved. A mucosal tolerance approach to treating patients with SLE has yet to be carried out. However, promising results have been demonstrated in lupus prone animals.
MS
Feeding of MS patients with bovine myelin demonstrated that MBP- and proteolipid protein (PLP)-specific proliferative responses were affected and TGF-β secreting Th3-type cells were present in peripheral blood of treated patients but not in untreated patients 128,129. However, a 515 patient, placebo-controlled, double-blind phase III trail of single-dose bovine myelin in relapsing-remitting MS did not show differences between placebo and treated groups in the number of relapses with a large placebo effect 129. Trials in MS with the MBP analog glatiramer acetate (GA), which is currently given by subcutaneous injection to MS patients, have shown promising results and induced regulatory T cells that mediate bystander suppression 130,131. However, a phase III trial of oral GA given daily at 5 and 50 mg verses placebo found no clinical or immunologic effects and magnetic resonance imaging did not show improvement of affected areas in the brain. Phase II trial with 300 and 600 mg oral GA are currently in progress.
RA
A double-blind, placebo controlled phase II trial was carried out in 280 RA patients. Oral doses of liquid bovine type II collagen (CII) ranging from 0.025 to 10 mg demonstrated statistically significant positive effects in groups treated with the lowest dose 132. While oral CII at higher doses did not lead to significant clinical improvement although there was a higher prevalence of responders following oral CII compared to placebo. In another placebo-controlled trial of bovine collagen significant effects were seen in those receiving 0.5 mg but not in groups receiving 0.05 or 5 mg 133. These findings were consistent with findings in animal studies 89,134. Five double-blind phase II randomized studies of oral CII (Colloral) have been carried out. A total of 805 patients were treated with Colloral and 296 treated with placebo. A dose refinement study tested Colloral at 5, 20 and 60 μg. Weighted averages for the Paulus 20 and Paulus 50 responses were calculated for the 60 μg dose and placebo. A significant effect favoring 60 μg was observed for both the Paulus 20 and the Paulus 50 responses. Safety analysis demonstrated that Colloral was remarkably safe with no side effects. The magnitude of the clinical responses to Colloral appears to be on the same level as non-steroidal anti-inflammatory drugs for the majority of patients. However, there was a subgroup of patients who appeared to have a more significant response to the medication 132,135. On the basis of these data, a 760-patient phase III trial was performed comparing 60 μg of Colloral to placebo. However, no differences were observed. There was a large placebo effect in the control group. Clinical trials may be required to determine whether withholding no-steroidal anti-inflammatory drugs and prednisone will enhance the induction of oral tolerance in RA patients 136.
Oral CII was also tested in an open-label pilot study in juvenile RA with significant positive results and no toxicity observed 137. The absence of toxicity is an important feature for the clinical use of oral tolerization, especially in children for whom the long-term effects of immunosuppressive drugs is unknown. Oral CII in juvenile RA was associated with clinical improvement and decreased CII-specific IFN-γ and increased TGF-β 138.
Diabetes
Several trials of mucosal administration of recombinant human insulin as a therapy for type I diabetes are underway or already completed in Europe and the US. In France a double blind study is comparing oral insulin therapy and parenteral insulin therapy versus placebo in patients during the remission phase. 131 autoantibody positive diabetic patients aged 7–40 years were given 2.5 or 7.5 mg oral insulin daily or placebo for 1 year, in addition to subcutaneous insulin therapy. Findings in follow up showed that oral administration of insulin at these doses did not prevent the deterioration of beta-cell function or diminished titers of antibodies to insulin, glutamic acid decarboxylase or islet antigen 2 139. In Italy a multi-center double blind study is evaluating the effect of oral insulin verses placebo in diabetic patients who are treated with intensive insulin therapy by measuring cytokine and autoantibody responses to insulin. After 12 months of treatment there was a significantly higher level of TGF-β with reduced IFN-γ production in patients who received oral insulin compared to those who received placebo. Serum levels of IgG1 and IgG3 anti-insulin antibodies were also significantly lower in the patients treated with oral insulin. However, no clinical effect was observed and it was concluded that poor timing of initiation of treatment was responsible for the lack of positive effect on disease 140. In a double-blind, placebo controlled safety study in Finland (Type I Diabetes Prediction and Prevention Study, DIPP) insulin was given nasally to children who are at risk of developing diabetes. Insulin given nasally was well tolerated with low risk of hypoglycemia and no adverse effects were detected 141.
In the US a multi-center double-blind study is evaluating oral insulin therapy versus placebo in adults and children with recent-onset disease. No adverse effect was detected, and patients diagnosed after 20 years of age who were fed 1 mg insulin showed preserved β-cell function compared to patients who received placebo 142. In another double-blind, placebo controlled study oral insulin did not delay or prevent type I diabetes in nondiabetic relatives at risk for diabetes. However, subjects who received insulin orally had significantly lower autoantibodies against insulin compared to subjects who received placebo 143.
Uveitis
A pilot study in two patients, one with pars planitis and the other with Behcet’s disease, feeding of the retinal S-Ag resulted in these patients’ immunosuppressive medication being decreased and/or stopped. The trial yielded valuable information on dosage and expected immune responses and led to a larger randomized, masked study looking at the effect of feeding retinal antigens to uveitis patients 144.
SLE – taming the wolf by restoring self-tolerance?
New advances in the treatment of SLE have been documented in recent years. Most notably, a chimeric human-murine monoclonal antibody directed against CD20 (Rituximab) on B cells and their precursors but not against plasma cells, which do not have this surface marker was tested. The use of Rituximab has been widely used in the management of lymphoma, with a decent record of safety, and is well tolerated. Leandro 145 and Anolik 146 and their colleagues undertook the first studies of Rituximab in SLE and since then there have been many small open-label trials. The overwhelming consensus is that Rituximab has the potential to produce long remissions after only two to four infusions. However, results of a recent placebo controlled phase II/III clinical trial in SLE patients demonstrated no beneficial effect of Rituximab. This could be due to the formation of anti-chimeric antibody that hampered the efficacy of Rituximab 147. There are various protocols in use that combine Rituximab with intravenous cyclophosphamide and methylprednisolone, and we do not know whether maintenance of immunosuppressant is needed after Rituximab to prevent B cell re-accumulation and possible subsequent disease flares. There are several trails in progress to address these issues 148–151. The precise mechanism(s) of action of Rituximab remain unclear. In addition, humanized monoclonal anti-B-cell antibodies are in clinical trials and Dorner and colleagues’ findings 152 suggest that infusion of Epratuzumab, a fully human anti-CD22 monoclonal antibody, is safe in patients with lupus and reduces disease effectively in the short term. We await data on the long term clinical effects of Epratuzumab. Furthermore, in a phase II placebo controlled clinical trial, infusion of anti-B-lymphocyte stimulator (BLys) human monoclonal antibody (Belimumab) which neutralizes soluble BLys used in plasma cell formation in SLE patients showed a significant reduction in CD20+ B cells and plasma cells over a 76 week follow-up period with no increase in adverse events including infections 153.
So far there have been no clinical trials in SLE patients to evaluate the effect of mucosal administration of self-antigens or monoclonal antibodies on disease. In fact, unlike other autoimmune diseases, studies on mucosal tolerance in animal models of SLE have been relatively limited. Initial studies in animals suggested that oral tolerance is defective in the lupus prone (NZB×NZW)F1 (BWF1) mouse 154. In a recent study oral administration of OVA failed to inhibit the secondary IgG response after systemic immunization, again suggesting defective oral tolerance in BWF1 mice prone to developing lupus 155. As a novel approach to disease therapy we have carried out the first studies of nasal tolerance using a critical lupus autoantigen in mice that spontaneously develop a lupus like disease 19. In the early studies we used histone peptide H471 (derived from histone H4, position 71–93) expressing a dominant T cell epitope in the mononucleosome 60 to induce tolerance to itself and to the whole protein by nasal instillation in (NZB×SWR)F1 (SNF1) mice. We deliberately chose to deliver the antigen via the nasal cavity rather than orally because the nasopharyngeal environment which lacks the high acidity and proteolytic enzymes of the gut, is less degrading to proteins, and particularly peptides. Also, the respiratory mucosa is more accessible for small amounts of antigen than the gut mucosa. This is advantageous when expensive peptides or purified proteins are used as tolerogens.
Young pre-nephritic female SNF1mice were nasally dosed with the H471 peptide dissolved in PBS for 5 consecutive days (4 μg per day) (day –12 to –8). On day 0, each mouse received 100 μg H471 or mononucleosome emulsified in complete Freund’s adjuvant (CFA) intradermally and on day 10, T cell responses to H471 were tested in vitro. To examine the effect on disease progression and severity, similar young pre-nephritic female SNF1 mice were nasally dosed with H471 or irrelevant control peptide at 2-wk intervals until 32 weeks of age at which point disease related pathology and immune responses were examined. Nasal administration of H471 in mice markedly reduced subsequent T cell proliferative response to the peptide 19. The mechanism behind the observed T cell hyporesponsiveness was T cell anergy as increasing the in vitro concentration of antigen or adding exogenous IL-2 could reverse the anergic state of the T cells. Subsequent studies on nasal tolerance showed that T cell anergy induced by nasal administration of H471 peptide in lupus prone NZB mice was a result of antigen presentation by immature B cells that lack CD80 and CD86 expression 156.
One of the major obstacles in developing treatment for systemic autoimmune syndromes such as lupus has been to design a therapy that allows the suppression or elimination of autoimmunity against multiple self-antigens and/or tissues. Thus it was encouraging to learn that T cells from mice nasally tolerized to H471 also demonstrated significantly reduced proliferative response to mononucleosomes 19. Suppression of immune responses to mononucleosomes may prevent or delay the development of autoreactivity against other antigens such as DNA and histones. This was demonstrated in histopathological studies in SNF1 mice that were nasally treated with the H471 peptide over a long period of time (3 months) showing a significantly lower incidence of severe glomerulonephritis compared to mice treated with control peptide. The significant improvement in the severity of disease pathology as a result of nasal treatment of mice with histone peptide was associated with downregulation of autoantibody production 19.
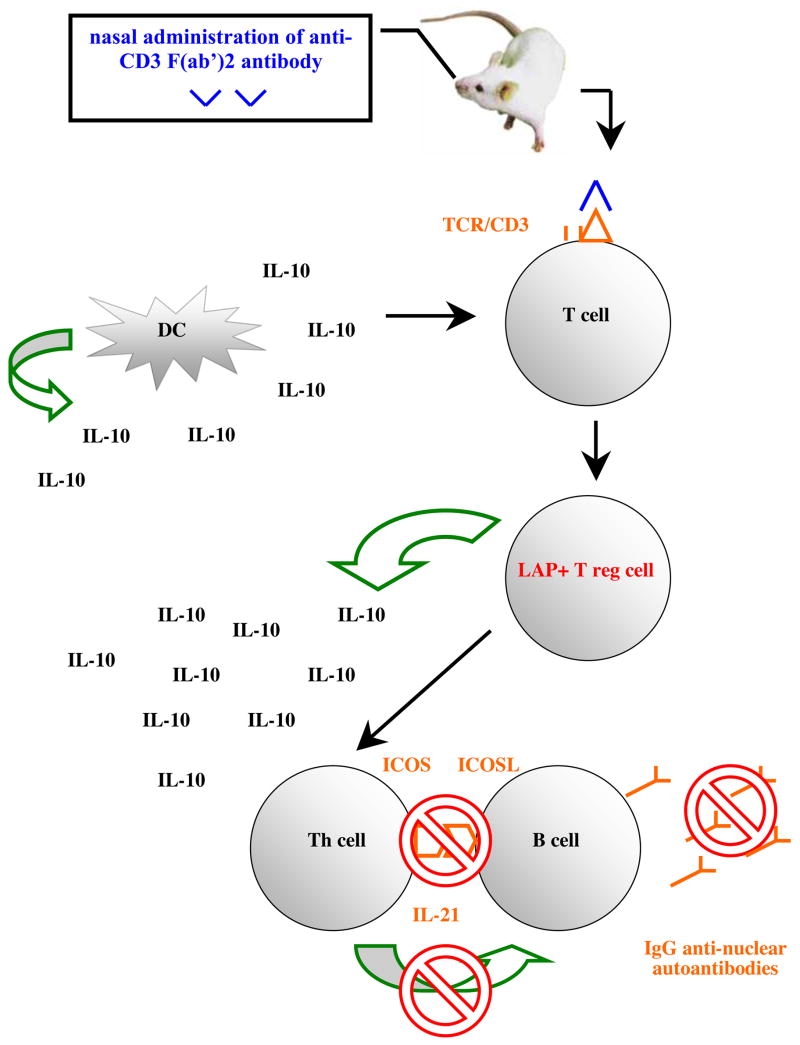
One potential drawback with a peptide-based mucosal therapy in SLE is that not all patients are sensitized to the peptide thus restricting its applicability. Therefore we recently carried out studies in two different strains of lupus prone mice, SNF1 and BWF1, with a CD3-specific monoclonal antibody that has been previously shown to generate inducible regulatory T cells and suppress EAE 21. Nasal or oral administration of anti-CD3 monoclonal antibody attenuated lupus development and arrested on-going disease. Nasal anti-CD3 induced a CD4+CD25−LAP+ regulatory T cell that secreted high levels of IL-10 and suppressed disease in vivo via IL-10 and TFG-β dependent mechanisms whereas suppression of lupus by oral anti-CD3 was associated with an increase in TGF-β secreting CD4+CD25−LAP+ regulatory T cell. Animals treated with nasal anti-CD3 had diminished antibody reactivity in autoantigen microarrays and ELISA, and significantly less glomerulonephritis. Disease suppression was associated with a significant downregulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cell function and IL-21 expression (Wu et al in press). In contrast to animals with lupus where IL-10 ameliorates disease, several studies have demonstrated that IL-10 is markedly upregulated in SLE patients and its levels correlate with disease activity 157,158. This suggests that IL-10 plays a role in the pathogenesis of SLE. In a small open-labeled study in SLE patients infusion of anti-IL-10 monoclonal antibody was beneficiary in 5 out of 6 patients 159. However, treatment with anti-IL-10 antibody did not lead to a decrease in circulating anti-DNA antibodies and the generalized improvement in patients treated with anti-IL-10 was due to an autoantibody independent mechanism 159. The high level of IL-10 in SLE patients could come from damaged tissues as a mechanism to suppress inflammation. Thus the role of IL-10 in the pathogenesis of SLE is unclear. We hypothesize that production of IL-10 by CD4+CD25−LAP+ regulatory T cells in lymphoid tissues results in suppression of helper T cell function thereby inhibiting B cell activation and autoantibody production (diagram 2).
Diagram 2.
IL-10 secreting LAP+ regulatory T cells suppress the function of follicular helper T cells leading to disruption of Th-B cell cognate interaction, suppression of autoantibody production and disease pathology.
In animal models of mucosal tolerance in which the subjects are typically tolerized before disease induction, there is usually a significant reduction in the quality and measure of pathology. This paradigm is, however, not applicable to clinical situations involving lupus because it is impossible to make a diagnosis before the patient has already developed disease. Thus, it is particularly encouraging to learn that nasal or oral anti-CD3 in 7 months old female SNF1 and BWF1 mice with persistent proteinuria markedly reduced disease severity and progression and significantly improved survival compared to mice treated with isotype control antibody. We observed no mitogenic effect of nasal or oral anti-CD3 antibody in mice and no evidence of cytokine release syndrome (wasted appearance, ruffled fur) even after 30 nasal administrations. We performed our experiments with an F(ab’)2 antibody to eliminate any potential side effects related to the Fc portion of the molecule that might occur after multiple administrations of the antibody. No anti-F(ab’)2 antibody response was seen in mice treated nasally or orally with the antibody (Wu et al in press). Thus nasal or oral administration of CD3-specific antibody would appear to be clinically applicable for chronic therapy, with few expected side effects such as cytokine release syndromes and anti-globulin responses. CD3-specific antibody would seem to be safe for human nasal or oral administration, given the long experience with intravenous CD3-specific antibody in humans, though nasal or oral CD3-specific antibody has never been tested in humans.
Concluding remarks
A specific and side effect-free treatment for SLE remains elusive. Novel treatment strategies that are disease specific and can reduce the possibility of compromising normal protective immunity and immune surveillance have been the focus of many immunologists. Despite the limited volume of research, data suggest that mucosal-based therapy holds good potential in the treatment of SLE. However, there are many important areas that need to be investigated and understood before the theory turns to reality. The unknown areas include factors that determine the efficacy of treatment and the immunological mechanism(s) behind disease suppression. There has not been a new drug approved to treat SLE for over 40 years. In light of a new millennium we hope that a relief for lupus patients is no more than a sniff or a sip away.
Acknowledgments
I would like to express my gratitude to Professors Howard L. Weiner, Byron H. Waksman, Peter H. Schur and George C. Tsokos at Harvard Medical School for their support and critical assessment of this manuscript. I would also like to thank the NIH for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amoura Z, Piette JC, Bach JF, Koutouzov S. The key role of nucleosomes in lupus. Arthritis Rheum. 1999;42:833–43. doi: 10.1002/1529-0131(199905)42:5<833::AID-ANR1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Theofilopoulos AN, Dixon FJ. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 3.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 4.Ngan J, Kind LS. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978;120:861–5. [PubMed] [Google Scholar]
- 5.Vaz NM, Maia LC, Hanson DG, Lynch JM. Inhibition of homocytotropic antibody responses in adult inbred mice by previous feeding of the specific antigen. J Allergy Clin Immunol. 1977;60:110–5. doi: 10.1016/0091-6749(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 6.Miller SD, Hanson DG. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979;123:2344–50. [PubMed] [Google Scholar]
- 7.Mowat AM, Strobel S, Drummond HE, Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982;45:105–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson DG, et al. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55:526–32. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- 9.Richman LK, Chiller JM, Brown WR, Hanson DG, Vaz NM. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978;121:2429–34. [PubMed] [Google Scholar]
- 10.Titus RG, Chiller JM. Orally induced tolerance. Definition at the cellular level. Int Arch Allergy Appl Immunol. 1981;65:323–38. [PubMed] [Google Scholar]
- 11.Hanson DG, Miller SD. Inhibition of specific immune responses by feeding protein antigens. V. Induction of the tolerant state in the absence of specific suppressor T cells. J Immunol. 1982;128:2378–81. [PubMed] [Google Scholar]
- 12.Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol. 1988;140:440–5. [PubMed] [Google Scholar]
- 13.Lider O, Santos LM, Lee CS, Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989;142:748–52. [PubMed] [Google Scholar]
- 14.Fishman-Lobell J, Friedman A, Weiner HL. Different kinetic patterns of cytokine gene expression in vivo in orally tolerant mice. Eur J Immunol. 1994;24:2720–4. doi: 10.1002/eji.1830241122. [DOI] [PubMed] [Google Scholar]
- 15.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 16.Weiner HL, Gonnella PA, Slavin A, Maron R. Oral tolerance: cytokine milieu in the gut and modulation of tolerance by cytokines. Res Immunol. 1997;148:528–33. doi: 10.1016/s0923-2494(98)80146-6. [DOI] [PubMed] [Google Scholar]
- 17.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 18.Wu HY, Weiner HL. Oral tolerance. Immunol Res. 2003;28:265–84. doi: 10.1385/IR:28:3:265. [DOI] [PubMed] [Google Scholar]
- 19.Wu HY, Ward FJ, Staines NA. Histone peptide-induced nasal tolerance: suppression of murine lupus. J Immunol. 2002;169:1126–34. doi: 10.4049/jimmunol.169.2.1126. [DOI] [PubMed] [Google Scholar]
- 20.Prakken BJ, et al. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002;46:1937–46. doi: 10.1002/art.10366. [DOI] [PubMed] [Google Scholar]
- 21.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4(+)CD25(−)LAP(+) T cells. Nat Med. 2006;12:627–35. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 25.Taams LS, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji NM. Antigen-specific CD4(+) regulatory T cells in the intestine. Inflamm Allergy Drug Targets. 2006;5:191–201. doi: 10.2174/187152806778256043. [DOI] [PubMed] [Google Scholar]
- 27.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2002;99:8832–7. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annacker O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S, Lechler RI. CD4+CD25+ regulatory T-cell therapy for allergy, autoimmune disease and transplant rejection. Inflamm Allergy Drug Targets. 2006;5:239–42. doi: 10.2174/187152806779010981. [DOI] [PubMed] [Google Scholar]
- 33.Viehl CT, et al. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13:1252–8. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 34.Strauss L, Bergmann C, Whiteside TL. Functional and phenotypic characteristics of CD4(+)CD25(high)Foxp3(+) Treg clones obtained from peripheral blood of patients with cancer. Int J Cancer. 2007 doi: 10.1002/ijc.23001. [DOI] [PubMed] [Google Scholar]
- 35.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 36.Aseffa A, et al. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002;169:3232–41. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 37.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 38.Kojima A, Tanaka-Kojima Y, Sakakura T, Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976;34:550–7. [PubMed] [Google Scholar]
- 39.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–76. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–86. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 42.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 43.Abramowicz D, et al. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation. 1989;47:606–8. doi: 10.1097/00007890-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–71. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 46.McHugh RS, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 47.Morris GP, Kong YC. Interference with CD4+CD25+ T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J Autoimmun. 2006;26:24–31. doi: 10.1016/j.jaut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–2. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 51.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 52.Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 53.Wu HY, Staines NA. A deficiency of CD4+CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004;13:192–200. doi: 10.1191/0961203303lu1002oa. [DOI] [PubMed] [Google Scholar]
- 54.Bagavant H, Tung KS. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175:944–50. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 55.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 56.Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN. Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol. 2007;601:113–9. [PubMed] [Google Scholar]
- 57.Franz B, et al. Low number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosus. Arthritis Rheum. 2007;56:1910–20. doi: 10.1002/art.22699. [DOI] [PubMed] [Google Scholar]
- 58.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 59.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–9. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 60.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183:2459–69. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247–55. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 62.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–58. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 63.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black x New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542–8. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 64.Hahn BH, et al. Cellular and molecular mechanisms of regulation of autoantibody production in lupus. Ann N Y Acad Sci. 2005;1051:433–41. doi: 10.1196/annals.1361.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc Natl Acad Sci U S A. 2006;103:8810–5. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharabi A, Azulai H, Sthoeger ZM, Mozes E. Clinical amelioration of murine lupus by a peptide based on the complementarity determining region-1 of an autoantibody and by cyclophosphamide: similarities and differences in the mechanisms of action. Immunology. 2007;121:248–57. doi: 10.1111/j.1365-2567.2007.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cottrez F, Groux H. Specialization in tolerance: innate CD(4+)CD(25+) versus acquired TR1 and TH3 regulatory T cells. Transplantation. 2004;77:S12–5. doi: 10.1097/01.TP.0000106471.23410.32. [DOI] [PubMed] [Google Scholar]
- 68.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T-cells. Journal of Experimental Medicine. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huber S, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 72.Huber S, Schramm C. TGF-beta and CD4+CD25+ regulatory T cells. Front Biosci. 2006;11:1014–23. doi: 10.2741/1859. [DOI] [PubMed] [Google Scholar]
- 73.Weiner HL, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Inobe J-i, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cellular Immunology. 1997;178:62–68. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 75.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis as associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. Journal of Experimental Medicine. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 77.Melamed D, Fishman-Lovell J, Uni Z, Weiner HL, Friedman A. Peripheral tolerance of Th2 lymphocytes induced by continuous feeding of ovalbumin. Int Immunol. 1996;8:717–24. doi: 10.1093/intimm/8.5.717. [DOI] [PubMed] [Google Scholar]
- 78.Neurath MF, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183:2605–16. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faria AM, et al. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–45. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 80.Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P. 'Educated' dendritic cells act as messengers from memory to naive T helper cells. Nat Immunol. 2004;5:615–22. doi: 10.1038/ni1077. [DOI] [PubMed] [Google Scholar]
- 81.Gonnella PA, Chen YH, Waldner H, Weiner HL. Induction of oral tolerization in CD86 deficient mice: a role for CD86 and B cells in the up-regulation of TGF-beta. J Autoimmun. 2006;26:73–81. doi: 10.1016/j.jaut.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–57. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maron R, Melican NS, Weiner HL. Regulatory Th2-type T cell lines against insulin and GAD peptides derived from orally- and nasally-treated NOD mice suppress diabetes. J Autoimmun. 1999;12:251–8. doi: 10.1006/jaut.1999.0278. [DOI] [PubMed] [Google Scholar]
- 84.Aroeira LS, Cardillo F, De Albuquerque DA, Vaz NM, Mengel J. Anti-IL-10 treatment does not block either the induction or the maintenance of orally induced tolerance to OVA. Scand J Immunol. 1995;41:319–23. doi: 10.1111/j.1365-3083.1995.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 85.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 86.Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001;2:671–2. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]
- 87.Inobe J, et al. IL-4 is a differentiation factor for transforming growth factor-beta secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–90. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 88.Thorbecke GJ, Schwarcz R, Leu J, Huang C, Simmons WJ. Modulation by cytokines of induction of oral tolerance to type II collagen. Arthritis Rheum. 1999;42:110–8. doi: 10.1002/1529-0131(199901)42:1<110::AID-ANR14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Michael JG. Orally inducible immune unresponsiveness is abrogated by IFN-γ treatment. Journal of Immunology. 1990;144:4163–4165. [PubMed] [Google Scholar]
- 90.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–57. [PubMed] [Google Scholar]
- 91.Eaton AD, Xu D, Garside P. Administration of exogenous interleukin-18 and interleukin-12 prevents the induction of oral tolerance. Immunology. 2003;108:196–203. doi: 10.1046/j.1365-2567.2003.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nelson PA, et al. Effect of oral beta interferon on subsequent immune responsiveness. Ann N Y Acad Sci. 1996;778:145–55. doi: 10.1111/j.1749-6632.1996.tb21123.x. [DOI] [PubMed] [Google Scholar]
- 93.Soos JM, Mujtaba MG, Subramaniam PS, Streit WJ, Johnson HM. Oral feeding of interferon tau can prevent the acute and chronic relapsing forms of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;75:43–50. doi: 10.1016/s0165-5728(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 94.Soos JM, et al. Cutting edge: oral type I IFN-tau promotes a Th2 bias and enhances suppression of autoimmune encephalomyelitis by oral glatiramer acetate. J Immunol. 2002;169:2231–5. doi: 10.4049/jimmunol.169.5.2231. [DOI] [PubMed] [Google Scholar]
- 95.Xiao BG, Bai XF, Zhang GX, Link H. Suppression of acute and protracted-relapsing experimental allergic encephalomyelitis by nasal administration of low-dose IL-10 in rats. J Neuroimmunol. 1998;84:230–7. doi: 10.1016/s0165-5728(97)00264-6. [DOI] [PubMed] [Google Scholar]
- 96.Slavin AJ, Maron R, Weiner HL. Mucosal administration of IL-10 enhances oral tolerance in autoimmune encephalomyelitis and diabetes. Int Immunol. 2001;13:825–33. doi: 10.1093/intimm/13.6.825. [DOI] [PubMed] [Google Scholar]
- 97.Li HL, et al. Nasal tolerance to experimental autoimmune myasthenia gravis: tolerance reversal by nasal administration of minute amounts of interferon-gamma. Clin Immunol Immunopathol. 1998;87:15–22. doi: 10.1006/clin.1997.4495. [DOI] [PubMed] [Google Scholar]
- 98.Gautam SC, Chikkala NF, Battisto JR. Oral administration of the contact sensitizer trinitrochlorobenzene: initial sensitization and subsequent appearance of a suppressor population. Cell Immunol. 1990;125:437–48. doi: 10.1016/0008-8749(90)90097-b. [DOI] [PubMed] [Google Scholar]
- 99.Hoyne GF, Callow MG, Kuhlman J, Thomas WR. T-cell lymphokine response to orally administered proteins during priming and unresponsiveness. Immunology. 1993;78:534–40. [PMC free article] [PubMed] [Google Scholar]
- 100.Hoyne GF, Thomas WR. T-cell responses to orally administered antigens. Study of the kinetics of lymphokine production after single and multiple feeding. Immunology. 1995;84:304–9. [PMC free article] [PubMed] [Google Scholar]
- 101.Mowat AM, Steel M, Leishman AJ, Garside P. Normal induction of oral tolerance in the absence of a functional IL-12-dependent IFN-gamma signaling pathway. J Immunol. 1999;163:4728–36. [PubMed] [Google Scholar]
- 102.Mengel J, et al. Anti-gamma delta T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol Lett. 1995;48:97–102. doi: 10.1016/0165-2478(95)02451-4. [DOI] [PubMed] [Google Scholar]
- 103.Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–8. [PubMed] [Google Scholar]
- 104.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33 – 35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. Eur J Immunol. 1999;29:4060–71. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 105.McMenamin C, McKersey M, Kuhnlein P, Hunig T, Holt PG. Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390–4. [PubMed] [Google Scholar]
- 106.Kweon MN, et al. Lack of orally induced systemic unresponsiveness in IFN-gamma knockout mice. J Immunol. 1998;160:1687–93. [PubMed] [Google Scholar]
- 107.Lee HO, et al. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology. 2000;119:129–38. doi: 10.1053/gast.2000.8542. [DOI] [PubMed] [Google Scholar]
- 108.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garside P, Steel M, Liew FY, Mowat AM. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995;7:501–4. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- 110.Barone KS, Jain SL, Michael JG. Effect of in vivo depletion of CD4+ and CD8+ cells on the induction and maintenance of oral tolerance. Cell Immunol. 1995;163:19–29. doi: 10.1006/cimm.1995.1094. [DOI] [PubMed] [Google Scholar]
- 111.Desvignes C, Bour H, Nicolas JF, Kaiserlian D. Lack of oral tolerance but oral priming for contact sensitivity to dinitrofluorobenzene in major histocompatibility complex class II-deficient mice and in CD4+ T cell-depleted mice. Eur J Immunol. 1996;26:1756–61. doi: 10.1002/eji.1830260814. [DOI] [PubMed] [Google Scholar]
- 112.Mattingly PC, Mowat AG. Zinc sulphate in rheumatoid arthritis. Ann Rheum Dis. 1982;41:456–7. doi: 10.1136/ard.41.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lider O, et al. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989;83:752–6. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller A, al-Sabbagh A, Santos LM, Das MP, Weiner HL. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol. 1993;151:7307–15. [PubMed] [Google Scholar]
- 115.von Herrath MG, Dyrberg T, Oldstone MB. Oral insulin treatment suppresses virus-induced antigen-specific destruction of beta cells and prevents autoimmune diabetes in transgenic mice. J Clin Invest. 1996;98:1324–31. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.den Haan JM, Bevan MJ. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr Opin Immunol. 2001;13:437–41. doi: 10.1016/s0952-7915(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 117.Maecker HT, et al. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J Immunol. 2001;166:7268–75. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 118.Wang R, Ramaswamy S, Hu D, Cantor H. Definition of a novel binding site on CD8 cells for a conserved region of the MHC class Ib molecule Qa-1 that regulates IFN-gamma expression. Eur J Immunol. 2001;31:87–93. doi: 10.1002/1521-4141(200101)31:1<87::aid-immu87>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 119.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 120.Vistica BP, et al. CD8 T-cells are not essential for the induction of “low-dose” oral tolerance. Clin Immunol Immunopathol. 1996;78:196–202. doi: 10.1006/clin.1996.0029. [DOI] [PubMed] [Google Scholar]
- 121.Grdic D, Hornquist E, Kjerrulf M, Lycke NY. Lack of local suppression in orally tolerant CD8-deficient mice reveals a critical regulatory role of CD8+ T cells in the normal gut mucosa. J Immunol. 1998;160:754–62. [PubMed] [Google Scholar]
- 122.Gonnella PA, Waldner HP, Weiner HL. B cell-deficient (mu MT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166:4456–64. doi: 10.4049/jimmunol.166.7.4456. [DOI] [PubMed] [Google Scholar]
- 123.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 124.Trop S, et al. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746–55. doi: 10.1002/hep.510290334. [DOI] [PubMed] [Google Scholar]
- 125.Samsonov D, Trop S, Alper R, Diment J, Ilan Y. Enhancement of immune tolerance via induction of NK1.1 positive liver-associated-lymphocytes under immunosuppressive conditions. J Hepatol. 2000;32:812–20. doi: 10.1016/s0168-8278(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 126.Margenthaler JA, Landeros K, Kataoka M, Flye MW. CD1-dependent natural killer (NK1.1(+)) T cells are required for oral and portal venous tolerance induction. J Surg Res. 2002;104:29–35. doi: 10.1006/jsre.2002.6400. [DOI] [PubMed] [Google Scholar]
- 127.Ishimitsu R, Yajima T, Nishimura H, Kawauchi H, Yoshikai Y. NKT cells are dispensable in the induction of oral tolerance but are indispensable in the abrogation of oral tolerance by prostaglandin E. Eur J Immunol. 2003;33:183–93. doi: 10.1002/immu.200390021. [DOI] [PubMed] [Google Scholar]
- 128.Fukaura H, et al. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weiner HL, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–4. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 130.Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer 1. Proc Natl Acad Sci U S A. 1999;96:3842–7. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weiner HL. Oral tolerance with copolymer 1 for the treatment of multiple sclerosis. Proc Natl Acad Sci U S A. 1999;96:3333–5. doi: 10.1073/pnas.96.7.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barnett ML, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–7. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 133.Choy EH, et al. Control of rheumatoid arthritis by oral tolerance. Arthritis Rheum. 2001;44:1993–7. doi: 10.1002/1529-0131(200109)44:9<1993::AID-ART347>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 134.Yoshino S. Downregulation of silicone-induced chronic arthritis by gastric administration of type II collagen. Immunopharmacology. 1995;31:103–8. doi: 10.1016/0162-3109(95)00038-5. [DOI] [PubMed] [Google Scholar]
- 135.Trentham DE, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 136.Postlethwaite AE. Can we induce tolerance in rheumatoid arthritis? Curr Rheumatol Rep. 2001;3:64–9. doi: 10.1007/s11926-001-0052-z. [DOI] [PubMed] [Google Scholar]
- 137.Barnett ML, Combitchi D, Trentham DE. A pilot trial of oral type II collagen in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39:623–8. doi: 10.1002/art.1780390413. [DOI] [PubMed] [Google Scholar]
- 138.Myers LK, et al. Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum. 2001;44:1775–81. doi: 10.1002/1529-0131(200108)44:8<1775::AID-ART313>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 139.Chaillous L, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–9. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 140.Monetini L, et al. Cytokine profile and insulin antibody IgG subclasses in patients with recent onset type 1 diabetes treated with oral insulin. Diabetologia. 2004;47:1795–802. doi: 10.1007/s00125-004-1521-5. [DOI] [PubMed] [Google Scholar]
- 141.Kupila A, et al. Intranasally administered insulin intended for prevention of type 1 diabetes--a safety study in healthy adults. Diabetes Metab Res Rev. 2003;19:415–20. doi: 10.1002/dmrr.397. [DOI] [PubMed] [Google Scholar]
- 142.Ergun-Longmire B, et al. Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci. 2004;1029:260–77. doi: 10.1196/annals.1309.057. [DOI] [PubMed] [Google Scholar]
- 143.Skyler JS, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068–76. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 144.Nussenblatt RB, et al. Intraocular inflammatory disease (uveitis) and the use of oral tolerance: a status report. Ann N Y Acad Sci. 1996;778:325–37. doi: 10.1111/j.1749-6632.1996.tb21140.x. [DOI] [PubMed] [Google Scholar]
- 145.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–7. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 146.Anolik JH, et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–9. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 147.Albert D, et al. Variability in the biological response to anti-CD20 B-cell depletion in SLE. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 148.Eisenberg R. Targeting B cells in SLE: the experience with rituximab treatment (anti-CD20) Endocr Metab Immune Disord Drug Targets. 2006;6:345–50. doi: 10.2174/187153006779025757. [DOI] [PubMed] [Google Scholar]
- 149.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66:1933–48. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 150.Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev. 2006;5:18–24. doi: 10.1016/j.autrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 151.Looney RJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 152.Dorner T, et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Belimumab: anti-BLyS human monoclonal antibody, anti-BLyS monoclonal antibody, BmAb, human monoclonal antibody to B-lymphocyte stimulator. Drugs R D. 2008;9:197–202. doi: 10.2165/00126839-200809030-00008. [DOI] [PubMed] [Google Scholar]
- 154.Carr RI, Tilley D, Forsyth S, Etheridge P, Sadi D. Failure of oral tolerance in (NZB X NZW)F1 mice is antigen specific and appears to parallel antibody patterns in human systemic lupus erythematosus (SLE) Clin Immunol Immunopathol. 1987;42:298–310. doi: 10.1016/0090-1229(87)90018-3. [DOI] [PubMed] [Google Scholar]
- 155.Akadegawa K, et al. Breakdown of mucosal immunity in the gut and resultant systemic sensitization by oral antigens in a murine model for systemic lupus erythematosus. J Immunol. 2005;174:5499–506. doi: 10.4049/jimmunol.174.9.5499. [DOI] [PubMed] [Google Scholar]
- 156.Wu HY, Monsonego A, Weiner HL. The mechanism of nasal tolerance in lupus prone mice is T-cell anergy induced by immature B cells that lack B7 expression. J Autoimmun. 2006;26:116–26. doi: 10.1016/j.jaut.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Houssiau FA, et al. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 158.Park YB, et al. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- 159.Llorente L, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]