Abstract
Cyclophosphamide-based regimens are front-line treatment for numerous pediatric malignancies, however current dosing methods result in considerable interpatient variability in tumor response and toxicity. In this pediatric population, our objectives were to 1. quantify and explain the pharmacokinetic variability of cyclophosphamide, and two of its metabolites, hydroxycyclophosphamide (HCY) and carboxyethylphosphoramide mustard (CEPM); 2. apply a population pharmacokinetic model to describe the disposition of cyclophosphamide and these metabolites. A total of 196 blood samples were obtained from 22 children with neuroblastoma receiving intravenous (IV) cyclophosphamide (400 mg/m2/day) and topotecan. Blood samples were quantitated for concentrations of cyclophosphamide, HCY and CEPM using liquid chromatography-mass spectrometry and analyzed using nonlinear mixed effects modeling with NONMEM software system. After model building was complete, the area under the concentration-time curve (AUC) was computed using NONMEM. Cyclophosphamide elimination was described by noninducible and inducible routes with the latter producing HCY. Glomerular filtration rate (GFR) was a covariate for the fractional elimination of HCY and its conversion to CEPM. Considerable interpatient variability was observed in the AUC of cyclophosphamide, HCY and CEPM. These results represent a critical first step in developing pharmacokinetic-linked pharmacodynamic studies in children receiving cyclophosphamide to determine the clinical relevance of the pharmacokinetic variability in cyclophosphamide and its metabolites.
Keywords: Cyclophosphamide, metabolite, population pharmacokinetics, pediatric, neuroblastoma
INTRODUCTION
Cyclophosphamide is the cornerstone of curative chemotherapy regimens in over 50% of newly diagnosed pediatric cancer patients. Dose intensification of cyclophosphamide is associated with improved outcome in many of these patients, but is also associated with increased adverse drug reactions (ADRs). The pressing need for improving the safety of cyclophosphamide is highlighted by the impact of a minor dosing modification to the VAC (vincristine/dactinomycin/cyclophosphamide) regimen leading to unexpected increase in ADRs in children less than 3 years old.1 Thus, there is a need to delineate factors in cyclophosphamide metabolism that may impact both response and ADRs to cyclophosphamide-based combination chemotherapy regimens. In adults, there is substantial interpatient variability in the cyclophosphamide area under the plasma concentration-time curve (AUC), and an even greater variability in the AUC of its metabolites when cyclophosphamide is dosed based on body surface area (BSA) or weight.2, 3 Children have more rapid cyclophosphamide clearance normalized to BSA (i.e., ml/min/m2) relative to adults, although the mechanism is unidentified.4–8
Cyclophosphamide predominantly undergoes hepatic elimination, with 25% (range 5–30%) of an IV dose excreted unchanged in the urine.3 A major fraction of cyclophosphamide dose (70%) is metabolized to hydroxycyclophosphamide (HCY) by multiple cytochrome P450 enzymes.3 A minor fraction of cyclophosphamide dose (5%) is metabolized to dechloroethylcyclophosphamide (DCCY) by CYP3A4/5.9 The metabolite HCY exists in equilibrium with its tautomer–aldophosphamide, and the concentrations of these two metabolites are assayed together.10 HCY undergoes intracellular transport, subsequently releasing phosphoramide mustard which covalently cross-links DNA. Phosphoramide mustard does not readily cross cell membranes and thus makes the transport of HCY critical.3 Hydroxycyclophosphamide is metabolized by aldehyde dehydrogenase 1A1 (ALDH1A1) to nontoxic carboxyethylphosphoramide mustard (CEPM).3 CEPM is the predominant urinary metabolite of cyclophosphamide.3
A substantial challenge in seeking a better understanding of cyclophosphamide pharmacokinetics and pharmacodynamics has been the difficulty in quantitating HCY plasma concentrations.10 HCY is extremely unstable in biologic fluids (in vitro half-life in plasma < 3 minutes) and only recently have more clinically applicable methods been developed.11–14 These methods are accurate, sensitive and specific and have since provided reliable tools to advance our understanding of the pharmacokinetic/dynamics of cyclophosphamide and its metabolites in predominantly adult patients receiving myeloablative dose cyclophosphamide prior to hematopoietic cell transplantation.2, 15–19 In this study, we sought to be the first to characterize HCY pharmacokinetics and to develop a population pharmacokinetic model of cyclophosphamide, HCY, and CEPM in children receiving multiple daily doses of cyclophosphamide.
METHODS
Patient population
Twenty-two children (17 male and 5 female) were included in this study. The pharmacokinetics were evaluated after the first cycle of fractionated non-myeloablative dose cyclophosphamide with topotecan, administered under a limited institution pilot protocol, ANBL02P1 which was conducted by the Children’s Oncology Group (Duarte, CA). Institutional Review Board approval was obtained at each individual participating institution, specifically Children’s Hospital and Regional Medical Center (Seattle, WA), Children’s Memorial Hospital Medical Center at Chicago (Chicago, IL), St. Jude Children’s Research Hospital (Memphis, TN) and University of California San Francisco (San Francisco, CA). Children from the Children’s Hospital at Westmead could participate in the clinical trial but were excluded from the cyclophosphamide pharmacokinetic study due to the extended time period (i.e., >48 hours (h)) necessary to transport the plasma samples from Australia to the United States. Prior to study conduct, parental written informed consent was obtained for all children and written assent for those children over 7 years of age was obtained.
All children were diagnosed with neuroblastoma or ganglioneuroblastoma and had no prior systemic therapy. All children had adequate renal (i.e., serum creatinine ≤ 1.5 mg/dL, and creatinine clearance or radioisotope glomerular filtration rate (GFR) ≥ 60 ml/min/1.73 m2) and liver (i.e., total bilirubin ≤ 1.5 mg/dL and alanine aminotransferase < 300 units/L) function. Prior to chemotherapy administration, radioisotope GFR was determined using each individual institution’s procedures.
Chemotherapy regimen
Cyclophosphamide was infused through a central venous access catheter over 30 minutes at a dose of 400 mg/m2/day in those children weighing greater than 12 kilograms or 13.3 mg/kg/day in those children weighing 12 kilograms or less. The body weight and BSA used to calculate doses were based on individual institutional guidelines. Cyclophosphamide was administered daily for 5 days. Cyclophosphamide doses were not adjusted based on pharmacokinetic data. Immediately after each cyclophosphamide infusion was completed, a 30-minute topotecan infusion began and was repeated daily after each dose of cyclophosphamide. Topotecan doses were pharmacokinetically targeted after doses 1 and 3. The success of topotecan targeting and clinical response will be reported in a separate manuscript.
Cyclophosphamide pharmacokinetic sampling and analysis
The blood volume for cyclophosphamide pharmacokinetic analysis was limited by the young age of the patient population and the blood volume needed for pharmacokinetically targeting topotecan doses. Cyclophosphamide pharmacokinetic blood samples were drawn from the lumen not used to infuse the cyclophosphamide dose. Pharmacokinetic blood samples were drawn immediately before, upon completion of the 30-minute infusion, and at 3, 6, and 24 h after the start of the first and fourth cyclophosphamide infusions. The volume of blood drawn was 1 mL for the first four samples and 2 mL for the fifth sample (i.e., 24 h post infusion).
The sample processing procedures were derived from those developed in hematopoietic cell transplant recipients.13, 14 Aliquots of each sample were placed into tubes containing either phenylhydrazine for analysis of HCY or ethylenediaminetetraacetic acid for analysis of cyclophosphamide and CEPM. The tubes were inverted 3 to 4 times, stored at 4°C for a maximum of 1 h and centrifuged. The supernatant was then decanted and stored at −70°C within 1 h of sample collection.
All samples were shipped on dry ice to our laboratory within 3 months. Cyclophosphamide, HCY, and CEPM concentrations in plasma were quantitated separately using the methods of Kalhorn et al13, 14 with minor modifications as described in Table 1. In the case of CEPM, negative ion detection at m/z 293 was used. This ion, although lesser in abundance, has a lower incidence of background interference, allowing for more sensitive detection of CEPM. The HCY-phenylhydrazine derivative was detected in the negative ion mode as the chloride adduct at m/z 401.14
Table 1.
Quantitation of cyclophosphamide and metabolites by liquid chromatography-mass spectrometry
| Analyte | Matrix | Sample preparation | Charge/Ion | Limit of detection | |
|---|---|---|---|---|---|
| FHCRC | Yule et al4–8 | ||||
| Cyclophosphamide | 150 μL plasma | Protein precipitation 1.ml acetonitrile | Positive/261 | 0.6 μM13 | 1 μM |
| HCY | 500 μL whole blood + 1 mL phenylhydrazine | Solid phase extraction | Negative/401 | 0.25 μM14 | Not assessed |
| CEPM | 100 μL plasma | Protein precipitation 150 μL acetonitrile | Negative/293 | 0.25 μM14 | 3 μM |
Population Pharmacokinetic Modeling
Given the small number of blood samples available in each subject, nonlinear mixed effects modeling was used to describe this pharmacokinetic dataset. The nonlinear mixed effects approach explicitly models the time course of the data and the hierarchical statistical variability which is present among subjects and within subjects. Pharmacokinetic models of cyclophosphamide metabolism were fit to all pharmacokinetic data (i.e., cyclophosphamide, HCY and CEPM) from all patients simultaneously by use of the first-order method in the nonlinear mixed-effects modeling software NONMEM (version V, double precision).20 Between-subject variability (BSV) of parameters was modeled by use of a lognormal model. Residual unknown variability (RUV) was estimated by use of additive models for cyclophosphamide and CEPM and a combination of proportional and additive errors for HCY. Individual parameters were calculated by use of the POSTHOC option (empirical Bayes estimates) of NONMEM. A nominal significance level of 0.05 (linked to a difference in objective function value of at least 3.86 points for one additional parameter) was assumed to aid in covariate model selection.
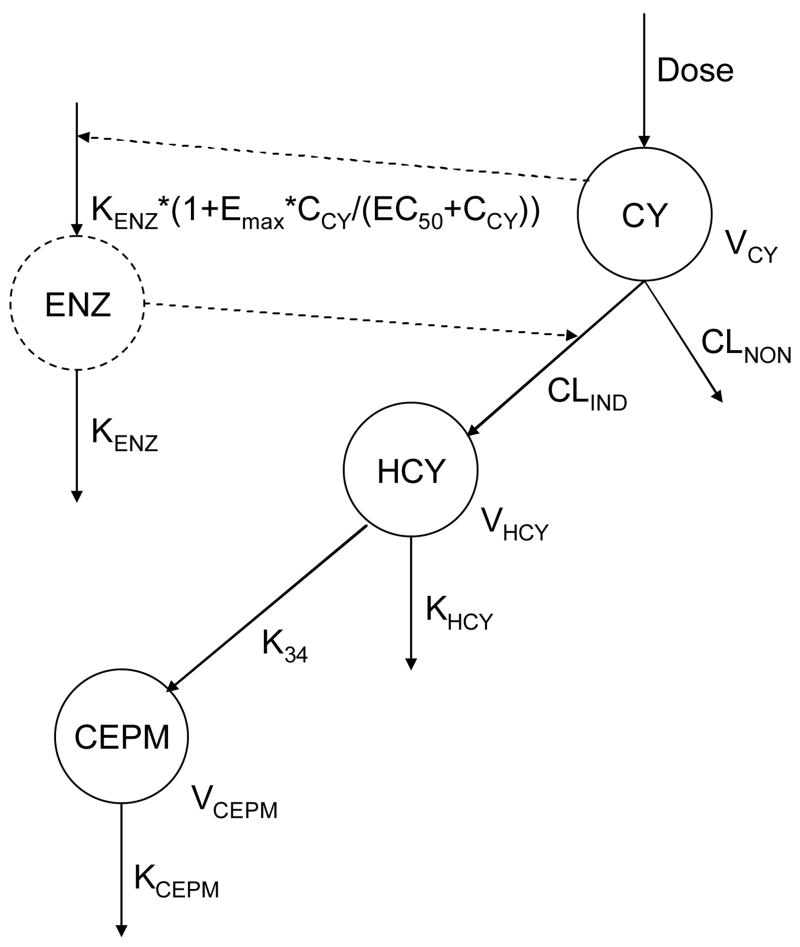
The pharmacokinetic model framework we used is depicted in Figure 1 and its components have been previously described.18, 21 The definition of the different parameters of this model is explained in Table 3. This integrated model simultaneously describes the pharmacokinetics of cyclophosphamide, HCY and CEPM and includes autoinduction of cyclophosphamide to HCY. The concentrations are expressed in μmol/L of cyclophosphamide (with molecular weight 261.1 g/mol), HCY (277.1 g/mol) and CEPM (293 g/mol). Because the model, expressed as a system of four differential equations, is by definition a “mass balance” equation, the modeling is performed in mass units (mg/m2) and the amounts are converted to μmol/L for comparison with data. Similarly, the EC50 is reported in μmol/L of cyclophosphamide, but is converted within the model to mass units.
Figure 1.
Metabolic schema for cyclophosphamide (CY), HCY and CEPM
See Table 3 for explanation of the parameters (and final modeled values). Note, the inducible enzyme formation rate depends on the modeled (time varying) concentration of cyclophosphamide CCY.
Table 3.
Estimates of population pharmacokinetic parameters of model for cyclophosphamide (CY), HCY and CEPM
| Parameter | Designation (units) | Estimate (SE%) | BSV (%) | CY | HCY | CEPM |
|---|---|---|---|---|---|---|
| Volume of distribution of CY | VCY (L/m2) | 13.1 (5.1) | 17.8 | |||
| Noninducible clearance of CY | CLNON (L·h−1·m−2) | 1.83 (30.6) | 61.7 | |||
| Initial inducible clearance of CY | CLIND (L·h−1·m−2) | 1.79 (28.2) | 65.7 | |||
| Enzyme degradation rate | KENZ (h−1) | 6.57E-3(55.3) | 126 | |||
| Volume of distribution of HCY | VHCY (L/m2) | 0.57, fixed | ||||
| HCY elimination rate | KHCY = α + β ·(GFR-GFR*)/GFR* (h−1) | 35.9 | ||||
| - typical value at median GFR | α (h−1) | 47.0 (20.9) | ||||
| - change in KHCY per unit GFR from median | β (h−1) | −26.6 (36.8) | ||||
| K34, CEPM formation rate from HCY | K34 = a + b·(GFR-GFR*)/GFR* (h−1) | 20.3 | ||||
| - typical value at median GFR | a (h−1) | 0.331 (9.2) | ||||
| - change in K34 per unit GFR from median | b (h−1) | −0.234 (14.1) | ||||
| Volume of distribution of CEPM | VCEPM (L/m2) | 0.57, fixed | ||||
| CEPM elimination rate | KCEPM (h−1) | 0.447 (16.0) | 38.1 | |||
| CY concentration of half-maximal induction | EC50 (μmol/L) | 0.6, fixed | ||||
| Maximum induction | EMAX | 5, fixed | ||||
| Residual error | ||||||
| Proportional error (%) | 24.7 | |||||
| Additive error (μmol/L) | 6.2 | 0.295 | 0.389 |
GFR is glomerular filtration rate (ml/min/1.73 m2). GFR* is the median GFR (i.e., 117 ml/min/1.73 m2).
Elimination of cyclophosphamide is described via a noninducible route and an inducible route, with the latter leading to HCY formation. Autoinduction of HCY formation was modeled by use of a hypothetic enzyme compartment as described previously.19, 21–23 The inducible clearance was proportional to the amount of enzyme in this compartment. The “initial” inducible clearance is defined as the inducible clearance at time 0. The enzyme followed a zero-order formation that was enhanced by cyclophosphamide with a maximum fold of induction (Emax)–type relationship and first-order degradation. The initial amount of enzyme was set to a dimensionless value of 1 at time 0.
The initial structural model (Figure 1), with three compartments for cyclophosphamide (CY) and metabolites HCY and CEPM as well as a fourth enzyme compartment to model the inducible clearance, was as described in Qiu and Yao et al21 and in Salinger et al.19
where the concentrations of cyclophosphamide, HCY and CEPM are and respectively and where the model parameters are described in Figure 1 and Table 3. The model departed from that of Qiu and Yao et al21 in the following ways. First, doses were based upon BSA rather than body weight (see “Chemotherapy Regimen” section above). In those two children whose cyclophosphamide dose was based upon weight, the actual dose (mg) administered was divided by that patient’s BSA. Weight and BSA are correlated in this pediatric population (r2=0.97), and thus we would expect similar results using weight-normalized dosing. Second, we made no assumption regarding the influence of the covariate age upon the base model. Third, the volume of distribution of HCY (VHCY) and CEPM (VCEPM) were both assumed based on our previous experience with cyclophosphamide modeling in hematopoietic cell transplant recipients.21, 23. In our previous cyclophosphamide modeling, VHCY and VCEPM could not be estimated23 and a value of 1 L was assumed for adults.21, 23 The average BSA in the original hematopoietic cell transplant population cyclophosphamide pharmacokinetic model was 1.76 m2,21 and thus VHCY and VCEPM were assumed equal to 1 L/1.76 m2 (0.57 L/m2).
As a validation of the model, we performed a visual predictive check wherein data were simulated for 4400 subjects (200 for each of the 22 measured covariate sets) using the final population pharmacokinetic model and the median and 90% prediction intervals plotted with the original data.
Statistical Analysis
Generalized estimating equations were used to evaluate the association of AUC with independent variables that included age, sex, BSA, GFR and day of CY administration. Each analysis was done separately for the AUCs of CY, HCY, and CEPM. SAS Version 9.1 (Cary, NC) was used for all statistical analyses.
RESULTS
Patient Population
Of the 22 patients studied, 17 were male and 5 were female. At the time of diagnosis, the median age was 3.16 years (range: 1.30 – 9.37). At the time of cyclophosphamide infusion, the BSA was 0.62 m2 (range: 0.45 – 1.47), actual body weight was 14.2 kg (range: 8.7 – 52.8), and GFR was 117 ml/min/1.73m2 (range: 78 – 238).
Quantitation of cyclophosphamide and metabolites
Twenty two and twenty patients, respectively, had pharmacokinetic sampling after the first and fourth cyclophosphamide dose. Cyclophosphamide and CEPM concentrations were detectable in 167 (85%) and 128 (65%), respectively, of 196 samples. HCY required separate bedside processing, and was detectable in 113 of 196 (58%) samples. The majority of samples which were below the limit of detection were the 24 h samples, despite obtaining 2 ml of blood in an effort to improve our ability to quantitate cyclophosphamide, HCY and CEPM. Of the 29 samples drawn at 24 h, no analytes could be quantitated in 23 samples, 5 had one analyte that was detectable and 1 was drawn after the subsequent dose was given (the latter of which was deleted from population pharmacokinetic modeling).
Population pharmacokinetic modeling
In Model 1 (see Table 2), we started by assuming a value for VCEPM = VHCY = 0.57 L/m2 and allowed the remaining pharmacokinetic parameters (i.e., CLIND, CLNON, VCY, KHCY, KENZ, K34 and KCEPM) to be estimated relative to this assumption. Estimates of most of the parameters, other than K34 and KHCY, were unaffected by the choice of VCEPM and VHCY value. The reported values of K34 and KHCY should be considered as “relative” to these volume assumptions.
Table 2.
Alternative models evaluated for cyclophosphamide, HCY and CEPM
| Model | Model description | Result | OFV |
|---|---|---|---|
| 1 | Initial model | Proper convergence and covariance estimation only with excellent starting values | 1152.2 |
| 2 | Dose not normalized by BSA | Inferior model | 1394.0 |
| 3 | Added random component to VCY in Model 1 | Significantly decreased OFV, decreased variability (both BSV and RUV) and improved precisions | 1079.0 |
| 4 | Model 3 but KHCY a linear model in GFR | Decreased OFV and variability (both BSV and RUV) over Model 3 | 1058.6 |
| 5 | Model 3 but K34 a linear model in GFR | Decreased OFV and variability (both BSV and RUV) over Model 3 | 1063.4 |
| 6 | Model 3 but CLNON a linear model in gender | No improvement over Model 3. Gender provides no predictive value | 1079.0 |
| 7 | Both KHCY and K34 linear models in GFR | Significantly improved OFV (over Models 3, 4 and 5). Final model | 1041.6 |
OFV, minimum value of objective function. BSV, Between-subject variability. RUV, Residual unknown variability
Various alternative models were evaluated, and the results are summarized in Table 2. Model 2 evaluated the actual dose administered, not normalized for BSA – essentially testing removal of BSA as a covariate. In this model the fixed volumes (VCEPM = VHCY = 0.57 L/m2) were multiplied by the mean subject BSA of 0.681 m2 to convert to units of liters (L). While this conversion may not be optimal, the clear underperformance of Model 2, as measured by increased objective function value (OFV), did not warrant further investigation.
In Model 3, we replaced the fixed-effects model for parameter VCY with a mixed-effects model with lognormal random effect. This resulted in a highly significant improvement in OFV (i.e., decrease of 73.2 points) versus Model 1.
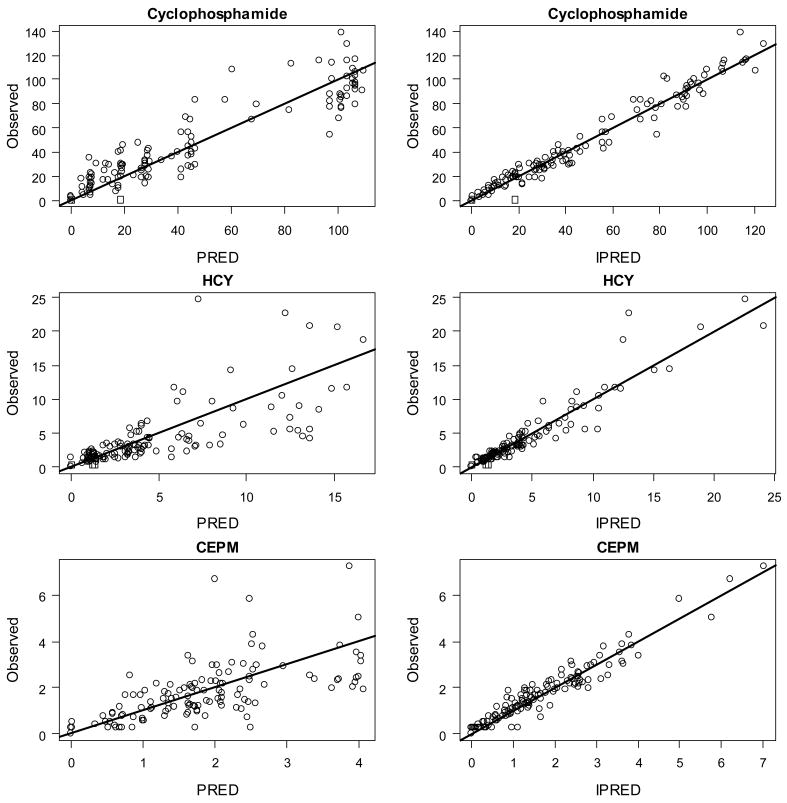
Using Model 3, we performed a covariate analysis by visual assessment of plots and by regressions of the seven individually varying parameter values (CLIND, CLNON, VCY, KHCY, KENZ, K34 and KCEPM) and the corresponding random effects with the available covariates (i.e., age, gender, weight, BSA, and pre treatment GFR). The three most-promising parameter-covariate relationships were KHCY vs. GFR (r2=0.31, p=0.004), K34 vs. GFR (r2=0.25, p=0.01), and CLNON vs. gender (r2=0.13, p=0.05). We tested each of these by postulating a linear relationship between parameters and covariates in Models 4, 5 and 6, respectively. Models 4 and 5 both showed significant (p < 0.05) improvement in OFV versus Model 3, while Model 6 did not. Model 7 included linear functions of GFR for both KHCY and K34. This resulted again in a significant improvement over both Models 4 and 5 (at p < 0.05), and provided the final model. The final parameter estimates are summarized in Table 3. Figure 2 shows plots of observed vs. population predicted (PRED) and individually predicted (IPRED) concentrations of cyclophosphamide, HCY and CEPM for the final model.
Figure 2.
Observed vs. Population Predicted (PRED) and Individually Predicted (IPRED) concentration (μM) of cyclophosphamide, HCY and CEPM.*
*Population Predicted (PRED) are predictions using population-level parameter values (Table 3). Individually Predicted (IPRED) are predictions using individual-level parameter values (not reported). Solid lines have slope=1. Data points denoted with a □ were BQL.
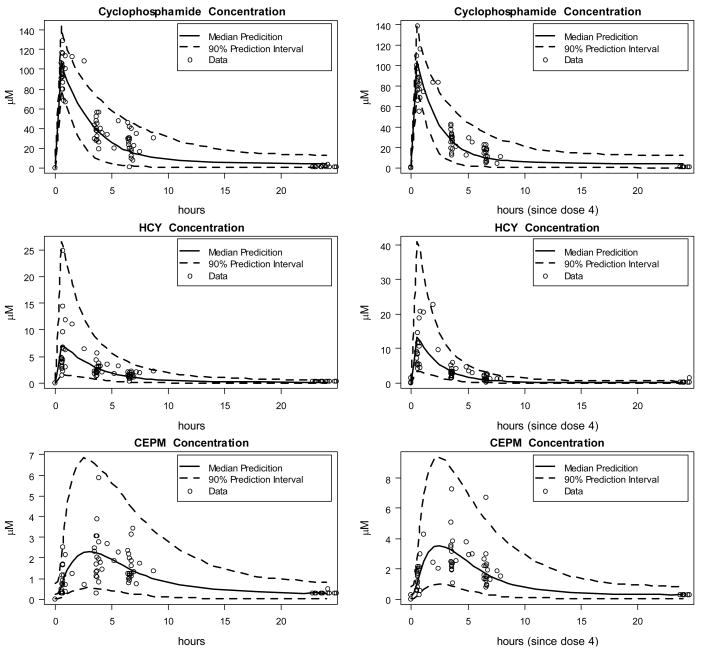
Figure 3 provides model validation in the form of a visual predictive check. The median and 90% prediction intervals were plotted with the original data for cyclophosphamide and its metabolites HCY and CEPM after the first and fourth dose. The visual predictive check shows generally good agreement between model and data. That fact that fewer than 10% of data points lie outside the 90% prediction interval might indicate a slight overestimation of the variability. While this may be a consequence of the relatively small dataset, it is more likely the result of employing only a diagonal model covariance matrix. However, the data did not support estimation of parameter covariances.
Figure 3.
Model validation via a visual predictive check: observed data is compared with median prediction and 90% prediction intervals from 4400 subjects simulated from the final model.
AUC of cyclophosphamide, HCY and CEPM in children
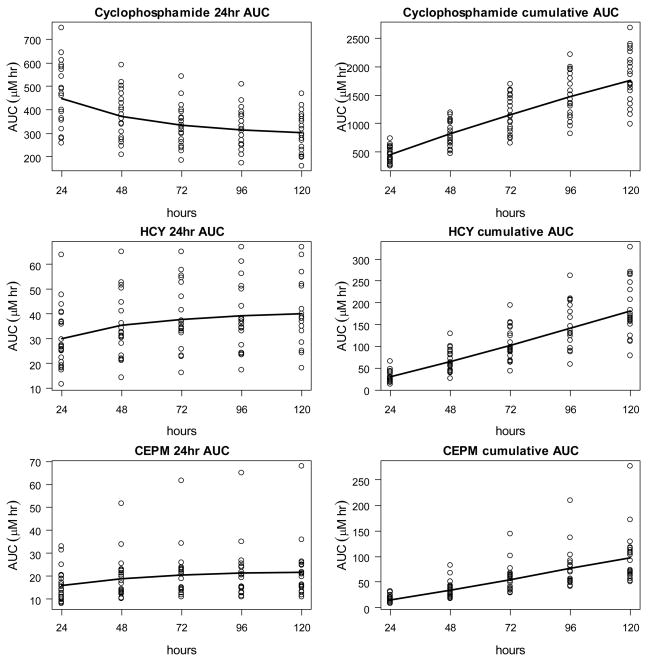
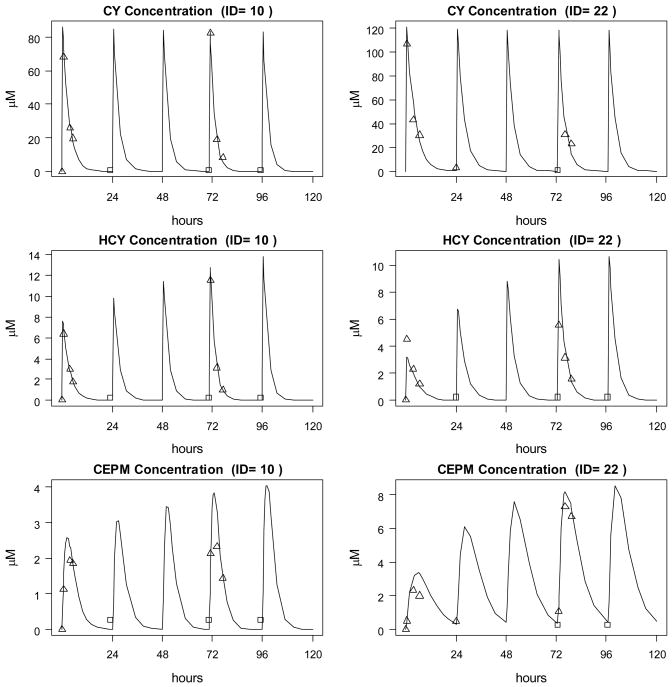
The final model (Model 7) was applied to compute the AUC of cyclophosphamide, HCY and CEPM after each of the five cyclophosphamide doses. The resulting AUCs for cyclophosphamide, HCY and CEPM are shown in Figure 4 and provided in Table 4 for the whole patient population. Figure 5 contains examples of the pharmacokinetic model fit for two patients to the cyclophosphamide, HCY and CEPM concentration-time data.
Figure 4.
Modeled cumulative and 24 hour interval AUC for cyclophosphamide, HCY and CEPM plotted for each individual. The curves show the mean AUC.
Table 4.
The AUC of cyclophosphamide (CY) and its metabolites on day 1 and 4 of cyclophosphamide administration
| Dose | AUC of CY (μM*h) | AUC of HCY (μM*h) | AUC of CEPM (μM*h) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age (Yr) | Sex | Weight (kg) | BSA (m2) | GFR (ml/min/1.73 m2) | mg/kg | mg/m2 | Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 4 |
| 1 | 1.93 | M | 15.6 | 0.67 | 125 | 400 | 357 | 254 | 18.4 | 27.4 | 12.4 | 18.5 | |
| 2 | 1.30 | M | 8.7 | 0.45 | 238 | 13.3 | 257 | 569 | 510 | 36.5 | 50 | 9.3 | 13.4 |
| 3 | 3.22 | M | 14.7 | 0.63 | 145 | 400 | 461 | 291 | 24.9 | 37.6 | 11.3 | 17.1 | |
| 4 | 5.51 | F | 13.3 | 0.61 | 148 | 400 | 487 | 286 | 43.8 | 56.1 | 21.8 | 28 | |
| 5 | 3.53 | F | 13.6 | 0.63 | 109 | 400 | 582 | 393 | 22.4 | 36.8 | 10.5 | 17.3 | |
| 6 | 1.83 | F | 12.8 | 0.54 | 78 | 400 | 591 | 348 | 29.5 | 34.6 | 23.9 | 28.3 | |
| 7 | 3.10 | M | 15.1 | 0.63 | 116 | 400 | 643 | 380 | 25.8 | 38.5 | 18.1 | 27.9 | |
| 8 | 3.43 | M | 12.4 | 0.58 | 112 | 400 | 746 | 404 | 40.7 | 51.7 | 20 | 25.4 | |
| 9 | 3.48 | M | 15.3 | 0.68 | 190 | 400 | 468 | 344 | 36.8 | 61.6 | 16.8 | 28.3 | |
| 10 | 9.02 | M | 52.8 | 1.47 | 141 | 400 | 277 | 209 | 27 | 33.9 | 14.5 | 18.2 | |
| 11 | 1.89 | M | 14.4 | 0.57 | 78 | 400 | 317 | 246 | 11.4 | 17.3 | 9.8 | 14.9 | |
| 12 | 8.36 | F | 24.3 | 0.91 | 99 | 400 | 611 | 316 | 27.3 | 34.3 | 13.9 | 17.6 | |
| 13 | 2.26 | M | 12.7 | 0.57 | 101 | 400 | 282 | 234 | 40.2 | 42.3 | 38.8 | 40.9 | |
| 14 | 2.49 | M | 12.5 | 0.57 | 158 | 400 | 541 | 441 | 25.5 | 37.6 | 16.1 | 24 | |
| 15 | 9.37 | M | 32.9 | 1.14 | 100 | 400 | 399 | 278 | 17.9 | 23.8 | 13 | 17.4 | |
| 16 | 2.46 | M | 18.1 | 0.73 | 161 | 400 | 400 | 224 | 26.6 | 32.8 | 10.3 | 12.7 | |
| 17 | 3.24 | M | 18.6 | 0.74 | 173 | 400 | 280 | 209 | 47.7 | 56 | 19.2 | 22.6 | |
| 18 | 2.41 | M | 14 | 0.6 | 115 | 400 | 363 | 309 | 19.4 | 24.2 | 23.6 | 29.9 | |
| 19 | 1.44 | F | 9.7 | 0.48 | 110 | 13.3 | 269 | 258 | 171 | 20.3 | 23.4 | 13.1 | 15.1 |
| 20 | 3.77 | M | 13.2 | 0.58 | 98 | 400 | 392 | 364 | 35.5 | 37.2 | 29.3 | 30.9 | |
| 21 | 2.55 | M | 13.3 | 0.6 | 134 | 400 | 354 | 326 | 63.8 | 66.4 | 23.6 | 24.6 | |
| 22 | 4.18 | M | 17.5 | 0.62 | 118 | 400 | 493 | 379 | 17.3 | 35.2 | 36.4 | 76.6 | |
Figure 5.
Concentration-Time profiles and simulations of cyclophosphamide (CY), HCY and CEPM for two study patients. Data points represented by a □ were BQL and have been set to QL/2.
Considerable interpatient variability was observed in cyclophosphamide AUC (i.e., 2.9 fold variability after dose 1 and 3.0 fold variability after dose 4). For the AUC of cyclophosphamide, the median was 431 μM*h (range: 258–746) on day 1 and 313 μM*h (range: 171–510) on day 4. The cyclophosphamide AUC was associated with age (p=0.03), BSA (p=0.028), and day of administration (p=0.0001). The cyclophosphamide clearance, calculated based on the AUCs in Table 4, was 3.58 L/h/m2 (range: 1.73–5.53) after dose 1 and 4.86 L/h/m2 (range: 1.93–7.37) after dose 4 (Table 5). Model-predicted initial inducible clearance (i.e., the clearance value extrapolated to before the first dose, without circulating cyclophosphamide) was 1.77 L/h/m2.
Table 5.
Comparison to previously reported pharmacokinetic results
| Author | Subjects | CY Dose (mg/m2) | Timing | CY Clearance (L/h*m2) | Subjects with detectable CEPM AUC | CEPM AUC (μM*h) |
|---|---|---|---|---|---|---|
| Tasso 19924 | 9 | 125–1500 | Variable | 1.48 (0.6–5.2) | 7 | 0.17c (ND–74.7) |
| Yule 19955 | 36 | 368–2490 | Variable | NA | 28 | 105c (ND–1210) |
| Yule 19966 | 38 | 370–2490 | Dose 1 | 2.9 (1.2–106.1) | NA | NA |
| Yule 20017 | 13 | 600–2000 | Dose 1 | 3.1 (1.4–6.2) | 11 | 99 (ND–274) |
| Yule 20048 | 36 | 300–1000 | Dose 1 | 3.6 (2.1–5.4) | 8 | ND (ND–206) |
| McCune 2008 | 22 | 400 | Dose 1 | 3.58 (1.73–5.53) | 22 | 16.5 (9.3–38) |
| Dose 4 | 4.86 (1.93–7.37) | 20 | 23.3 (12.7–76.6) |
In comparison to cyclophosphamide AUC, there was greater variability in the AUC of HCY and CEPM (i.e., 3.8–6.0 fold variability). For the AUC of HCY, the median was 26.8 μM*h (range: 11.4–63.8) on day 1 and 37.0 μM*h (range: 17.3–66.4) on day 4. The HCY AUC was associated with GFR (p=0.031), and day of administration (p<0.0001). For the CEPM AUC, the median was 16.5 μM*h (range: 9.3–38) on day 1 and 23.3 μM*h (12.7–76.6) on day 4. The CEPM AUC was associated with day of administration (p=0.0032). There was negligible correlation (i.e., all R2<0.039) between AUCCY and AUCHCY after dose 1 or after dose 4 and between AUCCY and AUCCEPM after dose 1 or after dose 4.
DISCUSSION
This manuscript is the first to report considerable pharmacokinetic variability of the plasma concentrations of HCY in children receiving daily nonmyeloablative cyclophosphamide. Hydroxycyclophosphamide is transported intracellularly to release the active metabolite phosphoramide mustard, and thus may be a key determinant in the efficacy and toxicity of cyclophosphamide-containing regimens. Quantitation of the other cyclophosphamide metabolites was not feasible because of the limited blood volume per sample. This manuscript is also the first describing a population pharmacokinetic model of cyclophosphamide, HCY, and CEPM in children. The key findings of this pediatric study include the presence of considerable interpatient variability of cyclophosphamide AUC and its metabolites and the development of a population pharmacokinetic model with covariates to predict these AUCs. The population pharmacokinetic model identified baseline GFR as a covariate of HCY elimination rate and CEPM formation rate. By reducing unexplained variability, covariate identification can reduce the need for pharmacokinetic sampling, thereby facilitating the development of a limited sampling schedule feasible for adequately sized pharmacokinetic and pharmacodynamic studies. This work represents an essential step in advancing our understanding of risk factors for poor response or severe ADRs to cyclophosphamide in pediatric cancer patients.
Cyclophosphamide-based combination chemotherapy regimens are used to treat the majority of children with cancer. Traditional dosing methods based on single covariates, such as BSA, do not necessarily address the presence of variability sufficiently well.25, 26 The natural first step in obtaining an improved understanding of pharmacodynamic variability is to evaluate the interpatient variability in the systemic exposure of the parent drug and relevant metabolites. This is the main contribution of these results; however there are additional potential benefits. Specifically, pharmacokinetic-linked pharmacodynamic studies are hindered by the need for intense blood sampling schedules, and in the case of HCY, bedside processing. A population pharmacokinetic model provides a rigorously quantitative approach to incorporate population information (i.e., typical values and variability of drug disposition, together with explanatory covariates) in the determination of an individual’s systemic exposure and can reduce the need for individual-specific information. Population pharmacokinetics can potentially be used to simultaneously describe the concomitant role of demographic covariates (e.g., age), and clinical covariates (e.g., GFR). Improved understanding of the determinants of variability can then lead to improved trial design. While the awareness of these issues has increased for drugs in the development phase, there is a still strong need to improve dosing for drugs that are already in use, such as cyclophosphamide. Understanding the sources of variability in young patients is of the utmost importance to optimize exposure to anti-tumor chemotherapy to ultimately achieve the maximum disease response with non-lethal toxicity. In addition, a population pharmacokinetic model facilitates development of an improved limited sampling schedule, which could decrease the needed blood volume and resource intensity of pharmacokinetic-linked pharmacodynamic trials.27
Cyclophosphamide clearance agrees with the previously published values in pediatric populations (Table 5), suggesting that topotecan does not influence cyclophosphamide elimination.4, 6–8 Similarly, the autoinduction of cyclophosphamide clearance (resulting in decreased AUC for subsequent doses) agreed with previous reports.17, 28–30 In addition, the current convention of dosing children less than 12 kg on a mg/kg basis (i.e., patients 2 and 19) appears to yield a comparable AUC to those larger children (i.e., >12 kg) who receive cyclophosphamide 400 mg/m2 (Table 4). The latter point is critically important, as children younger than 3 years of age experienced increased ADRs after what was anticipated to be a minor dosing modification to the VAC (vincristine/dactinomycin/cyclophosphamide) regimen.1 These pharmacokinetic data, along with the accompanying population pharmacokinetic model, represent initial steps towards the goal of optimizing cyclophosphamide dosing in such young children.
Using analytical methods developed for pharmacodynamic studies in myeloablative hematopoietic cell transplant patients (Table 1), we are the first to describe the pharmacokinetics of HCY in children. The interpatient variability in the AUC of HCY was substantial, that is 5.6 fold after dose 1 and 3.8 fold after dose 4 (Table 4). A similar trend was found in the AUC of CEPM (Table 4). In contrast to the reports of Yule et al that some children had no detectable CEPM concentration,4, 5, 7, 8 we were able to estimate the AUC of CEPM in all patients (Table 5) due to the increased sensitivity of our analytical method (Table 1) and the use of a population pharmacokinetic model (Figure 1). Notably, there was a weak correlation between the AUC of CY and the AUC of its metabolites, in agreement with previous reports.12, 16, 17, 31, 32 This suggests that the cyclophosphamide clearance (as AUC = dose/clearance) cannot be used to predict metabolite exposure as has been suggested.8, 33, 34
This is the first population pharmacokinetic model of cyclophosphamide and metabolite disposition in young children, specifically less than 10 years of age (i.e., 1.30 – 9.37 years). The majority of the population pharmacokinetic models were presumably (i.e., age not reported)35 or were exclusively from36 populations older than 10 years of age. However, our initial population pharmacokinetic model of cyclophosphamide in hematopoietic cell transplant patients receiving myeloablative dose cyclophosphamide (60 mg/kg/day × 2 days) with total body irradiation (CY/TBI) did include eight children whose age were less than 10 years old.21 In the CY/TBI population, age was the only covariate which improved model fitting and was inversely correlated with the noninducible clearance (CLNON) of cyclophosphamide.21 In agreement with this finding, the CLNON in the neuroblastoma patients was higher (i.e., 1.13 L/hr calculated from 1.83 L/h/m2 × 0.62 m2 median patient BSA) than that observed in the CY/TBI conditioned patients (i.e., 0.665 L/hr calculated from 0.00899 L/hr/kg21 × the mean patient body weight of 74 kg in that dataset). However, age was not a covariate for CLNON (Tables 2 and 3) within the narrow age range (i.e., < 10 years) of this neuroblastoma population. Regarding the initial inducible clearance of cyclophosphamide (CLIND), the CLIND was 1.11 L/h (1.79L/h/m2 × 0.62 m2 median patient BSA). This is lower than previously reported values from adult populations, which range from 2.11 L/h21 (i.e., 0.0286 L/h/kg × the mean patient body weight (74 kg) in that dataset) and 2.91 L/h.15, 35 This suggests that the relative contribution of the CLIND is lower in children, although further data are needed to confirm this finding. The remaining parameters compare favorably with population pharmacokinetic models of cyclophosphamide and its metabolites in adult populations. For example, the pediatric pharmacokinetic analysis provides a value of 46.5/h for KHCY, which is the HCY elimination rate. This large value can be perceived as suggesting a very rapid elimination, however, it is less than previously published values for adults from our group (i.e., 147/h)19, 21 and others (i.e., 118 to 169/h).35, 37 The assumed values of VHCY and VCEPM are in accordance with previous reports, as we have remarked in the results section.
GFR was a covariate for the fractional elimination of HCY and its conversion to CEPM (Tables 2 and 3). Further studies are needed to confirm these findings, as a physiologic rationale for GFR being a covariate with these two parameters is currently elusive. CEPM is the predominant urinary metabolite after cyclophosphamide administration and its AUC was dramatically higher in an anephric child.38 The conversion of HCY to CEPM is mediated by hepatic aldehyde dehydrogenase. This model predicted the individual and population concentrations well (Figure 2). It also allows for calculation of modeled AUCs of cyclophosphamide, HCY and CEPM after each of the five cyclophosphamide doses (Figures 3 and 4) while obtaining pharmacokinetic blood samples after dose 1 and 4 only. This population pharmacokinetic model needs to be validated in a separate pediatric population, especially due to our limited ability to quantitate all three analytes in many of the 24h samples. We plan to validate this model in a separate cohort of children receiving non-myeloablative dose cyclophosphamide and subsequently create an optimal limited sampling schedule to conduct pharmacokinetic and pharmacodynamic studies in children. Identifying pharmacodynamic relationships in future studies could lead to personalized cyclophosphamide dosing to achieve a target AUC to improve efficacy or decrease toxicity. We are currently employing this approach after myeloablative cyclophosphamide dosing in hematopoietic cell transplant recipients.
Pharmacodynamic studies are needed to determine the clinical relevance of this interpatient variability in the pharmacokinetics. Quantitation of cyclophosphamide and CEPM concentrations allowed for comparison to historical cyclophosphamide pharmacokinetic data within pediatric patients. It is also important to quantitate CEPM concentrations because elevated CEPM AUC is associated with a higher risk of liver toxicity in hematopoietic cell transplant recipients receiving myeloablative dose CY/TBI.2 Quantitation of HCY was critical, as its pharmacokinetics has been reported in only one anephric child to date.38 The majority of pharmacodynamic studies with cyclophosphamide and its metabolites have been conducted in patients receiving myeloablative cyclophosphamide for conditioning prior to hematopoietic cell transplantation.2, 15, 31 In this population, the pharmacodynamics of cyclophosphamide and its metabolites differ based on the conditioning regimen.31 In those receiving busulfan/cyclophosphamide, the AUC of cyclophosphamide, HCY and CEPM are not related to toxicity or response.31 However, in patients receiving CY/TBI, an elevated AUC of CEPM is associated with a higher risk of liver toxicity.2 This has led to ongoing studies of targeting cyclophosphamide doses based on the AUC of CEPM in hopes of lowering toxicity while maintaining the AUC of HCY in hopes of maintaining engraftment rates.18 These results suggest that the concentration – effect relationships may differ between combination chemotherapy regimens and thus, future pharmacokinetic and pharmacodynamic studies should be conducted in homogeneous disease populations treated with the same combination chemotherapy regimen. The development to this population pharmacokinetic model of cyclophosphamide and its metabolites is an integral step in improving the efficacy and toxicity of cyclophosphamide -based regimens in pediatric cancer patients. Despite its widespread use for over fifty years, there are still uncertainties regarding the optimal dose of cyclophosphamide as shown by the increased rate of ADRs after a minor dosing modification to the VAC from mg/kg to mg/m2 dosing of vincristine/dactinomycin/cyclophosphamide in young children.1 This pharmacokinetic data, along with the accompanying population pharmacokinetic model, are first steps in determining if there are select populations of children which may need different cyclophosphamide doses. In addition, the creation of a limited sampling schedule – which is essential for pharmacokinetic-pharmacodynamic children in infants and toddlers – may be possible after validation of this population pharmacokinetic model. A limited sampling schedule will greatly facilitate recruitment of enough children to conduct pharmacokinetic and pharmacodynamic studies, which are desperately needed in hopes of improving the efficacy of cyclophosphamide.
In conclusion, we have shown substantial interpatient variability in the pharmacokinetics of cyclophosphamide and its metabolites in children. Topotecan administration did not appear to affect the clearance of cyclophosphamide. The development of a population pharmacokinetic model with relevant covariates will provide a framework to evaluate the pharmacodynamics of cyclophosphamide, with the long-range goal of improved prediction of an individual child’s response and/or ADRs of cyclophosphamide-based regimens in this population.
Acknowledgments
The participation of the children and their families in these studies and the analytical expertise of Brian Phillips are greatly appreciated.
Supported by grants from the National Institutes of Health, National Cancer Institute (CA18029, CA87058-01A, CA98543); the National Institute of Biomedical Imaging and Bioengineering (EB001975); M01-RR-00037 (GCRC); Alex’s Lemonade Stand; Children’s Foundation
References
- 1.Arndt C, Hawkins D, Anderson JR, Breitfeld P, Womer R, Meyer W. Age is a risk factor for chemotherapy-induced hepatopathy with vincristine, dactinomycin, and cyclophosphamide. J Clin Oncol. 2004 May 15;22(10):1894–1901. doi: 10.1200/JCO.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 2.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003 Mar 1;101(5):2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 3.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44(11):1135–1164. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 4.Tasso MJ, Boddy AV, Price L, Wyllie RA, Pearson AD, Idle JR. Pharmacokinetics and metabolism of cyclophosphamide in paediatric patients. Cancer Chemother Pharmacol. 1992;30(3):207–211. doi: 10.1007/BF00686313. [DOI] [PubMed] [Google Scholar]
- 5.Yule SM, Boddy AV, Cole M, et al. Cyclophosphamide metabolism in children. Cancer Res. 1995;55(4):803–809. [PubMed] [Google Scholar]
- 6.Yule SM, Boddy AV, Cole M, et al. Cyclophosphamide pharmacokinetics in children. Br J Clin Pharmacol. 1996;41(1):13–19. doi: 10.1111/j.1365-2125.1996.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 7.Yule SM, Price L, Cole M, Pearson AD, Boddy AV. Cyclophosphamide metabolism in children following a 1-h and a 24-h infusion. Cancer Chemother Pharmacol. 2001;47(3):222–228. doi: 10.1007/s002800000220. [DOI] [PubMed] [Google Scholar]
- 8.Yule SM, Price L, McMahon AD, Pearson AD, Boddy AV. Cyclophosphamide metabolism in children with non-Hodgkin’s lymphoma. Clin Cancer Res. 2004 Jan 15;10(2):455–460. doi: 10.1158/1078-0432.ccr-0844-03. [DOI] [PubMed] [Google Scholar]
- 9.Ren S, Yang JS, Kalhorn TF, Slattery JT. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res. 1997;57(19):4229–4235. [PubMed] [Google Scholar]
- 10.Baumann F, Preiss R. Cyclophosphamide and related anticancer drugs. J Chromatogr B Biomed Sci Appl. 2001 Nov 25;764(1–2):173–192. doi: 10.1016/s0378-4347(01)00279-1. [DOI] [PubMed] [Google Scholar]
- 11.Anderson LW, Chen TL, Colvin OM, et al. Cyclophosphamide and 4-Hydroxycyclophosphamide/Aldophosphamide Kinetics in Patients Receiving High-Dose Cyclophosphamide Chemotherapy. Clin Cancer Res. 1996;2(9):1481–1487. [PubMed] [Google Scholar]
- 12.Chen TL, Kennedy MJ, Anderson LW, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4- hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Dispos. 1997;25(5):544–551. [PubMed] [Google Scholar]
- 13.Kalhorn TF, Ren S, Howald WN, Lawrence RF, Slattery JT. Analysis of cyclophosphamide and five metabolites from human plasma using liquid chromatography-mass spectrometry and gas chromatography- nitrogen-phosphorus detection. J Chromatogr B Biomed Sci Appl. 1999;732(2):287–298. doi: 10.1016/s0378-4347(99)00300-x. [DOI] [PubMed] [Google Scholar]
- 14.Kalhorn TF, Howald WN, Cole S, et al. Rapid quantitation of cyclophosphamide metabolites in plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 May 1;835(1–2):105–113. doi: 10.1016/j.jchromb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge ME, Huitema AD, Tukker AC, van Dam SM, Rodenhuis S, Beijnen JH. Accuracy, feasibility, and clinical impact of prospective Bayesian pharmacokinetically guided dosing of cyclophosphamide, thiotepa, and carboplatin in high-dose chemotherapy. Clin Cancer Res. 2005 Jan 1;11(1):273–283. [PubMed] [Google Scholar]
- 16.Slattery JT, Kalhorn TF, McDonald GB, et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol. 1996 May;14(5):1484–1494. doi: 10.1200/JCO.1996.14.5.1484. [DOI] [PubMed] [Google Scholar]
- 17.Ren S, Kalhorn TF, McDonald GB, Anasetti C, Appelbaum FR, Slattery JT. Pharmacokinetics of cyclophosphamide and its metabolites in bone marrow transplantation patients. Clin Pharmacol Ther. 1998 Sep;64(3):289–301. doi: 10.1016/S0009-9236(98)90178-3. [DOI] [PubMed] [Google Scholar]
- 18.McDonald GB, McCune JS, Batchelder A, et al. Metabolism-based cyclophosphamide dosing for hematopoietic cell transplant. Clin Pharmacol Ther. 2005 Sep;78(3):298–308. doi: 10.1016/j.clpt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Salinger DH, McCune JS, Ren AG, et al. Real-time dose adjustment of cyclophosphamide in a preparative regimen for hematopoietic cell transplant: a Bayesian pharmacokinetic approach. Clin Cancer Res. 2006 Aug 15;12(16):4888–4898. doi: 10.1158/1078-0432.CCR-05-2079. [DOI] [PubMed] [Google Scholar]
- 20.Beal SL, Sheiner LB, Boeckmann AJE. NONMEM Users Guides. Elliott City, Maryland, USA: Icon Development Solutions; pp. 1989–2006. [Google Scholar]
- 21.Qiu R, Yao A, Vicini P, et al. Diminishing the risk of nonrelapse mortality in hematopoietic stem cell transplantation: Prediction of exposure to the cyclophosphamide metabolite carboxyethylphosphoramide mustard. Clin Pharmacol Ther. 2004 Sep;76(3):270–280. doi: 10.1016/j.clpt.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Hassan M, Svensson US, Ljungman P, et al. A mechanism-based pharmacokinetic-enzyme model for cyclophosphamide autoinduction in breast cancer patients. Br J Clin Pharmacol. 1999;48(5):669–677. doi: 10.1046/j.1365-2125.1999.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huitema AD, Mathot RA, Tibben MM, Rodenhuis S, Beijnen JH. A mechanism-based pharmacokinetic model for the cytochrome P450 drug-drug interaction between cyclophosphamide and thioTEPA and the autoinduction of cyclophosphamide. J Pharmacokinet Pharmacodyn. 2001 Jun;28(3):211–230. doi: 10.1023/a:1011543508731. [DOI] [PubMed] [Google Scholar]
- 24.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006 Apr;55(4):515–524. doi: 10.1016/j.metabol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Gurney H. Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol. 1996;14(9):2590–2611. doi: 10.1200/JCO.1996.14.9.2590. [DOI] [PubMed] [Google Scholar]
- 26.Baker SD, Verweij J, Rowinsky EK, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst. 2002 Dec 18;94(24):1883–1888. doi: 10.1093/jnci/94.24.1883. [DOI] [PubMed] [Google Scholar]
- 27.Panetta JC, Iacono LC, Adamson PC, Stewart CF. The importance of pharmacokinetic limited sampling models for childhood cancer drug development. Clin Cancer Res. 2003 Nov 1;9(14):5068–5077. [PubMed] [Google Scholar]
- 28.Graham MI, Shaw IC, Souhami RL, Sidau B, Harper PG, McLean AE. Decreased plasma half-life of cyclophosphamide during repeated high- dose administration. Cancer Chemother Pharmacol. 1983;10(3):192–193. doi: 10.1007/BF00255760. [DOI] [PubMed] [Google Scholar]
- 29.Moore MJ, Hardy RW, Thiessen JJ, Soldin SJ, Erlichman C. Rapid development of enhanced clearance after high-dose cyclophosphamide. Clin Pharmacol Ther. 1988;44(6):622–628. doi: 10.1038/clpt.1988.203. [DOI] [PubMed] [Google Scholar]
- 30.Fasola G, Lo Greco P, Calori E, et al. Pharmacokinetics of high-dose cyclophosphamide for bone marrow transplantation. Haematologica Mar-Apr. 1991;76(2):120–125. [PubMed] [Google Scholar]
- 31.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following Targeted Oral Busulfan as Conditioning for Hematopoietic Cell Transplantation: Pharmacokinetics, Liver Toxicity, and Mortality. Biol Blood Marrow Transplant. 2007 Jul;13(7):853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Sladek NE, Doeden D, Powers JF, Krivit W. Plasma concentrations of 4-hydroxycyclophosphamide and phosphoramide mustard in patients repeatedly given high doses of cyclophosphamide in preparation for bone marrow transplantation. Cancer Treat Rep. 1984 Oct;68(10):1247–1254. [PubMed] [Google Scholar]
- 33.Ayash LJ, Wright JE, Tretyakov O, et al. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol. 1992 Jun;10(6):995–1000. doi: 10.1200/JCO.1992.10.6.995. [DOI] [PubMed] [Google Scholar]
- 34.Petros WP, Broadwater G, Berry D, et al. Association of high-dose cyclophosphamide, cisplatin, and carmustine pharmacokinetics with survival, toxicity, and dosing weight in patients with primary breast cancer. Clin Cancer Res. 2002 Mar;8(3):698–705. [PubMed] [Google Scholar]
- 35.de Jonge ME, Huitema AD, Beijnen JH, Rodenhuis S. High exposures to bioactivated cyclophosphamide are related to the occurrence of veno-occlusive disease of the liver following high-dose chemotherapy. Br J Cancer. 2006 May 8;94(9):1226–1230. doi: 10.1038/sj.bjc.6603097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joerger M, Huitema AD, Richel DJ, et al. Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet. 2007;46(12):1051–1068. doi: 10.2165/00003088-200746120-00005. [DOI] [PubMed] [Google Scholar]
- 37.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Integrated Population Pharmacokinetic Model of both cyclophosphamide and thiotepa suggesting a mutual drug-drug interaction. J Pharmacokinet Pharmacodyn. 2004 Apr;31(2):135–156. doi: 10.1023/b:jopa.0000034405.03895.c2. [DOI] [PubMed] [Google Scholar]
- 38.McCune JS, Adams D, Homans AC, Guillot A, Iacono L, Stewart CF. Cyclophosphamide disposition in an anephric child. Pediatr Blood Cancer. 2006 Jan;46(1):99–104. doi: 10.1002/pbc.20558. [DOI] [PubMed] [Google Scholar]