Abstract
The success of an invasive species becoming established in a new region often depends on its interactions with ecologically similar resident species. The propensity of the newly-established mosquito Aedes japonicus to inhabit rock pools throughout the eastern United States provides a natural setting for interspecific larval competition with the native Aedes atropalpus. A laboratory experiment conducted in simulated rock pools to evaluate larval interactions between and within these two species suggested that the performance of both species was more significantly impacted by intraspecific conditions than interspecific conditions of the same mosquito density. Aedes atropalpus was apparently more sensitive to larval densities than Ae. japonicus because it reproduces autogenously, requiring a lengthened period of larval development to obtain nutrient reserves for egg development, which may ultimately put Ae. atropalpus at a disadvantage under larval conditions of competition and limited resources. Excessively stressful experimental conditions, as evidenced by reduced body size, and thus fecundity and estimated finite rate of increase, may have obscured the effects of larval competition between these species. The impact of larval competition between these species in rock pool communities warrants further investigation under more ecologically realistic experimental conditions.
Keywords: invasive, mosquito, rock pool, competition
INTRODUCTION
Within its native range in Asia, Aedes japonicus (Theobald) larvae are found in a wide variety of natural and artificial containers, however rock pools appear to be the most favored habitat (LaCasse and Yamaguti 1950, Tanaka et al. 1979). Immatures of this species have occupied a similar ecological niche since its invasion of the United States in 1998 via used tire shipments (Peyton et al. 1999, Andreadis et al. 2001) from Japan (Fonseca et al. 2001). Aedes japonicus have since been detected along the east coast with reports as far south as Alabama (Mullen 2005), north to Maine (Foss and Dearborn 2001), and west to Missouri (Gallitano et al. 2006). This species appears to have become established on the west coast in Washington (Roppo et al. 2004) and has recently been detected in Mississippi, Nevada (Moore, personal communication), and Hawaii (Larish and Savage 2005). As an invasive species, the likelihood of Ae. japonicus establishing and propagating in its new range depends partly on resource availability and its ability to compete with ecologically similar resident species. Interspecific competition is instrumental in determining the outcome of an invasion, regardless of whether it promotes or limits the spread of a newly-established species, and may only be avoided if an invader is filling a vacant niche by exploiting a previously unoccupied habitat or unused resource (Williamson 1996).
The effects of interspecific competition due to severe crowding and limited resources among the larvae of container-inhabiting mosquitoes have been well documented (e.g., Livdahl and Willey 1991, Barrera 1996, Juliano 1998, Braks et al. 2004, Juliano et al. 2004, Costanzo et al. 2005); however the majority of these experimental investigations concerned the invasion of Aedes albopictus. Such studies have demonstrated both the success (O’Meara et al. 1995, Juliano et al. 2004) and failure (O’Meara et al. 1989, Lounibos et al. 2003) of invasive species to spread. Interspecific competition may potentially impact the overall population performance of container-inhabiting species by affecting growth, survivorship, and reproductive success (Juliano and Lounibos 2005). Under conditions of severe interspecific competition, such effects may lead to the decline or elimination of a resident species following the introduction of a competitively superior invader (e.g., Juliano 1998).
Due to the propensity of Ae. japonicus to inhabit rock pools, the indigenous mosquito most likely to be affected by the invasion of this species is the North American rock pool mosquito, Ae. atropalpus (Coquillett). The two species are sympatric in parts of the eastern United States. Frequent and abundant collections of Ae. japonicus larvae co-occurring with Ae. atropalpus in rock pools (Andreadis et al. 2001, Bevins 2007) indicate the potential for interspecific larval resource competition, which may limit the success of the recent invader (Juliano and Lounibos 2005). However, recent routine mosquito surveillance data and field studies have indicated that competitive displacement of Ae. atropalpus by Ae. japonicus may be occurring in rock pools in New Jersey (Scott et al. 2001), North Carolina (B. Harrison, personal communication), and Virginia (unpublished data). Because of the specialized primary larval habitat of Ae. atropalpus in rock pools, this species tends to be sparsely and irregularly distributed despite its large geographical range. Such conditions may promote the localized decline or extinction of this species in areas where these two species co-occur, particularly if Ae. japonicus is a superior competitor. However, as noted in Lounibos (2002), Ae. atropalpus has expanded its distribution by adapting to discarded tires and has itself been considered an invasive species. While interspecific larval resource competition is a likely mechanism for competitive displacement (Lounibos 2007), no findings on competition between these two species have been published. Thus a laboratory investigation was conducted on these species to detect intra- and interspecific effects of larval density on survivorship, development time, body size, and population growth.
MATERIALS AND METHODS
The experiment was conducted in an insectary at the Florida Medical Entomology Laboratory in Vero Beach, FL, under controlled conditions of 25.5±0.01° C, 86.7±0.1% RH, and a 12L:12D photoperiod, from October to November, 2006. Ambient temperature was monitored hourly for the duration of the experiment with a single Onset HOBO data logger (Onset Computer Corporation, HOBO Data Logger Company, Bourne, MA). The Ae. japonicus and Ae. atropalpus used in this experiment were harvested from eggs obtained from colonies maintained at the Headlee Research Laboratory Mosquito Research and Control Unit at Rutgers University in New Brunswick, NJ. The Ae. japonicus colony originated from larval collections from a horse farm in New Egypt, Ocean County, NJ, in 2000, and was supplemented with additional field-collected larvae from the same location in 2001 (L. McCuiston, personal communication). The Ae. japonicus colony has been maintained through the provision of bloodmeals from quail. The Ae. atropalpus colony originated from larvae collected in 1995 from Monmouth, Salem, Cumberland, and Burlington Counties, NJ, and has been maintained solely by autogenous reproduction (L. McCuiston, personal communication). The number of generations could not be obtained for either species.
To investigate inter- and intraspecific larval competition, the development of larvae in surrogate rock pools in different density-species combinations was monitored. Surrogate rock pools were constructed within plastic planters (91.4 × 18 × 12 cm) using a fast setting, high strength concrete mix consisting of coarse sand aggregate, and cement (Sakrete®, Charlotte, NC). Approximately 15 kg of concrete mixed with 1.6 liters of water were poured into each of six planters, and within each, five individual indentations (7 cm deep with a diameter of 11.5 cm) were made using glass canisters to create the rock pools. Field surveys in Fairfax County, VA, indicated that these species occur in rock pools of similar shape and size in nature. Once dry, each planter was completely flooded with water for 24 h to ensure structural reliability. A randomized complete block design was used for the experiment, with five density combinations of Ae. japonicus:Ae. atropalpus (20:0, 60:0, 0:20, 0:60, and 30:30) as treatments and individual planters as blocks. Each planter contained one replicate of each density-species composition combination in a separate rock pool, for a total of six replicates per treatment and 30 individual rock pools. Two months prior and up to the start of the experiment, surrogate rock pools were flooded with water to allow for any potentially toxic chemicals to leech out of the cement in an effort to reduce or prevent larval mosquito mortality. It should be noted that the selection of the density combinations used for this experiment was somewhat arbitrary, as natural densities were not available from field surveillance data. An extensive field survey of rock pools conducted in Northern Virginia from May to October, 2006 uncovered only four such habitats containing Ae. atropalpus, two of which were also inhabited by Ae japonicus larvae (unpublished data).
On 19 October, each planter was randomly labeled with a number, and each of its rock pools was randomly labeled with a letter corresponding to one of the five treatments. Food consisted of fallen pin oak leaves (Quercus palustris) that had been collected in Fairfax, VA, washed, and dried at room temperature for one week prior to quartering, weighing, and sorting. Four days prior to the start of the experiment, 1.0 g of leaves was added with 300 ml of distilled water to each of the 30 rock pools. The appropriate water level was marked in each rock pool, and was checked every five days for evaporation and refilled with distilled water as necessary. Each planter was covered with fiberglass screen (0.5 mm) and secured with a large rubber band to prevent entry of other macrofauna. In the laboratory, eggs of Ae. japonicus and Ae. atropalpus were synchronously hatched (Novak and Shroyer 1978), and 24 h later larvae were counted into aliquots of 20, 30, and 60. Within one h after counting, the larvae were distributed into appropriate rock pools.
Each rock pool was monitored daily for the presence of pupae, which were collected and housed singly in sealed 10 dram (36.7 ml) vials containing water from their respective rock pools. Each vial was labeled with the appropriate planter and treatment identifier and placed in a rack until adult eclosion. Upon emergence, adults were killed by freezing before scoring by container, species, sex, and day of emergence. The experiment ended on 26 November when the final adult emerged. Survivorship was calculated as the proportion of adults that emerged from the initial cohort of 1st instar larvae. Development time was calculated as the number of days from hatching to adult emergence. Adult body size was estimated from the length of one wing, which was removed from each female and measured under a dissecting microscope with an ocular micrometer (Packer and Corbet 1989).
Data analysis
Population growth correlates
Standardized multivariate analysis of variance (MANOVA, PROC GLM, SAS Institute 1989) techniques for related variables (Scheiner 2001) were used to evaluate the effects of inter- and intraspecific competition on Ae. atropalpus and Ae. japonicus. Separate MANOVAs with three different density-species composition combinations as treatments and six different planters as blocks were performed separately for each species (SAS Institute 1989, PROC GLM). Median development times and body size were used (rather than means) to meet assumptions of homogeneous variance and normality; survivorship data were not transformed. Standard canonical coefficients (SCC) were calculated for each MANOVA and used to assess the contribution of each population growth correlate to the main treatment effect (Scheiner 2001). Post hoc contrasts were performed among the three density-species combinations using significance levels adjusted with the sequential Bonferroni technique for the number of tests done to reduce type I error (experimentwise α = 0.05) (Rice 1989).
Composite Index of Performance (λ’)
The effects of inter- and intraspecific competition on Ae. atropalpus and Ae. japonicus were further evaluated by deriving composite indices of mosquito population performance (λ’) from the survivorship, female development time, and wing length from each density-species combination. This index is an analog of the finite rate of increase as modified from Livdahl and Sugihara (1984) by Juliano (1998):
where N0 is the initial number of females (assumed to be 50% of the cohort), AX is the number of females eclosing on day x, wX is the mean wing length of females eclosing on day x, and f(wX) is a function relating egg production to wing length. D is the time from adult eclosion to reproduction, taken as eight days for Ae. atropalpus and 12 days for Ae. japonicus (personal observation).
Values of λ’ greater than one indicate that the population is increasing, approximately equal to one that the population is stable, and less than one that the population is decreasing. If no individuals survive to reproduction, λ’ equals zero (Léonard and Juliano 1995, Grill and Juliano 1996). A regression relating female wing length to fecundity for Ae. japonicus was obtained from Armistead et al. (2008):
where wX is the wing length in millimeters on day x. Because no suitable equation could be derived from the literature, a size-fecundity regression for Ae. atropalpus was determined experimentally (see below). Randomization ANOVA (Manly 1991, 1997) was used to analyze λ’ as no transformation yielded data that met assumptions of normality and homogeneous variance. Posthoc comparisons of treatment means were not warranted.
Estimation of Ae. atropalpus fecundity from wing length
A regression for Ae. atropalpus was determined experimentally under controlled conditions of 26° C and 12h:12h L:D in an insectary at the Florida Medical Entomology Laboratory in Vero Beach, FL. Aedes atropalpus eggs were hatched simultaneously using a medium of Brewer’s yeast and lactalbumin, and 24 h later larvae were transferred into enamel pans containing approximately 1 liter of distilled water at a density of 100 per pan where they were reared under conditions of varying food availability. Food consisted of pin oak leaves (Q. palustris) from Fairfax, VA, that were collected and dried at room temperature before quartering, weighing, and sorting into 0.5, 1.5, and 3g allotments. These varying food levels were used to generate adult females with a large range of body size, with the specific goal of obtaining very small females, as adult body size is likely to be impacted under stressful larval developmental conditions. Four days prior to the start of the experiment, three allotments of each leaf size were added to the enamel pans. This period allowed for the leaves to soak and be colonized by microorganisms. Because nutrient levels provided in these diet regimes appeared insufficient for development, each larval cohort was fed 100 mg of an artificial diet consisting of one part Brewer’s yeast and one part lactalbumin every other day to ensure completion of development.
Upon pupation, mosquitoes were transferred to plastic cups containing distilled water and placed within 0.028 m3 (30.5 × 30.5 × 30.5 cm) rearing cages. Cotton soaked in a 20% sucrose solution was provided as a source of carbohydrates for adults at all times. Because Ae. atropalpus are obligatorily autogenous in their first egg cycle but may ingest blood for subsequent cycles, blood-feeding was unnecessary. Similar to procedures used by O’Meara and Krasnick (1970) and Telang and Wells (2004), females were killed by freezing 72 h post-eclosion. To provide an estimate of body size for each female, one wing was removed and measured under a dissecting microscope with an ocular micrometer (Packer and Corbet 1989). Ovaries were dissected out and the number of primary follicles matured beyond stage 2b of Clements, similar to Christopher’s stage II, (Christophers 1911, Clements 1992) were counted, as females with follicles advanced beyond this stage are considered autogenous. As others have determined that there is no significant difference between the number of mature follicles counted from dissections and the number of eggs matured (Telang and Wells 2004), this method of fecundity estimation was considered sufficient for Ae. atropalpus.
RESULTS
Population growth correlates
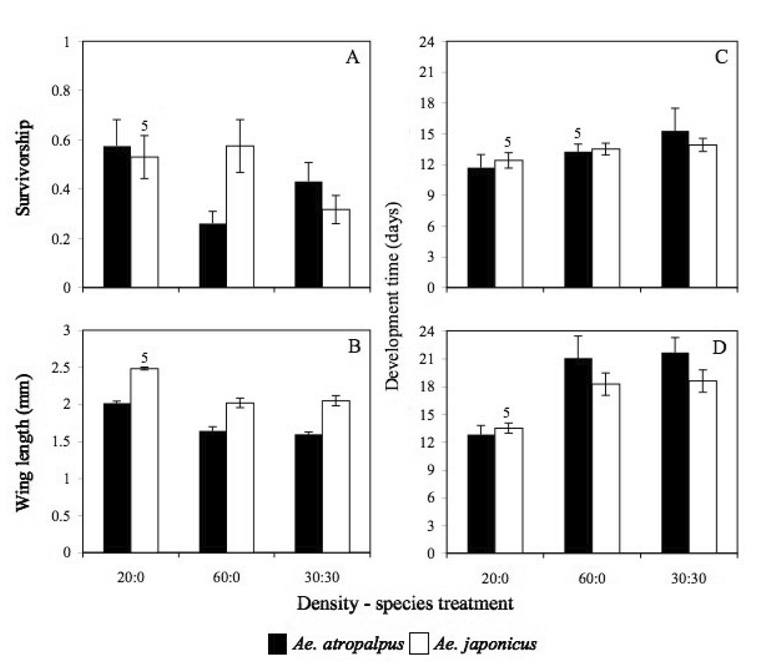
Population growth correlates for both Ae. atropalpus and Ae. japonicus were significantly impacted by density-species treatment but not by planter location (Figure 1, Table 1). Standardized canonical coefficients (Table 1) revealed that significant effects of treatment were primarily manifested in survivorship and female body size (as indicated by wing length) of Ae. atropalpus and female body size and male development time of Ae. japonicus. Posthoc pairwise contrasts based on the combination of all population growth correlates revealed that the population performance of both species was impacted most under high-density intraspecific conditions, which was significantly different than the high-density interspecific treatment and the low-density intraspecific treatment for both species. There was no significant difference between the 20:0 and 30:30 treatments for either species (Table 1).
Figure 1.
Population growth correlates for Ae. atropalpus and Ae. japonicus. (A) Survivorship (proportion of the original number of larvae surviving to adulthood). (B) Median wing lengths (mm). (C) Median time to adulthood (in days) for male and (D) female mosquitoes. Values are means ± SE (n = 6 for all treatments in A – D except as noted by numerals above the appropriate bars).
Table 1.
Multivariate analysis of variance (MANOVA) results, posthoc contrasts, and standardized canonical coefficients (SCC) for mosquito population growth correlates.
| Source | MANOVA |
SCC |
|||||
|---|---|---|---|---|---|---|---|
| Pillai’s Trace | df | P | Survival | Male dev. time | Female dev. time | Wing length | |
| Treatment | |||||||
| Ae. atropalpus | 1.31 | 8,22 | 0.0010 | 0.7559 | 0.1533 | 0.1693 | 2.2956 |
| Ae. japonicus | 1.04 | 8,22 | 0.0204 | 0.5008 | 1.1582 | 0.0546 | −1.9322 |
| Planter | |||||||
| Ae. atropalpus | 1.23 | 20,40 | 0.6022 | 0.4697 | −1.2863 | 0.8668 | −0.1508 |
| Ae. japonicus | 1.08 | 20,40 | 0.7617 | −1.8396 | −1.2702 | 1.8971 | 1.6297 |
| Treatment Contrasts | |||||||
| Ae. atropalpus | |||||||
| 20:0 vs 60:0 | 0.84 | 4,10 | 0.0006 | ||||
| 20:0 vs 30:30 | 0.44 | 4,10 | 0.1704 | ||||
| 60:0 vs 30:30 | 0.82 | 4,10 | 0.0008 | ||||
| Ae. japonicus | |||||||
| 20:0 vs 60:0 | 0.80 | 4,10 | 0.0015 | ||||
| 20:0 vs 30:30 | 0.23 | 4,10 | 0.5775 | ||||
| 60:0 vs 30:30 | 0.76 | 4,10 | 0.0039 | ||||
Aedes japonicus females were, in general, larger than Ae. atropalpus females. Median wing length of both species was noticeably reduced in the 60:0 and 30:30 treatments as compared to the 20:0 treatment (Figure 1). Survival of Ae. atropalpus was lowest in the high-density intraspecific treatment, while that of Ae. japonicus was impacted most in the presence of Ae. atropalpus (Figure 1). Mean survivorship of Ae. atropalpus was higher than that of Ae. japonicus in all instances except the 60:0 treatment. Development was completed faster among males and females of both species in the low-density intraspecifc treatment as compared to those in inter- or intraspecific high-density treatments (Figure 1).
Estimation of Ae. atropalpus fecundity from wing length
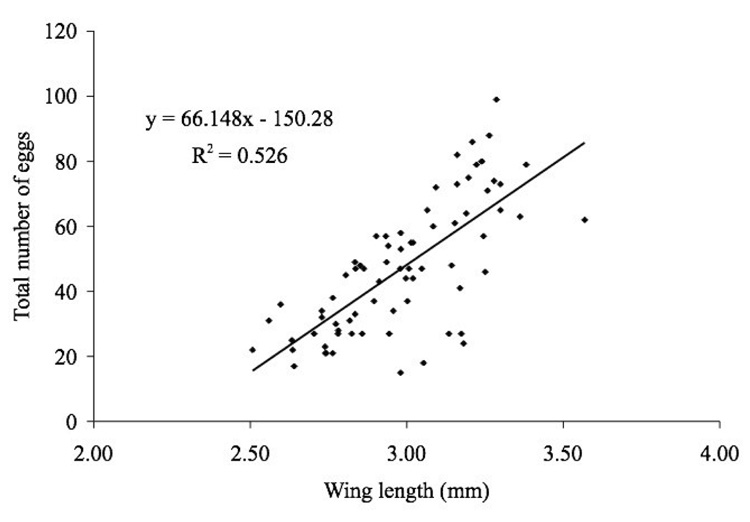
A total of 74 Ae. atropalpus females was examined, all of which contained mature follicles upon dissection, and total egg production per female ranged from 15 to 99 eggs. The mean ± SD total eggs produced per female was 47.41 ± 2.38 eggs. The average wing length of females was 2.99 ± 0.03 mm with a range of 2.51–3.57 mm. A single combined linear regression was obtained for all three larval diet regimes (SAS 1989) and the correlation of wing length and fecundity was determined for Ae. atropalpus (Figure 2). There was a positive correlation between wing length and the number of eggs produced per female (r2 = 0.526), and the following regression equation relating wing length to fecundity was obtained for Ae. atropalpus:
where wX is the wing length in millimeters on day x.
Figure 2.
Relationship between wing length (mm) and total eggs produced for Ae. atropalpus.
Estimated finite rate of increase (λ’)
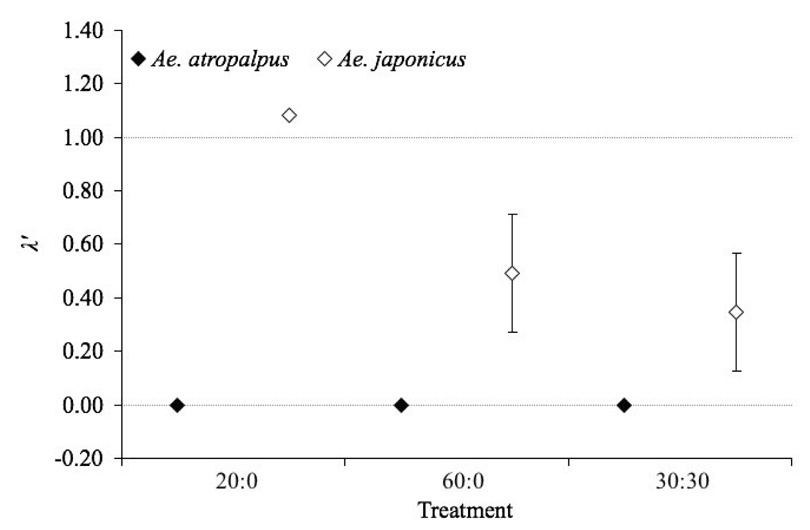
The mean estimated finite rate of increase of Ae. japonicus was not significantly affected by density treatments (F2,10 = 1.45, P = 0.2767). However, λ’ was less than one for both the 60:0 and 30:30 treatments, indicating population decline (Figure 3). Planter location did not affect λ’ for Ae. japonicus (F5,10 = 0.27, P = 0.8435). For Ae. atropalpus, λ’ was zero for all treatments (Figure 3).
Figure 3.
Estimates of population performance (λ’, an estimate of the finite rate of increase for the cohort) for female Ae. atropalpus and Ae. japonicus adults. Values are means ± SE (n = 6 for 60:0 and 30:30 treatments, n = 5 for 20:0 treatment). Randomization ANOVA did not indicate a significant difference in survivorship among treatments for Ae. japonicus. The line at λ’ = 1 is where the population is being replaced, neither increasing nor decreasing, and the line at λ’ = 0 is where no individuals survive to reproductive age.
DISCUSSION
These results suggest that the overall performance of both Ae. atropalpus and Ae. japonicus was most significantly impacted under high-density intraspecific conditions. However, when comparing treatments of the same mosquito density, it appears that survival was slightly better for Ae. atropalpus in the presence of Ae. japonicus than conspecifics, and that the survival of Ae. japonicus was reduced in the presence of Ae. atropalpus. Despite this finding, data suggest that the performance of Ae. atropalpus was more sensitive to larval densities, regardless of whether the other larvae were conspecifics or not, because it requires more resources for autogenous development.
The estimated finite rate of increase for Ae. japonicus was not detectably different under inter- or intraspecific conditions of the same mosquito density. However, the unusually short wing lengths from which the fecundity of females from many replicates was extrapolated to be zero are likely to have obfuscated the true value of λ’. The median wing lengths of females in these experiments, which ranged from approximately 2.0–2.5 mm, were noticeably smaller than those from previous field competition experiments of similar densities with Aedes albopictus (Skuse), where they were approximately 1 mm longer (Armistead et al. 2008). The mean estimated finite rate of increase, λ’, was estimated to be zero for all treatments for Ae. atropalpus, indicating that no individuals were able to reproduce autogenously, while that of Ae. japonicus did not significantly change between intra- and interspecific treatments at the same mosquito density. This suggests better overall population performance for this species, despite reduced survivorship across all experimental treatments. The λ’ values for Ae. atropalpus appear to be the result of small body sizes from which zero fecundity values were extrapolated for all treatments (Figure 3).
Considering the autogenous reproduction of Ae. atropalpus (O’Meara and Craig 1970, O’Meara and Krasnick 1970), in which the fecundity of this species depends on nutrient reserves obtained in the larval stage, these results are not surprising. It is interesting to note that male Ae. atropalpus, on average, developed faster than that of Ae. japonicus, but the reverse applied to females, indicating the need for females of the former species to lengthen larval development to accumulate nutrient reserves for egg production. This suggests that autogenous reproduction of Ae. atropalpus (O’Meara and Craig 1970, O’Meara and Krasnick 1970) may render this species more sensitive to competitive stress. However, while it is assumed that Ae. atropalpus are obligatorily autogenous in their first gonotrophic cycle, it is not known whether small females producing few or no eggs in an autogenous cycle will then seek a bloodmeal to initiate an anautogenous cycle. Thus, without accounting for the possibility of autogenous reproduction in both species, it is unclear whether Ae. japonicus has a reproductive advantage over Ae. atropalpus under these experimental conditions or in nature. Furthermore, the wing lengths of Ae. atropalpus in these experiments did not exceed 2.0 mm, having little or no overlap with the wing lengths used to generate the fecundity-body size regression. While a linear relationship between body size and fecundity was assumed, it is possible that this is not the case, and that very small females may have produced a few eggs. However, none of the small Ae. atropalpus from these experiments were dissected to directly measure fecundity to address this issue.
An attempt was made to simulate the ecological conditions experienced by these species in nature; however the poor population performances of both species, as evidenced by the declining or zero estimated finite rates of increase, suggest that perhaps these experimental conditions were excessively stressful. The use of increased or multiple levels of food, a different food source, or pulse delivery of food in this experiment may have provided experimental conditions more comparable to those in nature in which interspecific competition might be expressed. The primary detrital resource or microorganism (e.g., algae) that drives rock pool mosquito communities is unknown, as is the foraging efficiency and behavior, resistance to starvation, or tolerance to leaf tannins of either Ae. atropalpus or Ae. japonicus, which may also have affected their population performance. Furthermore, it is possible that the cement from which the rock pools were constructed imposed some toxic effect on the mosquito larvae, although it is important to note that survivorship of Ae. japonicus in this experiment did not differ from values observed in the field competition experiment of this species with Ae. albopictus (Armistead et al. 2008).
These results emphasize the importance of experimental conditions and suggest that multiple factors other than interspecific larval resource competition may be important in determining the current abundance and distribution of these species. The cold-hardiness of Ae. japonicus, particularly the ability of larvae to survive at low temperatures for extended time periods (Nakata 1962, Kamimura 1976), may confer an ecological advantage in obtaining resources in the presence of Ae. atropalpus, which diapauses in the egg stage (Hedeen 1953) and emerges later in the season. Furthermore, it may facilitate asymmetric intraguild predation (Edgerly et al. 1999) of newly-hatched Ae. atropalpus larvae by 4th instar Ae. japonicus; however the importance of this ecological process in structuring container-inhabiting mosquito communities has yet to be demonstrated for either species. Predation by diving beetles, which are prominent in rock pools (Shaw and Maisey 1961, James 1964a,b), may play a role. If Ae. atropalpus and Ae. japonicus tend to occupy different spaces within a rock pool (i.e., at the bottom, surface, or in the water column), selective predation (Griswold and Lounibos 2005, 2006) may be important in facilitating the invasion of the latter species in these habitats and should be investigated further. Finally, the tendency of Ae. atropalpus larvae to congregate under leaves and other organic debris at the bottom of rock pools (Hedeen 1953) may allow the species to safely inhabit rock pools fully exposed to the sun, which are more flood prone, therefore partitioning, at least to some extent, rock pool habitats with Ae. japonicus, which is less frequently found under such conditions (B. Byrd, personal communication).
These experimental findings appear ambiguous with respect to the nature of interspecific larval resource competition between Ae. atropalpus and Ae. japonicus because of the stressful experimental conditions. However, a slight competitive advantage for Ae. japonicus seems likely. Numerous factors that may potentially impact interactions among Ae. atropalpus and Ae. japonicus in rock pool communities have been proposed here, however it is important to note that variations in temperature (Lounibos et al. 2002), habitat (Bertness, 1984, Livdahl and Willey 1991), larval density, season (Teng and Apperson 2000), and oviposition attraction and repellency (Maire and Langis 1985, Zahiri et al. 1997) may also influence larval competition differently among mosquito species and warrant further research with respect to interactions between these two species. Ultimately, the outcome of interspecific competition between these two species depends on the frequency of their co-occurrence in both rock pools and other containers in nature. Such co-occurrences will depend on the seasonal distributions, habitat preferences, and overwintering behaviors of these two species in areas where they co-exist. Further investigation of these factors should be made on a large geographical scale, as they may ultimately influence the community structure of the rock pools in which these species coexist.
Acknowledgments
We thank L. McCuiston for providing the Ae. japonicus and Ae. atropalpus eggs used for this research. Thanks also to J. Arias for his input on experimental design, M. Reiskind for his technical assistance, G. O’Meara for advice in rearing Ae. atropalpus, and B. Harrison, B. Byrd, and J. Scott for their input on the ecology of Ae. japonicus. Thanks also to B. Harrison and G. O’Meara for reviewing an early draft of this manuscript. This work was supported by funds from National Institutes of Health grant AI-044793.
REFERENCES CITED
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J. Med. Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Armistead JA, Arias JR, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in Northern Virginia. J. Med. Entomol. 2008;45:629–637. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol. Entomol. 1996;21:117–127. [Google Scholar]
- Bertness MD. Habitat and community modification by an introduced herbivorous snail. Ecology. 1984;65:370–381. [Google Scholar]
- Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Díptera: Culicidae) in the Southern Appalachians, USA. J. Med. Entomol. 2007;44:945–952. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourençode-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Ann. Entomol. Soc. Am. 2004;97:130–139. [Google Scholar]
- Christophers SR. The develoment of the egg follicle in Anopheles. Paludism. 1911;2:73–87. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Vol. 1. London: Chapman and Hall; 1992. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2005;42:559–572. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerly JS, Willey MS, Livdahl T. Intraguild predation among treehole mosquitoes Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. J. Med. Entomol. 1999;36:394–399. doi: 10.1093/jmedent/36.3.394. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Campbell S, Crans WJ, Mogi M, Miyagi I, Toma T, Bullians M, Andreadis TG, Berry RL, Pagac B, Sardelis MR, Wilkerson RC. Aedes (Finlaya) japonicus (Diptera: Culicidae), a newly recognized mosquito in the United States: analyses of genetic variation in the United States and putative source populations. J. Med. Entomol. 2001;38:135–146. doi: 10.1603/0022-2585-38.2.135. [DOI] [PubMed] [Google Scholar]
- Foss KA, Dearborn RG. Technical Report No. 42. Augusta, Maine: Maine Department of Conservation, Maine Forest Service, Forest Health and Monitoring Division; 2001. Preliminary faunistic survey of mosquito species (Diptera: Culicidae) with a focus on population densities and potential breeding sites in greater Portland, Maine; 36 pp. [Google Scholar]
- Gallitano S, Blaustein L, Vonesh J. First occurrence of Ochlerotatus japonicus in Missouri. J. Vector Ecol. 2006;30:347–348. [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J. Anim. Ecol. 1996;65:63–76. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 2005;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeen RA. The biology of the mosquito Aedes atropalpus Coq. J. Kansas Entomol. Soc. 1953;26:1–10. [Google Scholar]
- James HG. Insect and other fauna associated with the rock pool mosquito Aedes atropalpus (Coq.) Mosq. News. 1964a;24:325–329. [Google Scholar]
- James HG. Predators of Aedes atropalpus (Coq.) (Diptera: Culicidae) and of other mosquitoes breeding in rock pools in Ontario. Can. J. Zool. 1964b;43:155–159. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K. On the Japanese species of the family Culicidae. In: Sasa M, editor. Science of Mosquitoes (Japanese) Tokyo, Japan: Hokuryukan; 1976. pp. 150–188. [Google Scholar]
- LaCasse WJ, Yamaguti S. 1950. Mosquito Fauna of Japan and Korea. Parts I and II. Mosquito Survey data on Japan and their application in the control of mosquito-borne diseases. Off. Surg., HQ 1 Corps APO 301 (Japan). [Google Scholar]
- Larish LB, Savage HM. Introduction and establishment of Aedes (Finlaya) japonicus japonicus (Theobald) on the island of Hawaii: Implications for arbovirus transmission. J. Am. Mosq. Contr. Assoc. 2005;21:318–321. doi: 10.2987/8756-971X(2005)21[318:IAEOAF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf litter and density on fitness and population performance of the hole mosquito Aedes triseraitus. Ecol. Entomol. 1995;20:125–136. [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. J. Anim. Ecol. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Competitive displacement and reduction. J. Am. Mosq. Contr. Assoc. Bull. (Suppl. 2) 2007;23(No 7):276–282. doi: 10.2987/8756-971x(2007)23[276:cdar]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Nishimura N, Escher RL. Interactions with native mosquito larvae regulate the production of Aedes albopictus from bromeliads in Florida. Ecol. Entomol. 2003;28:551–558. [Google Scholar]
- Lounibos LP, Suárez S, Menédez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Maire A, Langis R. Oviposition responses of Aedes (Ochlerotatus) communis (Diptera: Culicidae) to larval holding water. J. Med. Entomol. 1985;22:111–112. [Google Scholar]
- Manly BFJ. Randomization and Monte Carlo Methods in Biology. London: Chapman and Hall; 1991. 292 pp. [Google Scholar]
- Manly BFJ. RT: a program for randomization testing. Version 2.1. Cheyenne, WY: West Incorporated; 1997. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Ann. Rev. Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mullen GR. First report of Ochlerotatus japonicus in Alabama. Ala. Vector Manage. Soc. Newsl. 2005;15:2. [Google Scholar]
- Nakata G. Taxonomical and ecological studies on Japanese mosquitoes. Sanitary Injurious Insects. 1962;6:43–173. [Google Scholar]
- Novak RJ, Shroyer DA. Eggs of Aedes triseriatus and A. hendersoni: a method to stimulate optimal hatch. Mosq. News. 1978;38:515–521. [Google Scholar]
- O’Meara GF, Craig GB. Geographical variation in Aedes atropalpus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 1970;63:1392–1400. doi: 10.1093/aesa/63.5.1392. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Krasnick GJ. Dietary and genetic control of the expression of autogenous reproduction in Aedes atropalpus (Coq.) (Diptera: Culicidae) J. Med. Entomol. 1970;7:328–334. doi: 10.1093/jmedent/7.3.328. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Larson VL, Mook DH, Latham MD. Aedes bahamensis: its invasion of south Florida and association with Aedes aegypti. J. Am. Mosq. Contr. Assoc. 1989;5:1–5. [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae) Ecol. Entomol. 1989;14:297–309. [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J. Am. Mosq. Contr. Assoc. 1999;15:238–241. [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Roppo MR, Lilya JL, Maloney FA, Sames WJ. First occurrence of Ochlerotatus japonicus in the state of Washington. J. Am. Mosq. Contr. Assoc. 2004;20:83–84. [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. Version 6. Fourth edition. Volume 2. North Carolina, U.S.A: SAS Institute, Cary ; 1989. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2nd ed. Oxford, UK: Oxford Univsity Press; 2001. pp. 99–133. [Google Scholar]
- Scott JJ, Carle FL, Crans WJ. Ochlerotatus japonicus collected from natural rockpools in New Jersey. J. Am. Mosq. Contr. Assoc. 2001;17:91–92. [PubMed] [Google Scholar]
- Shaw FR, Maisey SA. The biology and distribution of the rockpool mosquito, Aedes atropalpus (Coq.) Mosq. News. 1961;24:12–16. [Google Scholar]
- Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae) Contrib. Am. Entomol. Inst. 1979;16:1–987. [Google Scholar]
- Telang A, Wells MA. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 2004;50:677–685. doi: 10.1016/j.jinsphys.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J. Med. Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Williamson M. Biological Invasions. New York, NY: Chapman and Hall; 1996. 256 pp. [Google Scholar]
- Zahiri N, Rau ME, Lewis DJ. Oviposition responses of Aedes aegypti and Ae. atropalpus (Diptera: Culicidae) females to waters from conspecific and heterospecific normal larvae and from larvae infected with Plagiorchis elegans (Trematoda: Plagiorchiidae) J. Med. Entomol. 1997;34:565–568. doi: 10.1093/jmedent/34.5.565. [DOI] [PubMed] [Google Scholar]