Abstract
Rheumatoid arthritis (RA) is a systemic inflammatory disease with a predilection for symmetrically distributed diarthroidal joints. It is clinically heterogeneous, with particular disease phenotypes defined according to a complex interplay of genes and the environment. In this chapter we first summarize current knowledge of RA genetic susceptibility, a field which has been transformed in recent years by powerful modern genotyping technologies. The importance of a recently described subclassification for the disease based upon the presence or absence of circulating autoantibodies to citrullinated peptides has further informed genetic studies, and we consider the implications for our understanding of RA pathogenesis. We then review the cellular and molecular processes that initiate and perpetuate joint destruction.
Keywords: rheumatoid arthritis, early, genetics, aetiology, pathogenesis
What genetic factors predispose to rheumatoid arthritis (RA)?
It has long been documented that RA clusters in families: the likelihood that a first-degree relative of a patient will share the diagnosis is 2–10 times the population prevalence of the disease [1]. The genetic basis of such clustering is confirmed by observations that RA concordance amongst monozygotic twins is approximately 15%, which is up to 5 times greater than in dizygotic twins. Twin studies permit an estimation of heritability (the extent to which liability to a condition in a population can be explained by genetic variation), and on the basis of British and Finnish populations the heritability of RA has been calculated as 60% [2].
HLA association
The longest-established, and strongest, genetic association of RA is with a particular set of alleles at the highly polymorphic human leukocyte antigen (HLA)-DRB1 locus on the short arm of chromosome 6 (6p21.3). This gene encodes major histocompatability complex (MHC) class-II β-chain molecules whose function is in the presentation of antigen to CD4+ T ‘helper’ cells. Alleles associated with RA encode a conserved amino acid sequence within the binding groove of the final MHC class-II heterodimer [3]. These ‘shared epitope’ (SE) alleles display a dose effect with respect to susceptibility, with homozygotic individuals carrying increased risk over heterozygotes, and particular compound heterozygote combinations representing a disproportionate risk that achieves odds ratios of >30 [4]. A similar pattern emerges in respect of disease severity [5]. Genome-wide sibling-pair linkage analyses have confirmed linkage at the HLA locus amongst American [6] and European [7], but not Japanese [8], populations, illustrating the importance of ethnic considerations when interpreting genetic studies. Recently, a distinct class-II MHC β-chain amino-acid sequence has been shown to be protective against both RA susceptibility and severity in a northern European population, independently of coexisting risk alleles [9]. The DRB1 allele that encodes this sequence may even confer protection to the offspring of a woman who carries it without itself being inherited: a phenomenon termed non-inherited maternal allele (NIMA) protection [10]. DRB1-gene promoter polymorphisms add an additional layer of complexity to the picture. For example, differential regulation of respective DRB1 allele expression, with the potential to influence MHC phenotype and hence RA susceptibility, may be determined by polymorphism within the trans-acting HLA class-II promoter gene MHC2TA [11].
Altogether, the HLA region encompasses 3000 kilobases of genomic DNA and, aside from MHC class-I and class-II genes, it contains a wide range of other immunologically relevant genes. Many studies have investigated possible additional associations of such genes with RA susceptibility, but they are complicated by the inevitable linkage disequilibrium of candidate loci in the region with the HLA-DRB1 gene itself (reviewed by Newton et al) [12]. By controlling for SE risk amongst pedigrees and controls, transmission disequilibrium testing of loci within HLA haplotypes has recently provided strong evidence that such associations indeed exist [13], but further work is needed to delineate the findings.
Non-HLA genetic association
The contribution that HLA-DRB1 status makes to the overall heritability of RA has been estimated at about a third [14], and the search for non-HLA disease associations has therefore been intense. Investigation of the candidate gene PTPN22, located on chromosome 1 (p13), provided the first definitive evidence of such an association [15], since extensively validated [16]. A missense C → T substitution at base position 1856 of this gene leads to the presence of tryptophan (W) in place of arginine (R) at residue 620 of the protein product, which is the lymphoid-specific protein tyrosine phosphatase, LYP. ‘R620W’ substitution appears to represent a ‘gain-in-function’ mutation which, through enhanced regulation of T-cell receptor (TCR) signalling during thymic selection, permits autoantigen-specific T cells to escape clonal deletion, thereby predisposing to autoimmunity [17]. Once more, the association does not extend to all ethnic groups, and the R620W variant is not seen in Asian populations [18].
Innovation in high-throughput biotechnology, coupled with the accumulation of large repositories of clinical material, has culminated in the completion of the first genome-wide association studies (GWAS). Hundreds of thousands of single-nucleotide polymorphisms (SNPs) may now be genotyped simultaneously amongst large sample sets in a cost-effective manner. In the pioneering example of the Wellcome Trust Case Control Consortium (WTCCC) GWAS, DNA samples of 2000 RA patients (and similar numbers of patients diagnosed with other common complex diseases) were genotyped for over 500,000 SNPs alongside 3000 pooled controls, all drawn from UK populations [19]. This study confirmed the association of both HLA-DRB1 and PTPN22 with RA, and strong evidence of association was suggested at nine additional loci. Only one of these, on chromosome 6 (6q23), has since been independently validated by association analyses in both UK [20] and US [21] populations. The intronic region concerned lies in proximity to the TNF-α-induced protein 3 (TNFAI3) gene, whose product functions as a negative regulator of nuclear factor κB (NFκB), a transcription factor involved in the propagation of many inflammatory pathways. In separate case–control cohorts from US and Swedish populations, the GWAS approach has identified a further region of association on the long arm of chromosome 9 (9q33–34), which contains genes encoding both tumour-necrosis-factor-associated factor-1 (TRAF-1) and C5 complement [22]. Both products may play a role in the propagation of inflammatory responses, and although the findings have been independently replicated in European cohorts [23,24], functional studies will be needed to further refine the TRAF-1/C5 association.
Smaller-scale SNP association analyses have generated conflicting results for several additional gene loci. The inconsistent finding of RA association with the +49G allele at the cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) gene [25], along with the finding of only nominal association at this locus in the WTCCC study, is probably influenced by differences in the demographics of the various populations studied. A meta-analysis of this association demonstrated a striking bias towards positive studies in Asian and Far-Eastern, rather than white Caucasian, populations [25], and taken together it seems likely that the polymorphism influences disease susceptibility predominantly in non-Europeans. CTLA-4 is a candidate gene for RA since its product, expressed exclusively by T-cells, has an important role in down-regulating CD28-mediated T-cell co-stimulation, thereby attenuating T-cell activation [26]. Another previously identified genetic association with RA that appears to be relatively restricted to Asian populations involves the PADI4 gene, situated on the short arm of chromosome 1 (1p36.13) [27]. It encodes peptidyl arginine deiminase isotype 4 (PAD4), which is predominantly expressed by bone marrow and white blood cells, including those found within the rheumatoid synovium, where it catalyses the post-translational modification of arginine to citrulline [28]. Finally, robust evidence for allelic association of RA with signal transducer and activator of transcription-4 (STAT-4) gene polymorphism has recently emerged [24,29]. STAT-4, encoded on chromosome 2, is known to be up-regulated in RA synovium and, following the ligation of surface-expressed IL-12 receptor on CD4+ T cells, plays a role in skewing the effector phenotype of this cell type during maturation [24,30].
Further considerations
A range of other potential genetic associations with RA remains the subject of investigation, and stands to provide additional important insight into the pathogenesis of the disease. However, based on recent discoveries, the effect of any newly confirmed genetic association is likely to be small in terms of attributable risk, attaining an odds ratio of little more than 1. Whilst HLA and PTPN22 together probably make up about half of the stated genetic contribution to RA susceptibility, it is hard to see how the additive effects of additional minor genetic determinants might together make up the remaining 50% of the disease's heritability. As our understanding of the environmental risk factors for developing RA continues to grow [31], it is increasingly apparent that gene–environment interactions explain this phenomenon to a significant extent. Similarly, distinct genetic risk factors may provide multiplicative, rather than merely additive, combined risk because of their compound molecular consequences.
Autoantibodies and the genetic epidemiology of RA
The importance of ACPA (anti-cyclic citrullinated peptide antibody; anti-CCP) status in stratifying risk for RA has been a highly significant discovery. The peptidyl arginine deiminase-dependent conversion of positively-charged arginine to neutral citrulline occurs ubiquitously in health, taking place preferentially at supranormal intracellular calcium concentrations [28]. By contrast, the presence of circulating ACPAs, detectable using now widely available assays, is >95% specific for RA, with a sensitivity of around 65% [32]. Moreover, the demonstration that ACPA predates the clinical onset of arthritis by several years in some patients [33], that it predicts a more severe, erosive natural history [34], and that citrullinated peptides are present in the rheumatoid synovium [35], all imply a possible pathogenetic role for the autoantibody.
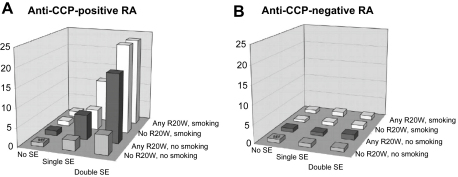
The remarkable observation has recently been made that with respect to the three most robust risk factors for the disease – HLA SE [36], PTPN22 [37] and smoking [38] – the predominant effect is seen only in ACPA-positive patients (Fig. 1). At least in the case of HLA SE, the primary risk factor appears to be ACPA status itself, in turn influenced by genotype, rather than the presence of the SE per se [39]. It has since been demonstrated that tobacco exposure confers a multiplicative risk of RA to SE-positive individuals, apparently by an ACPA-induced mechanism [40]. A large-scale collaborative epidemiological study duplicated these findings in Swedish, Dutch and US populations, showing that smoking–SE interaction increased risk, and that the combined risk conferred by coincidence of relevant HLA SE and PTPN22 alleles was also multiplicative rather than additive [41]. It should be noted, however, that the smoking–SE interaction was not reproduced in an independent large US cohort [42], and scope for identifying additional environmental interactors therefore remains. No such effects were seen amongst the ACPA-negative RA patients studied. Associations between RA and CTLA-4 [37], as well as the newly-defined 6q23 and 9q33–34 polymorphisms [20,23], have again been shown to be strongest amongst ACPA-positive individuals.
Fig. 1.
Histograms of odds ratios for developing anti-CCP-positive (A) and anti-CCP-negative (B) rheumatoid arthritis (RA) for different combinations of absence or presence of R620 W PTPN22, single or double HLA-DRB1 shared epitope (SE) alleles, and smoking (see text). CCP, cyclic citrullinated peptide. Reproduced from Kallberg et al Am J Hum Genet 2007;80:867–75) with permission.
ACPA-positive disease: the ‘real’ RA?
It is tempting to draw the conclusion that ACPA status delineates two distinct subsets of a clinically defined phenotype which happens to satisfy the ACR criteria for RA [43], with all of the latter's well-documented shortcomings. One might infer that the histological characteristics of the two conditions should differ, and this has recently been eloquently demonstrated: ACPA-positive synovium appears to be characterized by denser lymphocyte infiltrations and a higher rate of joint destruction, whereas more extensive fibrotic changes are apparent in ACPA-negative tissue [44]. Recently a small randomized placebo-controlled trial of methotrexate in undifferentiated arthritis demonstrated a potential for the drug to delay or prevent progression to RA amongst the ACPA-positive but not ACPA-negative subgroups [45], lending further credence to the concept that these might represent distinct entities within an overlapping clinical phenotype.
What might we conclude about the induction of autoimmunity in ACPA-positive RA? One salient observation is that deimination of arginine to citrulline greatly increases the affinity of putative peptide autoantigens for the positively charged binding groove common to the class-II MHC β-chain products which incorporate the SE [46]. As recently highlighted by Klareskog and colleagues [47], cigarette smoke is known to activate lung tissue macrophages and induce apoptosis, providing the increased calcium concentration necessary for such PAD4-mediated neoantigen formation to take place [48]. It is known that citrullinated peptides are capable of inducing T-cell responses in mice [49], and additional failures of central tolerance in humans, facilitated through either PTPN22 or CTLA-4 polymorphism, might strengthen the potential for immune dysregulation such that B cells were ‘helped’ to produce ACPA. Against such a background, a subsequent citrullination event within the joint might be sufficient to trigger an autoimmune arthropathy [47].
This model has attractive elements, but fails to account for a number of additional observations. RA is predominantly a disease of the joint, yet citrullination is a ubiquitous process that is not tissue-specific: what leads to localization of the disease to the synovium? Additionally, neither peptidyl arginine deiminase I (PADI) enzyme expression [50] nor any of the wide range of potentially autoantigenic citrullinated epitopes identified within RA synovia appears to be specific for the disease. Neither have disease-specific citrulline-reactive T cells yet been convincingly demonstrated in humans. Crucially, therefore, whether citrullination in RA in fact represents the initiating step in disease induction, an upstream indicator of immune dysregulation, or indeed a downstream ‘bystander’ phenomenon, has yet to be ascertained, and continues to be the subject of intense investigation.
The elephant in the room: ACPA-negative RA
It seems that, for all that we have gained through a growing understanding of ACPA-positive RA, we now need to ‘unlearn’ all that we thought we knew about its ACPA-negative counterpart. And it is the latter condition which remains the hardest to define and the most likely to be subject to delayed diagnosis. It might be argued that investigation of this subset should not be a priority, since patients characteristically experience a less ‘aggressive’ disease in terms of the development of radiographic progression [34]. However, it should be borne in mind that ACPA-negative RA may be less responsive to conventional treatments [45] and, especially where intervention is delayed, ultimately susceptible to poorer outcomes than ACPA-positive disease. Although genetic associations with this subset of RA patients have begun to emerge [51], they remain unconfirmed and warrant further investigation.
How is synovitis initiated and what leads to chronicity?
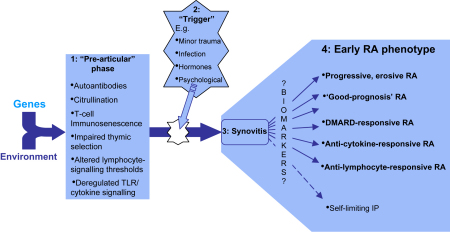
The striking heritability of RA, together with the presence of autoantibodies that precede clinical onset in a subset of patients, supports a pathogenetic model in which autoimmune propensity is long-established by the time joint inflammation is ‘triggered’ (Fig. 2). This concept is further supported by observations in animal models of arthritis. In the SKG mouse [52], for example, a mutation in the ZAP-70 gene leads to autoimmune propensity via impaired thymic T-cell selection, but arthritis cannot be induced in the absence of circulating pro-inflammatory cytokines [53]. Amongst rhesus monkeys in whom arthritis was artificially induced, microscopic joint inflammation was demonstrable prior to the clinical onset of disease, and evidence for an analogous ‘preclinical’ phase in humans comes from the observation that histological synovitis is manifest in the clinically uninvolved joints of RA patients [54]. Interestingly, comparative histology indicates that the synovitis of early RA shares many of the pathological features of established disease [55]. Whilst no single cell type or molecular pathway exclusively encapsulates the RA disease process, the relative importance of each probably varies between individuals in what is a heterogeneous disease (Fig. 2). Areas of particular current interest with regard to the initiation and persistence of synovitis are discussed here.
Fig. 2.
Various pathogenetic pathways account for the heterogeneity of the early rheumatoid arthritis (RA) phenotype. During both the pre-articular phase (1) and the triggering of synovitis itself (2), genetic and environmental determinants together induce a combination of pathogenetic pathways whose common outcome is a syndrome of synovitis ultimately recognizable as RA (3). The relative contributions of the various pathways in turn define an individual's disease phenotype (4). It is hoped that the search for biomarkers will yield a means of stratifying early RA into prognostically and therapeutically relevant subsets. TLR, ‘Toll-like’ receptor; DMARD, disease-modifying anti-rheumatic drug; IP, inflammatory polyarthritis.
An ‘arthritogenic autoantigen'?
Enriched populations of mature antigen-presenting dendritic cells (DCs) are seen in established RA lesions [56], and animal work supports a role for them in the induction of autoimmune arthritis [57]. The validity of a model in which DC antigen presentation is similarly central to the initiation of human disease has not been confirmed however, largely because the identity of a dominant arthritogenic autoantigen has remained elusive. One candidate is human cartilage glycoprotein 39 (HC gp-39) which, only when processed by antigen-presenting cells expressing the SE, has been shown to yield a unique set of immunodominant peptides capable of inducing proliferative human T-cell responses ex vivo [58]. Interestingly, T-cell responses against HC gp-39 have since been associated with production of immunoregulatory interleukin 10 (IL-10) in DRB1*04-positive healthy controls but pro-inflammatory cytokines in DRB1*04-positive RA patients [59]. Other similarly implicated autoantigens include human type-II collagen and aggrecan. Exposure to Epstein–Barr virus (EBV) and greater EBV viral loads are associated with RA such that infection by this pathogen has been mooted as a potential arthritogenic trigger; whilst intriguing mechanisms have been proposed, they remain unconfirmed, however [60]. DC might alternatively contribute to the circumvention of central tolerance by priming the immune system to respond to self antigen that has been post-translationally modified, as might occur following citrullination.
Fibroblast-like synoviocytes in disease induction
Although not as efficient at processing and presenting antigen as DCs, other cells of the innate immune system may be better placed to initiate an inflammatory process within the joint. Like DCs, resident fibroblast-like synoviocytes (FLSs) express members of the ‘Toll-like’ receptor (TLR) family [61] which function in the front line of host defence. As well as recognizing specific structures of invading pathogens called pathogen-associated molecular patterns (PAMPs) [62], TLRs have a range of endogenous ligands, including hyaluronan fragments [63], and high-mobility-group box 1 protein (HMGB-1) [64], which may appear transiently in the healthy synovium. TLR-2 or -4 engagement in human RA synovial tissue cultures has recently been shown to induce pro-inflammatory mediators, and the specific signalling pathways involved are being elucidated [65]. FLSs are also capable of secreting chemokines, in turn abundant in established RA lesions and largely responsible for the recruitment and retention of leukocytes at these sites [66]. Examples are CCL2, whose receptor is found on T cells, monocytes, DCs and basophils, and granulocyte chemotactic protein (GCP-2), both of which have been shown to be up-regulated in vitro upon TLR-2 ligation by FLS [67]. This cell type appears to play an additional role in the spatial organization of lymphocytes, once recruited, through the secretion of stromal-cell-derived factor-1 (SDF-1; CXCL12) [68]. Both FLSs and synovial macrophages can produce CXCL8 (IL-8), which recruits T cells, basophils and neutrophils, and macrophages may also be involved in B-cell recruitment via the secretion of CXCL13 (B-cell-attractant-1; BCA-1). Finally, FLSs have been shown capable of autoantigen presentation to T cells [69]. It is apparent that FLSs represent an important and previously overlooked player in synovitis initiation, propagation or both, and synovial stromal cells are as a whole instrumental in creating a microenvironment that favours inflammatory-cell retention and the perpetuation of immune pathology in RA [70]. Indeed, as we learn more of the molecular processes that initiate inflammation, there is optimism that TLR signalling pathways will provide novel therapeutic targets for the treatment of the condition [71].
Vascular factors
The role of the synovial vasculature in the pathogenesis of RA has also gained prominence in recent years. One tantalizing hypothesis, which arises from elegant mouse work, is that changes in local vascular permeability might provide a route via which a systemic autoimmune propensity becomes focused on the synovium [72]. In any event angiogenesis within the hypertrophic synovial sublining is an important early event in RA, driven by the increased metabolic demands and hypoxia of the expanding inflammatory tissue [73]. New vessels in the RA pannus are of a characteristic branching morphology [74]. This is thought to result from the unfavourable co-expression by intimal FLSs and vascular endothelial cells (ECs) of the angiogenic factor VEGF (vascular endothelial growth factor), and the angiopoietin-1/2–TIE2 complex [73]. VEGF and angiopoietin-2 together promote the invasive proliferation of ECs, and inhibition of this process may be one mechanism of anti-TNFα drug activity [75]. It is hoped that the complex paracrine pathways that promote angiogenesis may yield additional therapeutic targets [76].
The role of adaptive immunity
T cells are one of the most abundant cell types in RA synovium, comprising 30–50% of synovial tissue cells [77]. The majority are CD4+, although CD8+ T cells are also present and may be of pathogenetic importance [78]. At least amongst ACPA-positive individuals, the established genetic associations heavily implicate the CD4+ T ‘helper’ cell in the disease process. Naïve CD4+ T cells can differentiate into at least four broadly classified functional subgroups. In health, Th1 cells secrete interferon γ (IFNγ) and defend against intracellular bacteria; IL-4-secreting Th2 cells combat extracellular parasites; and the newly identified Th17 subset appears to defend against extracellular bacteria such as Klebsiella pneumoniae; a further subset of regulatory T cells (T-regs) has a vital role in immune tolerance and the modulation of established immune responses.
It now seems likely that Th17 cells represent a significant player in human autoimmune inflammation [79]. These cells survive in an environment characterized by IL-6, TGFβ and IL-23 [80], where they secrete IL-17. Through direct or indirect effects on various cell types, this highly pleiotropic cytokine can induce inflammation, angiogenesis, osteoclastogenesis, and breakdown of bone and cartilage. Several studies have demonstrated elevated IL-17 levels in blood and synovium of RA patients, with correlations between synovial levels and joint damage [81,82]. T-regs appear to be overrepresented in the synovium of RA patients, and evidence suggests that this may reflect their functional impairment within the autoimmune microenvironment they are supposed to suppress [83]. Moreover, a compelling body of evidence now suggests that the T-cell population as a whole is systemically abnormal in RA, having an excessive proliferative history; the result is a contracted T-cell receptor repertoire and an immunosenescent phenotype [84]. Intriguingly, such premature ageing of the immune system appears to predate the onset of clinical RA, and whether or not it represents a significant aetiological factor remains controversial.
The success of rituximab in the treatment of RA has confirmed the importance of B cells in disease pathogenesis [85]. Ectopic germinal-centre-like structures in the synovia of some patients create a spatially organized microenvironment ideally suited to humoral immune responses. B cells can process and present antigenic peptides to pre-primed CD4+ T cells, resulting in classical adaptive humoral responses, and similar cognate interactions with B cells may prime naive T cells in some cases [85]. In fact, co-ligation of the B-cell receptor (BCR) and TLR appears sufficient to induce B-cell activation and antibody production independent of T-cell ‘help’, and this pathway is a proven mechanism of rheumatoid factor (RF) autoantibody generation in experimental arthritis [86]. RF recognizes the Fc portion of IgG molecules and is a characteristic, although not specific, hallmark of human RA, with approximately 70% of patients being ‘seropositive’ [87]. Deposition of RF immune complexes occurs in the rheumatoid synovium, where these complexes fix complement, thereby reinforcing B-cell activation and perpetuating an Fc-receptor-mediated positive-feedback loop [85]. Rituximab therapy is associated with reductions in RF titres, but efficacy does not require complete autoantibody eradication, and neither is it absolutely confined to the seropositive population [88]. As with ACPA, the precise role of this autoantibody has therefore yet to be fully elucidated, but the RA synovium appears to provide a context in which B-cell tolerance may be broken, autoantibody production enhanced, and an aberrant immune response upheld.
A final common pathway
Cells of the monocyte–macrophage lineage and FLSs both represent important effectors of cartilage and bone destruction in RA. A number of immune pathways converge on these cell types, resulting in the deregulated production of pro-inflammatory cytokines such as TNFα and IL1β. TLR ligation [89,90], contact with activated T cells [91], cytokines including IL-17 [92], Fcγ-receptor ligation by immune complexes, and the action of serine proteinases secreted by mast cells and neutrophils probably all contribute [93]. Pro-inflammatory cytokines induce chondrocytes to secrete cartilage-degrading enzymes, including matrix metalloproteinases (MMP) 1 and 13 and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) 4 and 5. In fact, in the context of such an inflammatory milieu, macrophages and FLSs adopt an ability to produce cartilage-degrading enzymes directly. This is most evident at the interface between cartilage and inflamed synovium – the so-called cartilage–pannus junction (CPJ), accounting for the striking invasiveness of the inflammatory lesion in RA [94].
In the presence of macrophage colony-stimulating factor (M-CSF), and following engagement of surface receptor activator of NFκB (RANK) by RANK ligand (RANKL), macrophages coalesce into multinucleated syncytia or ‘giant cells’ uniquely equipped for bone resorption. Known as osteoclasts, these cells are now widely acknowledged to be the primary mediators of periarticular bone erosion in inflammatory arthritis [95]. Induced by pro-inflammatory cytokines, RANKL is expressed in abundance by TH17 cells and the activated FLSs of the CPJ. Its expression is also enhanced by the presence of pro-inflammatory cytokines and, it seems, TLR ligation [90].
Summary
Evolving insights into the pathogenesis of the clinical entity we call ‘rheumatoid arthritis’ indicate that we are on the verge of being in a position to ‘split’ a hitherto ‘lumped’ collection of conditions which share a degree of phenotypic overlap according to criteria that are of genuine aetiopathogenetic significance and clinical value (Fig. 2). There is every reason to hope that continued progress in this area will yield new paradigms for the treatment of the condition, with the choice of therapeutic agent being guided by the cellular and molecular peculiarities of the individual patient – the true realization of ‘personalized medicine’. Tools for stratifying RA into meaningful subsets in this way during the earliest phases of the disease are desperately needed, and are the subject of intense investigation. Researching the pathogenesis of early RA is not for the faint-hearted, but the potential gains in terms of outcomes for our patients, not to mention health economics, should make the pursuit worthwhile.
Practice points.
-
•
recently discovered genetic associations have provided new insights into RA pathogenesis
-
•
many associations relate to the subset of patients who are ACPA autoantibody-positive, suggesting distinct aetiologies for different disease subsets
-
•
genetic and environmental influences lead to deregulation of several cellular and molecular pathways, resulting in persistent joint inflammation and destruction.
Research agenda.
-
•
a better understanding of the various predisposing and triggering factors in early RA should yield biomarkers of prognostic and therapeutic utility for this clinically heterogeneous condition
-
•
the precise relevance of ACPA autoantibody production amongst a subset of RA patients remains an area of intense interest, despite considerable recent advances in the field; insights into the pathogenesis of ACPA-negative RA remain elusive.
Acknowledgement
Dr Pratt's work is funded by a Clinical Research Fellowship from the Arthritis Research Campaign.
References
- 1.John S., Myerscough A., Marlow A. Linkage of cytokine genes to rheumatoid arthritis. Evidence of genetic heterogeneity. Ann Rheum Dis. 1998;57:361–365. doi: 10.1136/ard.57.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacGregor A.J., Snieder H., Rigby A.S. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43:30–37. doi: 10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994;56:389–466. doi: 10.1016/s0065-2776(08)60456-3. [DOI] [PubMed] [Google Scholar]
- 4.Hall F.C., Weeks D.E., Camilleri J.P. Influence of the HLA-DRB1 locus on susceptibility and severity in rheumatoid arthritis. QJM. 1996;89:821–829. doi: 10.1093/qjmed/89.11.821. [DOI] [PubMed] [Google Scholar]
- 5.Turesson C., Schaid D.J., Weyand C.M. The impact of HLA-DRB1 genes on extra-articular disease manifestations in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1386–R1393. doi: 10.1186/ar1837. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jawaheer D., Seldin M.F., Amos C.I. Screening the genome for rheumatoid arthritis susceptibility genes: a replication study and combined analysis of 512 multicase families. Arthritis Rheum. 2003;48:906–916. doi: 10.1002/art.10989. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis F., Faure S., Martinez M. New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci U S A. 1998;95:10746–10750. doi: 10.1073/pnas.95.18.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiozawa S., Hayashi S., Tsukamoto Y. Identification of the gene loci that predispose to rheumatoid arthritis. Int Immunol. 1998;10:1891–1895. doi: 10.1093/intimm/10.12.1891. [DOI] [PubMed] [Google Scholar]
- 9.van der Helm-van Mil A.H.M., Huizinga T.W.J., Schreuder G.M.T. An independent role of protective HLA class II alleles in rheumatoid arthritis severity and susceptibility. Arthritis Rheum. 2005;52:2637–2644. doi: 10.1002/art.21272. [DOI] [PubMed] [Google Scholar]
- 10.Feitsma A.L., Worthington J., van der Helm-van Mil A.H.M. Protective effect of noninherited maternal HLA-DR antigens on rheumatoid arthritis development. Proc Natl Acad Sci U S A. 2007;104:19966–19970. doi: 10.1073/pnas.0710260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanberg M., Lidman O., Padyukov L. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet. 2005;37:486–494. doi: 10.1038/ng1544. [DOI] [PubMed] [Google Scholar]
- 12.Newton J.L., Harney S.M.J., Wordsworth B.P., Brown M.A. A review of the MHC genetics of rheumatoid arthritis. Genes Immun. 2004;5:151–157. doi: 10.1038/sj.gene.6364045. [DOI] [PubMed] [Google Scholar]
- 13.Kilding R., Iles M.M., Timms J.M. Additional genetic susceptibility for rheumatoid arthritis telomeric of the DRB1 locus. Arthritis Rheum. 2004;50:763–769. doi: 10.1002/art.20043. [DOI] [PubMed] [Google Scholar]
- 14.Deighton C.M., Walker D.J., Griffiths I.D., Roberts D.F. The contribution of HLA to rheumatoid arthritis. Clin Genet. 1989;36:178–182. doi: 10.1111/j.1399-0004.1989.tb03185.x. [DOI] [PubMed] [Google Scholar]
- 15.Begovich A.B., Carlton V.E.H., Honigberg L.A. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michou L., Lasbleiz S., Rat A.-C. Linkage proof for PTPN22, a rheumatoid arthritis susceptibility gene and a human autoimmunity gene. Proc Natl Acad Sci U S A. 2007;104:1649–1654. doi: 10.1073/pnas.0610250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vang T., Congia M., Macis M.D. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki E., Awata T., Ikegami H. Systematic search for single nucleotide polymorphisms in a lymphoid tyrosine phosphatase gene (PTPN22): association between a promoter polymorphism and type 1 diabetes in Asian populations. Am J Med Genet A. 2006;140:586–593. doi: 10.1002/ajmg.a.31124. [DOI] [PubMed] [Google Scholar]
- [19].Wellcome Trust Case Control C Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson W., Barton A., Ke X. Rheumatoid arthritis association at 6q23. Nat Genet. 2007;39:1431–1433. doi: 10.1038/ng.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plenge R.M., Cotsapas C., Davies L. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39:1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plenge R.M., Seielstad M., Padyukov L. TRAF1-C5 as a risk locus for rheumatoid arthritis – a genomewide study. N Engl J Med. 2007;357:1199–1209. doi: 10.1056/NEJMoa073491. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurreeman F.A.S., Padyukov L., Marques R.B. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med. 2007;4:e278. doi: 10.1371/journal.pmed.0040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barton A., Thomson W., Ke X. Re-evaluation of putative rheumatoid arthritis susceptibility genes in the post-genome wide association study era and hypothesis of a key pathway underlying susceptibility. Hum Mol Genet. April 22 2008 doi: 10.1093/hmg/ddn128. Advance Access Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han S., Li Y., Mao Y., Xie Y. Meta-analysis of the association of CTLA-4 exon-1 + 49A/G polymorphism with rheumatoid arthritis. Hum Genet. 2005;118:123–132. doi: 10.1007/s00439-005-0033-9. [DOI] [PubMed] [Google Scholar]
- 26.Sansom D.M., Walker L.S.K. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A., Yamada R., Chang X. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki A., Yamada R., Yamamoto K. Citrullination by peptidylarginine deiminase in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:323–339. doi: 10.1196/annals.1422.034. [DOI] [PubMed] [Google Scholar]
- 29.Remmers E.F., Plenge R.M., Lee A.T. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becskei A., Grusby M.J. Contribution of IL-12R mediated feedback loop to Th1 cell differentiation. FEBS Lett. 2007;581:5199–5206. doi: 10.1016/j.febslet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver J.E., Silman A.J. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35:169–174. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura K., Sugiyama D., Kogata Y. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [see comment] [DOI] [PubMed] [Google Scholar]
- 33.Nielen M.M.J., van Schaardenburg D., Reesink H.W. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 34.Meyer O., Nicaise-Roland P., Santos M.D. Serial determination of cyclic citrullinated peptide autoantibodies predicted five-year radiological outcomes in a prospective cohort of patients with early rheumatoid arthritis. Arthritis Res Ther. 2006;8:R40. doi: 10.1186/ar1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masson-Bessiere C., Sebbag M., Girbal-Neuhauser E. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 36.Huizinga T.W.J., Amos C.I., van der Helm-van Mil A.H.M. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum. 2005;52:3433–3438. doi: 10.1002/art.21385. [DOI] [PubMed] [Google Scholar]
- 37.Plenge R.M., Padyukov L., Remmers E.F. Replication of putative candidate-gene associations with rheumatoid arthritis in >4000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet. 2005;77:1044–1060. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stolt P., Bengtsson C., Nordmark B. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Helm-van Mil A.H.M., Verpoort K.N., Breedveld F.C. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–1121. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 40.van der Helm-van Mil A.H.M., Verpoort K.N., le Cessie S. The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum. 2007;56:425–432. doi: 10.1002/art.22373. [DOI] [PubMed] [Google Scholar]
- [41].Kallberg H., Padyukov L., Plenge R.M. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007;80:867–875. doi: 10.1086/516736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H.-S., Irigoyen P., Kern M. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56:1745–1753. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 43.Arnett F.C., Edworthy S.M., Bloch D.A. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 44.van Oosterhout M., Bajema I., Levarht E.W.N. Differences in synovial tissue infiltrates between anti-cyclic citrullinated peptide-positive rheumatoid arthritis and anti-cyclic citrullinated peptide-negative rheumatoid arthritis. Arthritis Rheum. 2008;58:53–60. doi: 10.1002/art.23148. [DOI] [PubMed] [Google Scholar]
- 45.van Dongen H., van Aken J., Lard L.R. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–1432. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 46.Hill J.A., Southwood S., Sette A. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- [47].Klareskog L., Stolt P., Lundberg K. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [see comment] [DOI] [PubMed] [Google Scholar]
- 48.Vossenaar E.R., Radstake T.R., van der Heijden A. Expression and activity of citrullinating pepridylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ireland J., Herzog J., Unanue E.R. Cutting edge: unique T cells that recognize citrullinated peptides are a feature of protein immunization. J Immunol. 2006;177:1421–1425. doi: 10.4049/jimmunol.177.3.1421. [DOI] [PubMed] [Google Scholar]
- 50.Foulquier C., Sebbag M., Clavel C. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 51.Verpoort K.N., van Gaalen F.A., van der Helm-van Mil A.H.M. Association of HLA-DR3 with anti-cyclic citrullinated peptide antibody-negative rheumatoid arthritis. Arthritis Rheum. 2005;52(10):3058–3062. doi: 10.1002/art.21302. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi N., Takahashi T., Hata H. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 53.Hata H., Sakaguchi N., Yoshitomi H. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kraan M.C., Versendaal H., Jonker M. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 55.Tak P.P., Smeets T.J., Daha M.R. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [see comment] [DOI] [PubMed] [Google Scholar]
- 56.Thomas R., Davis L.S., Lipsky P.E. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994;152:2613–2623. [PubMed] [Google Scholar]
- 57.Leung B.P., Conacher M., Hunter D. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol. 2002;169:7071–7077. doi: 10.4049/jimmunol.169.12.7071. [DOI] [PubMed] [Google Scholar]
- 58.Cope A.P., Patel S.D., Hall F. T cell responses to a human cartilage autoantigen in the context of rheumatoid arthritis-associated and nonassociated HLA-DR4 alleles. Arthritis Rheum. 1999;42:1497–1507. doi: 10.1002/1529-0131(199907)42:7<1497::AID-ANR25>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 59.van Bilsen J.H., van Dongen H., Lard L.R. Functional regulatory immune responses against human cartilage glycoprotein-39 in health vs. proinflammatory responses in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:17180–17185. doi: 10.1073/pnas.0407704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balandraud N., Roudier J., Roudier C. Epstein-Barr virus and rheumatoid arthritis. Autoimmun Rev. 2004;3(5):362–367. doi: 10.1016/j.autrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Seibl R., Birchler T., Loeliger S. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai T., Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Termeer C., Benedix F., Sleeman J. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J.S., Gamboni-Robertson F., He Q. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- [65].Sacre S.M., Andreakos E., Kiriakidis S. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol. 2007;170:518–525. doi: 10.2353/ajpath.2007.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vergunst C.E., Tak P.P. Chemokines: their role in rheumatoid arthritis. Curr Rheumatol Rep. 2005;7:382–388. doi: 10.1007/s11926-005-0026-7. [DOI] [PubMed] [Google Scholar]
- 67.Pierer M., Rethage J., Seibl R. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172:1256–1265. doi: 10.4049/jimmunol.172.2.1256. [erratum appears in J Immunol. 2004 Feb 15;172:following 2703] [DOI] [PubMed] [Google Scholar]
- 68.Bradfield P.F., Amft N., Vernon-Wilson E. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8 + T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–2482. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 69.Tran C.N., Davis M.J., Tesmer L.A. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis Rheum. 2007;56:1497–1506. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- [70].Burman A., Haworth O., Hardie D.L. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–1700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Neill L.A.J. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3:396–403. doi: 10.1016/s1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 72.Binstadt B.A., Patel P.R., Alencar H. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7(3):284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 73.Paleolog E.M. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl. 3):S81–S90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Reece R.J., Canete J.D., Parsons W.J. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999;42:1481–1484. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 75.Markham T., Mullan R., Golden-Mason L. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy. J Am Acad Dermatol. 2006;54:1003–1012. doi: 10.1016/j.jaad.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 76.Veale D.J., Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol. 2006;20:941–947. doi: 10.1016/j.berh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Zvaifler N.J., Boyle D., Firestein G.S. Early synovitis – synoviocytes and mononuclear cells. Semin Arthritis Rheum. 1994;23(6 Suppl. 2):11–16. doi: 10.1016/0049-0172(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 78.Kang Y.M., Zhang X., Wagner U.G. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weaver C.T., Harrington L.E., Mangan P.R. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Bettelli E., Carrier Y., Gao W. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 81.Ziolkowska M., Koc A., Luszczykiewicz G. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 82.Kirkham B.W., Lassere M.N., Edmonds J.P. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- [83].Sarkar S., Fox D.A. Regulatory T cell defects in rheumatoid arthritis. Arthritis Rheum. 2007;56:710–713. doi: 10.1002/art.22415. [comment] [DOI] [PubMed] [Google Scholar]
- 84.Goronzy J.J., Weyand C.M. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- [85].Edwards J.C.W., Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 86.Leadbetter E.A., Rifkin I.R., Hohlbaum A.M. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [see comment] [DOI] [PubMed] [Google Scholar]
- 87.Carson D.A., Lawrance S., Catalano M.A. Radioimmunoassay of IgG and IgM rheumatoid factors reacting with human IgG. J Immunol. 1977;119:295–300. [PubMed] [Google Scholar]
- 88.Cohen S.B., Emery P., Greenwald M.W. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 89.Huang Q., Ma Y., Adebayo A., Pope R.M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 90.Kim K.-W., Cho M.-L., Lee S.-H. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110:54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Burger D., Dayer J.-M. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann N Y Acad Sci. 2002;966:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- 92.Hwang S.-Y., Kim J.-Y., Kim K.-W. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6:R120–R128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McInnes I.B., Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 94.Karouzakis E., Neidhart M., Gay R.E., Gay S. Molecular and cellular basis of rheumatoid joint destruction. Immunol Lett. 2006;106:8–13. doi: 10.1016/j.imlet.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Schett G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res Ther. 2007;9:203. doi: 10.1186/ar2110. [DOI] [PMC free article] [PubMed] [Google Scholar]