Abstract
The EL4 murine lymphoma cell line exists in variant phenotypes that differ with respect to responses to the tumor promoter phorbol 12-myristate 13-acetate (PMA1). Previous work showed that “PMA-sensitive” cells, characterized by a high magnitude of PMA-induced Erk activation, express RasGRP, a phorbol ester receptor that directly activates Ras. In “PMA-resistant” and “intermediate” EL4 cell lines, PMA induces Erk activation to lesser extents, but with a greater response in intermediate cells. In the current study, these cell lines were used to examine mechanisms of Raf-1 modulation. Phospho-specific antibodies were utilized to define patterns and kinetics of Raf-1 phosphorylation on several sites. Further studies showed that Akt is constitutively activated to a greater extent in PMA-resistant than in PMA-sensitive cells, and also to a greater extent in resistant than intermediate cells. Akt negatively regulates Raf-1 activation (Ser259), partially explaining the difference between resistant and intermediate cells. Erk activation exerts negative feedback on Raf-1 (Ser289/296/301), thus resulting in earlier termination of the signal in cells with a higher level of Erk activation. RKIP, a Raf inhibitory protein, is expressed at higher levels in resistant cells than in sensitive or intermediate cells. Knockdown of RKIP increases Erk activation and also negative feedback. In conclusion, this study delineates Raf-1 phosphorylation events occurring in response to PMA in cell lines with different extents of Erk activation. Variations in the levels of expression and activation of multiple signaling proteins work in an integrated fashion to modulate the extent and duration of Erk activation.
Keywords: tumor promoter, mitogen-activated protein kinase, Akt, Erk, RKIP
1.0 INTRODUCTION
Raf serine/threonine kinases belong to the MAPKKK family [1]. A-Raf, B-Raf, and Raf-1 (or c-Raf) are the three known Raf isoforms in mammalian cells. While A-Raf and B-Raf display tissue-specific patterns of expression, Raf-1 is widely expressed. The downstream target of Raf-1 isoforms is MEK, a dual function kinase that activates the Erk kinases. Raf kinases are involved in “normal” physiological processes such as cellular metabolism, cell cycle progression, and cell death, as well as in tumorigenesis. Raf-1 is frequently activated in tumors, through either over-expression or mutation [2]. Thus, a comprehensive understanding of the process of Raf activation/deactivation is important.
Raf-1 activity needs to be tightly regulated to control fundamental cellular processes. The events that regulate Raf activation are only partially understood, but they are known to involve multiple phosphorylations and dephosphorylations that trigger conformational changes [1]. Previous studies have documented both positive and negative effects of Raf-1 phosphorylation. For example, p21 activated kinase (PAK) and Src phosphorylate Raf-1 on positive regulatory sites to augment Raf-1 activity [3]. Negative regulation of Raf-1 has also been reported, in that phosphorylation of Ser259 can inactivate Raf-1, probably by promoting the formation of a Raf auto-inhibitory complex via association with 14-3-3 [4]. In addition, protein-protein interactions, including those involving spatial regulation of the signaling components [5], can regulate Raf-1 activity.
Previous studies in our lab have established the EL4 lymphoma cell line as a model for investigating the regulation of Erk activation. This cell line exists in characterized variants that vary with respect to PMA-induced Erk activation [6]. Recent work established that the “PMA-sensitive” phenotype is conferred largely by expression of RasGRP, a Ras GEF that is directly activated by PMA [7]. Sensitive cells are characterized by a very high level of Erk activation in response to PMA, resulting in cell cycle arrest [8]. “PMA-resistant” cells show much lower levels of Erk activation in response to PMA, express low levels of RasGRP, and proliferate in the presence of PMA. “Intermediate” cells likewise express little RasGRP, but exhibit higher levels of PMA-induced Erk activation than is seen in resistant cells. The differences in response between resistant and intermediate cells are a particular focus of the current study.
The work described here examines the signaling steps responsible for the varying levels of PMA response between EL4 cell lines. Of the many pathways proposed to regulate Raf-1 activation, three were of particular interest. It has been reported that phospho-Akt can negatively regulate Raf-1 activation in some cell types [9]. Since Akt is constitutively active in PMA-resistant cells [10], we hypothesized that varying levels of phospho-Akt contribute to the distinct PMA-induced Raf/Erk activation profiles between EL4 cell lines. Second, Erk feedback appears to be a key regulatory step of the Raf-1 activation process, with alternative scenarios proposed. Erk-1 can phosphorylate Raf-1 Ser289/296/301 and stabilize the active form of Raf-1 by attenuating its inactivation [3]. In contrast, phosphorylation of Raf Ser296 and 301 can contribute to negative regulation of Raf-1 [4], with phosphorylation of Ser338 blocking auto-inhibition by Erk [11]. We hypothesized that differences in levels of Erk activation between EL4 cell lines might not only reflect differences in Raf-1 activation, but also contribute to Raf-1 regulation. Third, Raf-interacting proteins, including PKCs and the Raf-1 inhibitory molecule RKIP, comprise key regulatory elements of the Raf/MAPK cascade [1]. Having previously examined the roles of PKC isoforms in EL4 cells [8], we turned to the potential role of RKIP. Thus, in the current study, the regulatory events regulating Erk activation were addressed in a comprehensive manner by investigating the roles of multiple phosphorylation sites of Raf-1 in response to a single stimulus.
2.0 EXPERIMENTAL PROCEDURES
2.1 Cell culture
EL4 cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Atlanta Biologicals), as previously described [7]. PMA-sensitive cell lines were maintained in suspension culture dishes; PMA-resistant and intermediate cells were maintained in standard tissue culture dishes. For experiments, all cell types were grown on standard tissue culture plastic (6-well plates) for at least 24 hours prior to the experiment.
2.2 Immunoblotting
Antibodies were obtained from the following sources: phospho-Erk, Promega (Madison, WI); Erk-1, Santa Cruz Biotechnology (Santa Cruz, CA); phospho-Akt, Akt, phospho-c-Raf (Ser259), phospho-c-Raf (Ser289/296/301), phospho-c-Raf (Ser338), RKIP, actin, and c-Raf, Cell Signaling Technology Inc. (Beverly, MA).
EL4 cells were treated with and without 100nM PMA as indicated for each figure. After treatment, cells were collected; adherent cells were harvested using a cell scraper. Cells were lysed in an ice-cold buffer containing 20 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 30 mM sodium pyrophosphate, 100 μM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Extracts were sedimented by centrifugation at 10,000g for 10 min at 4°C to remove insoluble material. Samples equalized for protein (100 μg), as determined by Coomassie Protein Assay (Pierce), were separated by SDS-PAGE on 10% Laemmli gels, transferred to PVDF paper, and incubated with antibodies. Blots were developed using enhanced chemiluminescence reagents (Amersham).
2.3 siRNA Experiments
RKIP siRNA, control siRNA, siRNA transfection reagent, and transfection medium were obtained from Santa Cruz Biotechnology. Protein levels for RKIP, phospho-Raf (Ser259, Ser289/296/301, Ser338), c-Raf, phospho-Erk, and total Erk (immunoblotting) were assessed in cell extracts using the methods described above.
Cells, grown to 60 to 80% confluence, were incubated with RKIP siRNA or control siRNA for 5 to 7 hours in the absence of serum. FBS was then added to a final concentration of 10%; cells were incubated for an additional 18 to 24 h under cell culture conditions. The medium was then changed to RPMI with 10% FBS, and the cells were incubated for an additional 48 h before incubation with and without 100 nM PMA.
3.0 RESULTS
3.1 Acute effects of PMA on Raf phosphorylation in clonal EL4 cell lines
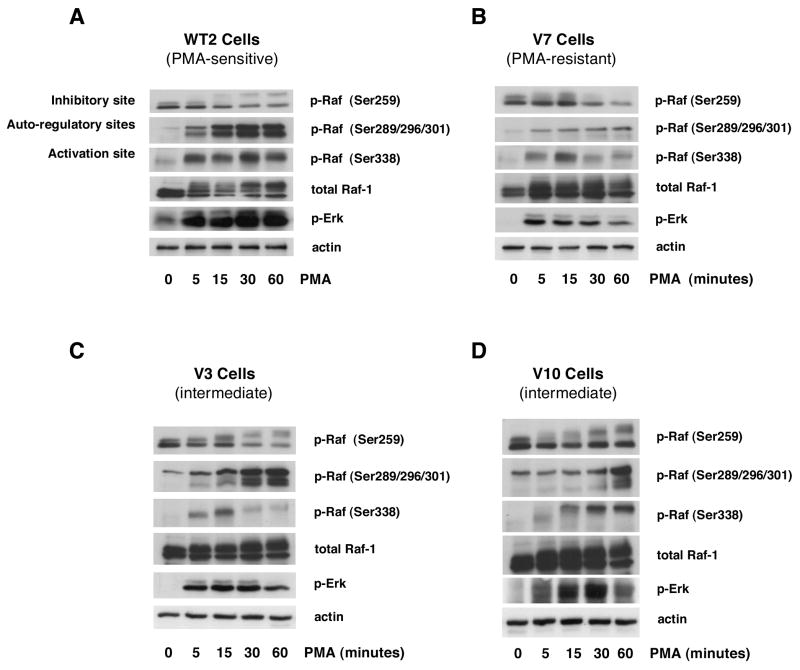
Time course experiments were performed with several EL4 cell lines to examine PMA-induced Raf phosphorylation at several regulatory sites (Figure 1). As shown in panel A, PMA induces strong Raf Ser338 (activation site) phosphorylation in WT2 (PMA-sensitive) cells that begins within 5 minutes and is maximal at 30 minutes. In V7 (PMA-resistant) cells, the weaker Raf Ser338 phosphorylation induced by PMA is maximal at 15 minutes and then declines (Figure 1B). Phosphorylation at Ser338 is likewise weak in the intermediate cells V3 (Figure 1C) and V10 (Figure 1D), although the time course varies between the two lines. Raf Ser259 is phosphorylated in the resting state in all cells tested. Raf Ser259 phosphorylation decreases after 15–30 minutes of PMA treatment in WT2, V7, and V3 cells. PMA-induced Raf Ser289/296/301 phosphorylation is much more robust in WT2 than in V7 cells. In WT2 cells, Raf Ser289/296/301 phosphorylation begins within 5 minutes of PMA treatment, and is maintained at a high level for at least 60 minutes. In V7 cells, the phosphorylation is very weak but follows a similar time course. In both V3 and V10, which are intermediate phenotype cell lines, levels of PMA-induced Raf Ser259, Ser289/296/301 and Ser338 phosphorylation are between those of WT2 and V7 (Figures 1C and 1D). The responses of V3 and V10 are not identical, but for both cell lines are “intermediate” between WT2 and V7. PMA-induced Erk activation was previously characterized in these cell lines [6,7], consistent with the phospho-Erk results shown for reference in Figure 1. Taken together, these data suggest that patterns of PMA-induced phosphorylation of multiple Raf residues are distinct between EL4 cell phenotypes.
Figure 1. Time course of the acute effects of PMA on Raf phosphorylation in EL4 cell lines.
WT2 (panel A), V7 (panel B), V3 (panel C), and V10 (panel D) cells were treated with or without 100 nM PMA for the indicated times. Immunoblots were performed on whole-cell lysates for phospho-Raf (Ser259, Ser289/296/301, Ser338), c-Raf, phospho-Erk, and actin. All incubations were done in the same experiment; blots for the four cell lines were exposed in parallel.
3.2 Long-term effects of PMA on Raf phosphorylation in clonal EL4 cell lines
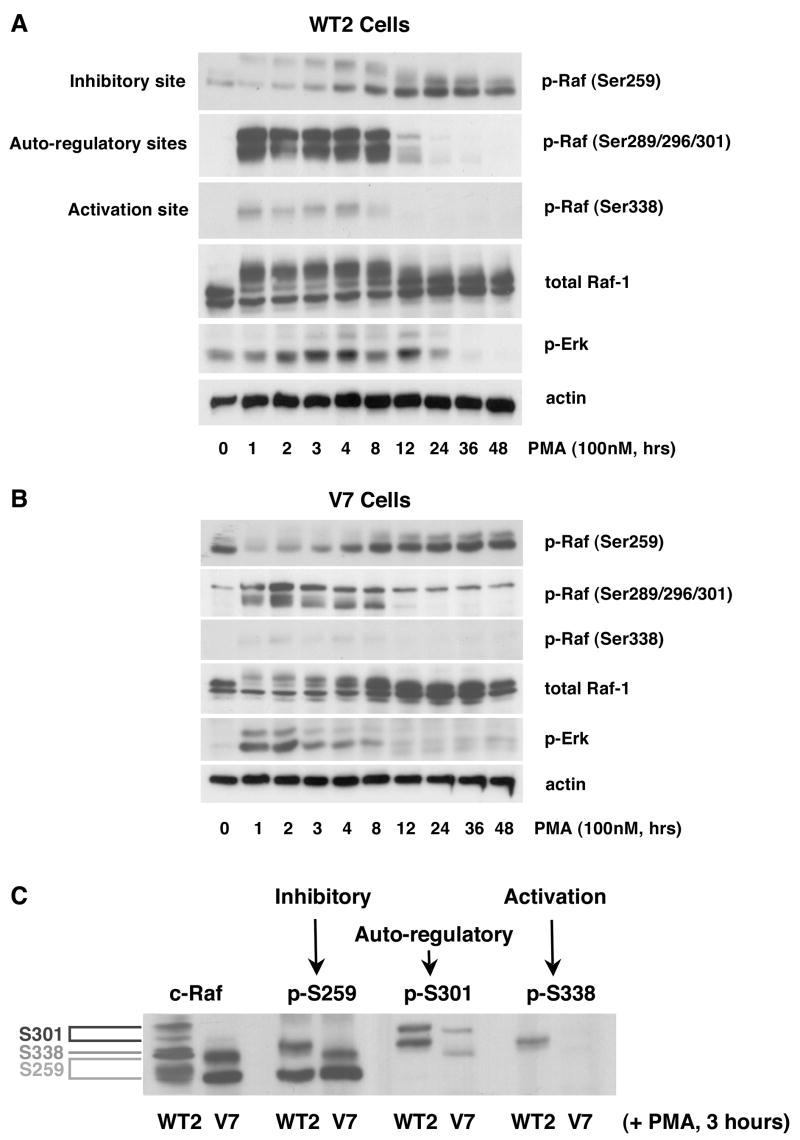
Since PMA-induced responses in EL4 cells extend beyond 60 minutes [7], we tested the long-term effects of PMA on Raf phosphorylation/dephosphorylation. Phosphorylation at Ser259 occurs as Ser338 is being dephosphorylated in both WT2 and V7 cells (Figures 2A and 2B), beginning at 3 hours and peaking at 12–24 hours, at which time Ser338 is completely dephophosphorylated. In WT2 cells, PMA-induced Raf Ser338 phosphorylation is maintained for 4 hours and then declines to basal levels by 12 hours after PMA addition (Figure 2A). In V7 cells, PMA-induced Raf Ser338 phosphorylation is very weak but also disappears after 12 hours of PMA stimulation (Figure 2B). The basal level of Raf Ser259 phosphorylation is higher in V7 than in WT2 cells, consistent with a negative regulatory effect of phospho-Ser259. Following an initial decrease in Raf Ser259 phosphorylation, a response that is quite prominent in V7 cells, p-Ser259 reappears after 3–4 hours in both WT2 and V7 cells and then gradually increases. Raf Ser289/296/301 is phosphorylated by 1 hour after PMA addition, and persists for 8 hours in both WT2 and V7 cells. The magnitude of this phosphorylation is much higher in WT2 than V7 cells, consistent with the higher level of Erk activation observed in WT2 cells. These data establish that multiple residues are phosphorylated and dephosphorylated in a coordinated manner, over the long term, to regulate the overall Raf activation process.
Figure 2. Time course of the long-term effects of PMA on Raf phosphorylation in EL4 cell lines.
WT2 (panel A) and V7 (panel B) cells were incubated for the indicated times with 100 nM PMA. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Raf (Ser259, Ser289/296/301, Ser338), c-Raf, phospho-Erk, and actin. In panel C, WT2 and V7 cells were treated with PMA for 3 hours. Whole-cell extracts, equalized for protein, were run on the same gel. Blots were probed for phospho-Raf (Ser259, Ser289/296/301, Ser338), and c-Raf to show the physical location of multiple Raf species. All incubations were done in the same experiment; the blots were exposed in parallel.
In blots for total Raf, multiple species appear between 1 and 8 hours of PMA treatment (e.g., note difference between 0 and 8 hours in Figure 2A). In order to differentiate the effects of the phosphorylation of specific residues with respect to Raf “gel shifts”, we prepared cell extracts treated with PMA for 3 hours (which show the full spectrum of bands), ran them on a single gel, and blotted for phospho-Raf (Ser259, Ser289/296/301, Ser338) and total Raf-1. The results show that Raf phosphorylated on Ser289/296/301 has the slowest migration on SDS-PAGE, and appears as the top two bands. Raf phosphorylated on Ser259 has the fastest mobility on gel, and appears as the bottom two bands. Raf phosphorylated on Ser338 is the single band in the middle (Figure 2C). The blot for total Raf-1 is expected to reveal species phosphorylated on multiple sites, as well as species phosphorylated on sites not recognized by the phospho-specific antibodies, and thus does not match exactly with the phospho-specific blots. Nonetheless, these data help to explain differences in the gel mobility of Raf-1 between cell lines (e.g., Figures 1 and 2).
3.3 PI3K/Akt regulation of PMA-induced Raf/Erk phosphorylation
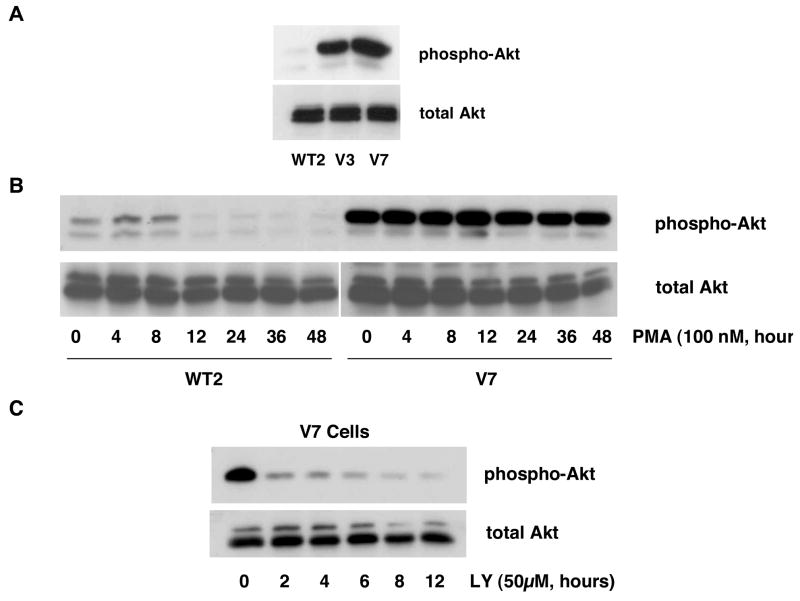
In order to test whether the PI3K/Akt signaling pathway is involved in regulation of the Raf/Erk cascade in EL4 cells, we examined basal Akt phosphorylation in representative EL4 cells (Figure 3A). The results show that there is strong constitutive Akt phosphorylation in PMA-resistant cells (V7), while basal phospho-Akt is low in PMA-sensitive cells (WT2), and moderate in intermediate cells (V3).
Figure 3. Constitutive Akt phosphorylation in EL4 cell lines.
In panel A, whole-cell extracts from WT2, V3, and V7 cells, equalized for protein, were immunoblotted for phospho-Akt and total Akt. In panel B, WT2 and V7 cells were incubated with 100 nM PMA for indicated times. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Akt and total Akt. In panel C, V7 cells were incubated with 50 μM LY294002 for indicated times. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Akt and total Akt.
To test whether constitutive Akt phosphorylation is affected by prolonged PMA treatment, we performed a time course examining phospho-Akt levels upon PMA stimulation (Figure 3B). The weak constitutive phospho-Akt signal decreased further after 12 hours of PMA treatment in WT2 cells. PMA had no detectable effect on Akt phosphorylation in V7 cells. Thus, PMA does not cause changes in Akt phosphorylation during the early time course of PMA-induced Erk activation.
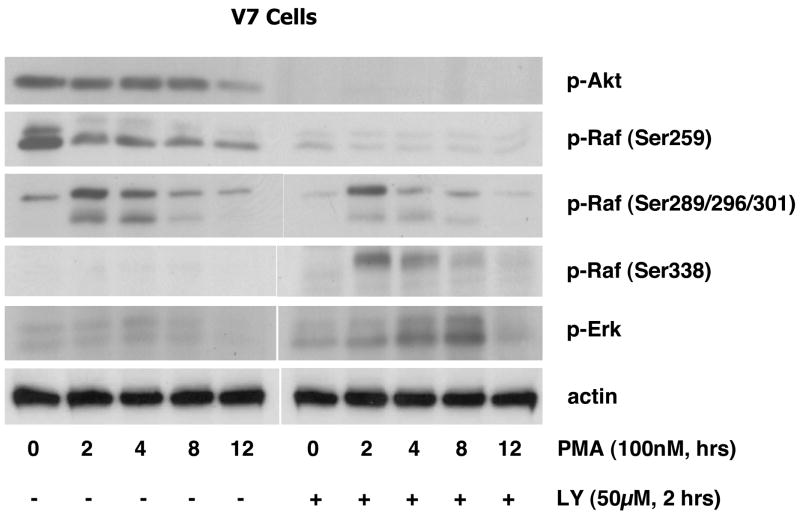
Next, to examine whether constitutive Akt phosphorylation is mediated by PI3K, we treated V7 cells with LY294002, a PI3K inhibitor. Incubation for two hours with 50μM LY294002 suppresses Akt phosphorylation (Figure 3C). Thus, constitutive Akt phosphorylation in EL4 cells is mediated by PI3K and can be blocked by LY294002.
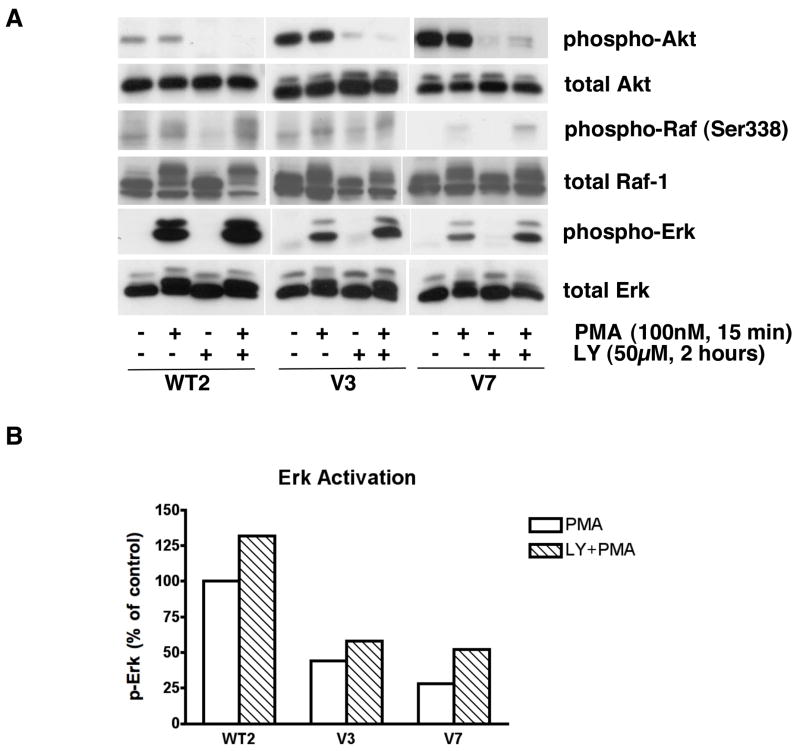
It has been reported that a negative feedback loop involving Raf and Akt exists, in which Akt phosphorylates Raf Ser259 and thereby inhibits the Raf/Erk signaling pathway [9]. To test whether this situation exists in EL4 cells, we examined the effects of LY294002 on PMA-induced Raf/Erk activation (Figure 4). PMA treatment did not alter Akt phosphorylation after 15 minutes. As shown in Figure 4A, negative regulation of Erk activation by PI3K/Akt exists in EL4 cells. In other words, there is enhanced Raf Ser338 and Erk activation in all of the EL4 cells tested when the PI3K/Akt pathway is blocked by LY294002. Erk is activated by PMA to a more similar level between resistant and intermediate cells after LY294002 treatment (Figures 4A and 4B), although (as expected) the response is still not as strong as that seen in PMA-sensitive cells.
Figure 4. Effects of LY294002 on Raf and Erk activation in EL4 cell lines.
In panel A, the indicated cell lines were incubated in the absence and presence of 50 μM LY294002 for 2 hours, and then treated with or without PMA for 15 minutes. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Akt, total Akt, phospho-Raf (Ser338), c-Raf, phospho-Erk, and total Erk. In panel B, data from panel A were quantified by densitometry and expressed as activated Erk in each cell line upon PMA treatment with/without LY294002 incubation. WT2 treated with PMA was defined as 100% for quantification.
To further examine the role of Akt, we studied the time course of PMA-induced Raf (Ser259, Ser289/296/301, Ser338) and Erk phosphorylation with and without LY294002 treatment in V7 cells. As shown in Figure 5, phosphorylation of Raf Ser259 is blocked following the inhibition of phospho-Akt by LY294002, with concomitant enhancement of Raf Ser338 and Erk phosphorylation. There is attenuation of the lower band of phospho-Raf Ser289/296/301 upon LY294002 treatment, suggesting that this species is dually phosphorylated on Ser259 and Ser289/296/301. These results demonstrate that PI3K/Akt signaling pathway negatively regulates the Raf/Erk cascade by phosphorylation of Raf Ser259.
Figure 5. Time course of the effects of LY294002 on PMA-induced Raf and Erk phosphorylation in V7 cells.
V7 cells were incubated in the absence and presence of 50 μM LY294002 for 2 hours, and then treated with PMA for the indicated times. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Akt, phospho-Raf (Ser259, Ser289/296/301, Ser338), phospho-Erk, and actin (loading control).
To test whether PTEN inactivation is responsible for the relatively high level of basal phospho-Akt in V7 cells, PTEN expression was analyzed by immunoblotting in representative EL4 cells (data not shown). WT2, V3, and V7 cells all express PTEN. There is no significant difference between PMA-sensitive and PMA-resistant EL4 cells with respect to total PTEN and constitutive phospho-PTEN. PMA does not affect PTEN phosphorylation within 15 minutes. Thus, differences in PTEN expression or phosphorylation are unlikely to be responsible for differences in constitutive Akt phosphorylation observed between EL4 cell lines.
3.4 Erk feedback regulation of PMA-induced Raf/Erk signaling
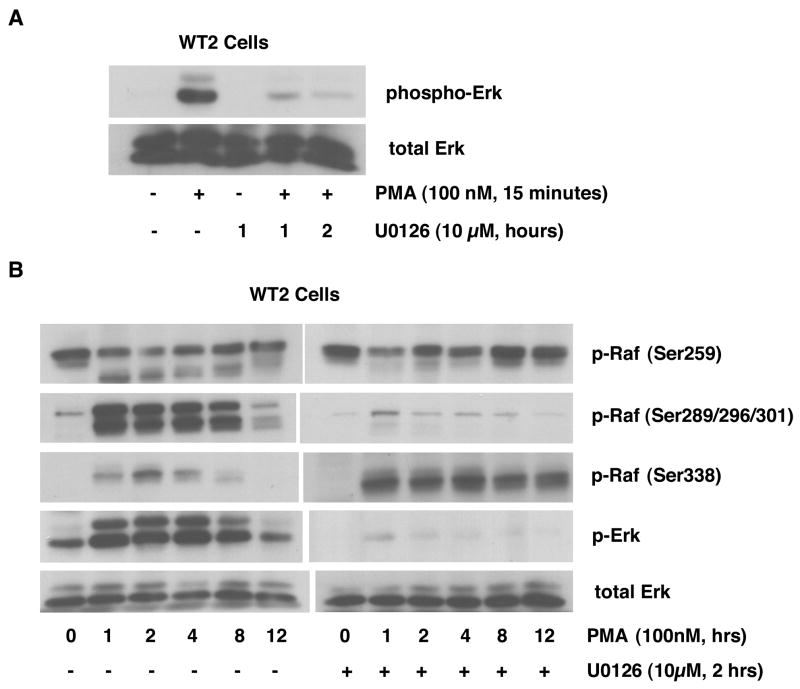
In order to further test the role of feedback auto-regulation by Erk on the Raf/Erk pathway, we used the MEK inhibitor U0126 to block Erk activation. WT2 cells, which have the strongest PMA-induced Erk activation, were utilized for this experiment to facilitate detection of U0126-induced changes. As shown in Figure 6A, PMA-induced Erk activation can be completely blocked by incubation with 10μM U0126 for 2 hours. This condition was used in subsequent experiments. We performed a long-term time course of PMA-induced Raf phosphorylation with and without U0126 treatment (Figure 6B). The results show that PMA-induced Erk activation is completely blocked by U0126. This effect is accompanied by attenuation of Raf phosphorylation on Ser289/296/301 and by enhanced Raf phosphorylation on Ser338 (both amplitude and duration). The phosphorylation of Raf Ser259 is not affected by U0126 treatment. The disappearance of the lower band in the presence of U0126 likely reflects the loss of a species dually phosphorylated on Ser259 and Ser289/296/301. These results confirm that Erk activation has an inhibitory effect on Raf, via phosphorylation of Ser289/296/301.
Figure 6. Effects of U0126 on PMA-induced Raf and Erk phosphorylation in WT2 cells.
In panel A, WT2 cells were incubated in the absence and presence of 10 μM U0126 for the indicated times, and then treated with or without PMA for 15 minutes. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Erk and total Erk. In panel B, WT2 cells were incubated in the absence and presence of 10 μM U0126 for 2 hours, and then treated with PMA for the indicated times. Whole-cell extracts, equalized for protein, were immunoblotted for phospho-Raf (Ser259, Ser289/296/301, Ser338), phospho-Erk and total Erk.
3.5 Effects of RKIP on PMA-induced Raf/Erk phosphorylation in V7 Cells
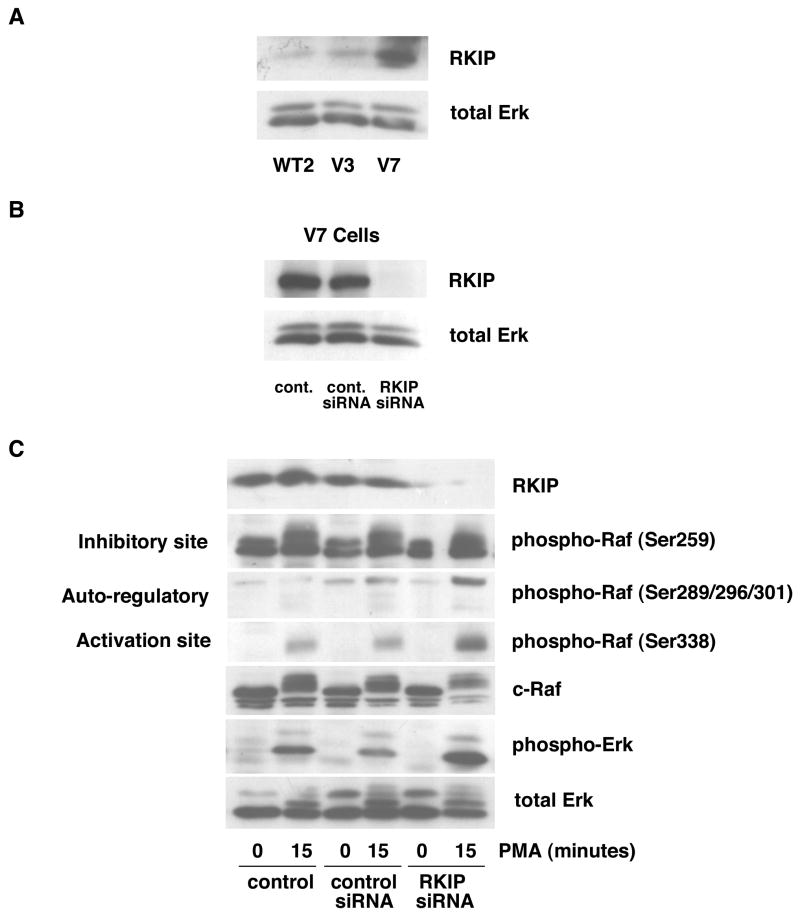
In order to examine the potential role of RKIP, we first tested levels of RKIP expression by immunoblotting in representative EL4 cell lines. The level of RKIP is higher in V7 cells as compared to WT2 and V3 cells (Figure 7A). Since RKIP selectively regulates Raf-1 activation [12], and Raf-1 is a key mediator of the Erk cascade, we explored the effects of RKIP on the phosphorylation of specific residues on Raf. To determine the role of RKIP, we tested the effects of RKIP depletion on PMA-induced Raf phosphorylation and Erk activation in V7 cells.
Figure 7. Effects of RKIP knockdown on PMA-induced Raf and Erk phosphorylation in V7 cells.
In panel A, whole-cell extracts (equalized for protein) from the indicated cell lines were immunoblotted for RKIP protein and for total Erk (loading control). In Panel B, V7 cells were incubated in the absence of siRNA (control) or in the presence of either a control siRNA or RKIP siRNA, as described in Experimental Procedures (section 2.3). The protein level for RKIP was assessed by immunoblot of whole-cell extracts, equalized for protein. In panel B, V7 cells were treated as in panel A, then incubated in the absence and presence of 100 nM PMA for 15 min. Levels of RKIP, phospho-Raf (Ser259, Ser289/296/301, Ser338), c-Raf, activated Erk (phospho-Erk), and total Erk (loading control) were assessed by immunoblotting of whole-cell extracts, equalized for protein.
First, we tested the effectiveness of RKIP siRNA (Figure 7B). V7 cells treated with RKIP siRNA demonstrated attenuated RKIP expression, while cells incubated with control siRNA show no difference as compared with untreated V7 cells. Next, the effects of RKIP knockdown on Erk activation were examined (Figure 7C). RKIP siRNA treatment potentiates PMA-induced Raf-1/Erk activation in V7 cells, as shown by increased Raf-1 Ser338 and Erk phosphorylation. Enhanced phosphorylation of Raf Ser289/296/301 is also observed in the absence of RKIP, which is expected considering that Ser289/296/301 phosphorylation is mediated by Erk feedback phosphorylation. The phosphorylation of Raf Ser259 is not affected by RKIP siRNA. Finally, basal Raf and Erk phosphorylations are not affected by RKIP knockdown. Thus, RKIP modulates PMA-induced Raf signaling directly, as well as indirectly via Erk feedback. The end result is for RKIP to limit the response of the cell to the PMA stimulus.
4.0 DISCUSSION
In this study, we utilized a characterized panel of EL4 cell lines to examine multiple regulatory steps that modulate Raf activation. The results show that negative regulation of Raf Ser259 by the PI3K/Akt pathway plays a role in the differences in PMA-induced Raf/Erk activation between PMA-resistant and intermediate cells. In addition, Erk-mediated feedback inhibition at Raf Ser289/296/301 contributes to the extent and duration of PMA-induced Raf Ser338 activation. Finally, our results show that expression of RKIP inversely correlates with PMA-induced Raf-1 and Erk activity in EL4 cells, providing an additional modulatory influence. This study illustrates that phosphorylations of different residues on Raf contribute to the Raf activation/deactivation process, and that multiple regulatory inputs are involved.
Phosphorylation/dephosphorylation of Raf plays a crucial role in the Raf activation process. Phosphorylation of Raf Ser338 correlates with Ras-mediated stimulation and is required for Raf activation [4]. Raf-1 activity is negatively regulated by phosphorylation of Ser259; Raf-1 activation is accompanied by dephosphorylation of this site [10]. Several studies have shown that phosphorylation of Raf Ser259 by protein kinase A results in negative regulation of Raf-1 [13–15]. Other investigations have suggested that inhibitory cross-talk, mediated by phosphorylation of Raf Ser259 by PI3K/Akt, is an important facet of Raf regulation [16,17]. Ser259 may also be phosphorylated as a result of JNK activation [18]. Our data support a significant role for the PI3K/Akt pathway in EL4 cells, in that the phosphorylation of Ser259 via Akt negatively regulates Raf Ser338 phosphorylation and subsequent activation of Raf-1. When basal Akt activity and phosphorylation of Raf Ser259 were blocked by a PI3K inhibitor, Raf Ser338 and Erk activation were enhanced. Raf and Erk were activated by PMA to a greater extent in resistant and intermediate cells in the presence of LY294002, although the extent of activation was still not as strong as that seen in PMA-sensitive cells. This is consistent with our previous finding that RasGRP expression is largely responsible for the very high level of PMA-induced Erk activation in PMA-sensitive EL4 cells. It is important to note that Ser259 phosphorylation does not directly interfere with Raf-1 activity, but rather regulates activity through protein-protein interactions [19]. Together, our data indicate that the negative regulation mediated by different levels of basal phospho-Akt activity contributes in part to the distinct patterns of PMA-induced Raf/Erk activation between resistant and intermediate EL4 cells.
The role of Erk feedback in Raf activation is complex. It has been reported that Raf Ser296 and 301 contribute to negative regulation of Raf-1 [4], and that phosphorylation of Ser338 in response to Ras activation blocks the auto-inhibition mediated by Erk [10]. Erk-mediated phosphorylation can promote disassembly of heterodimers between Raf-1 and B-Raf [20], which reduces Raf activity since such heterodimers have much higher kinase activity than Raf monomers. However, Erk activation can also provide a novel mechanism for a positive feedback of Raf-1 regulation [3]. In our study, we find that phosphorylations of Ser338 and Ser289/296/301 occur simultaneously. This suggests that phosphorylation of Ser289/296/301 by Erk counteracts the phosphorylation of Ser 338, which may be a key mechanism for preventing the over-amplification of a proliferative signal. A previous study indicated that in Swiss 3T3 cells, a MEK inhibitor increases Raf-1 activation by PDGF but not by EGF [21]. In this regard, it is possible that Erk feedback auto-inhibition is an agonist-dependent phenomenon. Our findings add another layer of complexity to the Raf-1 regulation mechanism and suggest that varying Erk activation levels can modulate the output from Raf-1.
Finally, we found that the basal level of RKIP expression is higher in V7 cells than in WT2 and V3 cells. RKIP functions as a negative modulator that controls the amplitude of Raf kinase activity rather than acting as a “switch” [22]. In mammalian cells, RKIP inhibits Raf-1 signaling to Erk, suppressing Raf-1-induced transformation [23]. Signaling downstream of MEK is attenuated when RKIP blocks the interaction between Raf and MEK [1]. In this study, we effectively suppressed RKIP expression in V7 cells by RKIP siRNA, and then studied the PMA-induced Raf/Erk phosphorylation status. Down-regulation of RKIP potentiated PMA-induced Raf-1/Erk activation in V7 cells, as shown by the increased Raf-1 Ser338 and Erk phosphorylation. Activation of Raf-1 is dependent on phosphorylation at Ser 338 by Ras [24]. Thus, inhibition of this phosphorylation event by RKIP would impair activation of downstream Erk activation. Our results also indicate that PMA-induced phosphorylation of Raf Ser289/296/301 is enhanced after RKIP knockdown, suggesting increased Erk feedback phosphorylation. Thus, RKIP modulates Raf signaling both directly and indirectly via Erk feedback, in both cases limiting the response of the cell to PMA stimuli The ability of RKIP depletion to potentiate PMA-induced Erk activation is consistent with results reported by others [25–27]. However, the site-specific analysis reported here reveals that phosphorylation of Raf Ser259 was not affected by RKIP, consistent with Ser259 being the site regulated by the PI3K/Akt cascade. These data support a residue-specific regulation of Raf by RKIP. Thus RKIP, acting as an inhibitor of the Raf-1/MAPK signaling cascade, plays a key role in regulating the Raf-1/Erk pathway.
6.0 CONCLUSIONS
In summary, our results demonstrate that multiple signaling proteins, including Akt, Erk, and RKIP, regulate PMA-induced Raf-1 phosphorylation in a residue-specific manner to generate a profile of Raf and Erk activation over time. Natural variations between clonal cell lines in the level of expression of proteins involved in Raf regulation modulate the extent and kinetics of Erk activation in response to PMA. This work provides an unusually detailed profile of the upstream phosphorylation events involved in PMA-mediated Erk activation.
Acknowledgments
This work was supported by a grant from the NIH (CA094144-01).
Footnotes
Abbreviations: The abbreviations used are: PMA, phorbol 12-myristate 13-acetate; RT-PCR, reverse transcriptase polymerase chain reaction; siRNA, small interfering RNA; WT, wild-type (PMA-sensitive) EL4 cells.
8.0 REFERENCES
- 1.Klysik J, Theroux SJ, Sedivy JM, Moffit JS, Boekelheide K. Cell Signal. 2008;20:1–9. doi: 10.1016/j.cellsig.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen P, McPhillips F, Monia BP, Smyth JF, Langdon SP. Int J Cancer. 2006;118:1565–1571. doi: 10.1002/ijc.21520. [DOI] [PubMed] [Google Scholar]
- 3.Balan V, Leicht DT, Zhu J, Balan K, Kaplun A, Singh-Gupta V, Qin J, Ruan H, Comb MJ, Gzivlon G. Mol Bio Cell. 2006;17:1141–1153. doi: 10.1091/mbc.E04-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hekman M, Fischer A, Wennogle LP, Wang YK, Campbell SL, Rapp UR. FEBS Lett. 2005;579:464–468. doi: 10.1016/j.febslet.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 5.Feng L, Xie X, Ding Q, Lo X, He J, Fan F, Liu W, Wang Z, Chen Y. Proc Natl Acad Sci USA. 2007;104:14348–14353. doi: 10.1073/pnas.0701298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku H, Meier KE. J Biol Chem. 2000;275:11333–11340. doi: 10.1074/jbc.275.15.11333. [DOI] [PubMed] [Google Scholar]
- 7.Han S, Knoepp SM, Hallman MA, Meier KE. Mol Pharmacol. 2007;71:314–322. doi: 10.1124/mol.106.028639. [DOI] [PubMed] [Google Scholar]
- 8.Sansbury HM, Wisehart-Johnson AE, Qi C, Fulwood S, Meier KE. Carcin. 1997:1817–1824. doi: 10.1093/carcin/18.9.1817. [DOI] [PubMed] [Google Scholar]
- 9.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 10.Knoepp SM, Chahal MS, Xie Y, Zhang Z, Brauner DJ, Hallman MA, Robinson SA, Han S, Imai M, Tomlinson S, Meier KE. Mol Pharmacol. 2008 doi: 10.1124/mol.107.040105. [DOI] [PubMed] [Google Scholar]
- 11.Tran NH, Wu X, Frost XJA. J Biol Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- 12.Trakul N, Rosner MR. Cell Res. 2005;15:19–23. doi: 10.1038/sj.cr.7290258. [DOI] [PubMed] [Google Scholar]
- 13.Bulow W, Dubben S, Engelhardt G, Hebel S, Plumaker B, Heine H. J Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 14.Dumaz N, Light Y, Marais R. Mol Cell Biol. 2002;22:3717–3728. doi: 10.1128/MCB.22.11.3717-3728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Mol Cell Biol. 2002;22:3237–3246. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. J Biol Chem. 2001;276:33630–33637. doi: 10.1074/jbc.M105322200. [DOI] [PubMed] [Google Scholar]
- 17.Laprise P, Langlois MJ, Boucher MJ, Jobin C, Rivard N. J Cell Physiol. 2004;199:32–39. doi: 10.1002/jcp.10432. [DOI] [PubMed] [Google Scholar]
- 18.Adler V, Bowne W, Michl J, Sookraj KA, Ikram K, Pestka S, Izotova L, Zenilman M, Friedman FD, Qu Y, Pincus MR. Ann Clin Lab Sci. 2008;38:47–56. [PubMed] [Google Scholar]
- 19.Light Y, Paterson H, Marais R. Mol Cell Biol. 2002;22:4984–4996. doi: 10.1128/MCB.22.14.4984-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rushworth LK, Hindley AD, O’Neill E, Kolch W. Mol Cell Biol. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 22.Rosner MR. Cell Div. 2007;2:1. doi: 10.1186/1747-1028-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Mol Cell Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trakul N, Menard RE, Schade GR, Qian Z, Rosner MR. J Biol Chem. 2005;26:24931–24940. doi: 10.1074/jbc.M413929200. [DOI] [PubMed] [Google Scholar]
- 25.Matheny SA, Chen C, Kortum RL, Razidlo GL, Lewis RE, Write MA. Nature. 2004;427:256–260. doi: 10.1038/nature02237. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, Sasaki M, Kuriyama M, Saito N, Shibuy M, Yoshimura A. Nat Cell Biol. 2003;5:427–432. doi: 10.1038/ncb978. [DOI] [PubMed] [Google Scholar]
- 27.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A. Nature. 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]