Abstract
An increase in corticotropin-releasing factor (CRF) is a putative factor in the pathophysiology of stress-related disorders. As CRF expression in the central nucleus of the amygdala (CeA) is important in adaptation to chronic stress, we hypothesized that unrestrained synthesis of CRF in CeA would mimic the consequences of chronic stress exposure and cause dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, increase emotionality and disrupt reproduction. To test this hypothesis, we used a lentiviral vector to increase CRF-expression site specifically in CeA of female rats. Increased synthesis of CRF in CeA amplified CRF and arginine vasopressin peptide concentration in the paraventricular nucleus of the hypothalamus, and decreased glucocorticoid negative feedback, both markers associated with the pathophysiology of depression. In addition, continuous expression of CRF in CeA also increased the acoustic startle response and depressive-like behavior in the forced swim test. Protein levels of gonadotropin-releasing hormone in the medial preoptic area were significantly reduced by continuous expression of CRF in CeA and this was associated with a lengthening of estrous cycles. Finally, sexual motivation but not sexual receptivity was significantly attenuated by continuous CRF synthesis in ovariectomized estradiol-progesterone-primed females. These data indicate that unrestrained CRF synthesis in CeA produces a dysregulation of the HPA axis, as well as many of the behavioral, physiological and reproductive consequences associated with stress-related disorders.
Keywords: CRF, CeA, emotionality, stress, sexual motivation, fertility
Introduction
Individuals adapt to stress exposure to restore homeostasis and maintain physical and emotional health. However, exposure to chronic stressors results in dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, mood-related disorders and a disruption in reproduction.1–4 This is of particular importance to women because the occurrence of stress-related mood disorders is more prevalent in women5 and stress-induced infertility in women, or functional hypothalamic anovulation,6 is associated with increased risks for cardiovascular disease, osteoporosis and dementia.6–8
A factor common to both stress-related psychopathology and reproductive dysfunction is the overactivity of central corticotropin-releasing factor (CRF). Notably, levels of CRF in cerebrospinal fluid (CSF) are elevated in depression and posttraumatic stress disorder (PTSD).9–11 In addition, CRF also disrupts gonadotropin-releasing hormone (GnRH) production and suppresses reproductive behavior.12–15 Thus, the inability to restrain central CRF is a precipitating factor in the stress-induced dysregulation of both of these systems.
CRF is heterogeneously distributed throughout the brain such as the paraventricular nucleus of the hypothalamus (PVN) and portions of the extended amygdala, including the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA).16–18 These regions are candidate sites wherein unconstrained CRF expression may be responsible for the disruption of affect and reproduction. During chronic stress, CRF is upregulated in CeA and BNST.19–21 In fact, as little as 24 h of increased glucocorticoid secretion stimulates the production of CRF in CeA.22 Furthermore, concentrations of arginine vasopressin (AVP) are increased whereas CRF levels are decreased in PVN in response to chronic stressors.23,24 This increase in AVP maintains adrenocorticotropic hormone (ACTH) release from the pituitary in the face of reduced CRF release from PVN.23–26 Thus, the downregulation of CRF in PVN and the upregulation of CRF in CeA are crucial for the adaptation to prolonged exposure to stressors.27–29 A disruption of this control may be involved in the development of a maladaptive response to chronic stress and result in disturbances in emotional regulation and reproduction.
In this study we hypothesized that a continuous production of CRF in CeA in female rats would dysregulate the HPA axis, increase anxiety behavior and disrupt reproduction. To test this, we used a lentiviral vector to site-specifically express CRF constitutively in CeA of female Sprague–Dawley rats and assessed changes in the regulation of the HPA axis, emotional and sexual behavior, and reproductive physiology.
Materials and methods
Production and testing of recombinant lentiviral vectors
Lentiviral vectors are extremely useful for in vivo studies in the CNS because they have a large insert capacity, generate little or no immune response, maintain expression for the life of the animal and can transduce nondividing cells, preferentially infecting neurons when injected into the brain.30–33 Lentiviral vectors have proved to be useful vehicles for efficient, long-term, stable gene delivery into the CNS without generating an immune response.34 Because these vectors are replication deficient, they do not leave the site of injection,33,35 making it possible to do site-specific studies such as the ones described in this analysis.
Plasmid construction
Viral vectors are derived from the HIV-based lentiviral backbones optimized by the laboratory of Dr Didier Trono.31 The Lenti-CMV-GFP viral plasmid is the ‘pCM02’ vector, which was a generous gift from the lab of Dr Joshy Jacob. PCM02 was created by inserting the 1.4 kb BamHI/XhoI fragment containing GFP-WPRE from the pHR′-CMV-GFP-WPRE plasmid36 into BamHI/XhoI sites of the pHR-GFP-SIN backbone in place of the green fluorescent protein (GFP) fragment.36 The resulting pCM02 lentivirus-packaging vector contains a cytomegalovirus (CMV) promoter driving GFP expression followed by a woodchuck posttranscriptional regulatory element (WPRE).
The Lenti-CMV-CRF-Ires-GFP virus (hereafter referred to as ‘LENTI-CMV-CRF’) was constructed as follows: The CRF coding sequence plasmid, a generous gift from Wylie Vale (Salk Institute), was digested with EcoRI and cloned into pIRES2-EGFP (5.3 Kb; Clontech Laboratories, Mountain View, CA, USA). The CRF-IRES-GFP segment was then double digested with BglII and HpaI and the lentiviral vector backbone pCMO2 was digested with EcoRI and BamHI. Both of these plasmids were incubated with T4 DNA polymerase (New England Biolabs, Ipswich, MA, USA), following the manufacturer's protocol, to make blunt ends and then ligated together following the manufacturer's protocol using DNA ligase (New England Biolabs). The final viral vector clone was restriction digest verified and tested for expression efficiency and coexpression of GFP and CRF as in Figure 1.
Figure 1.
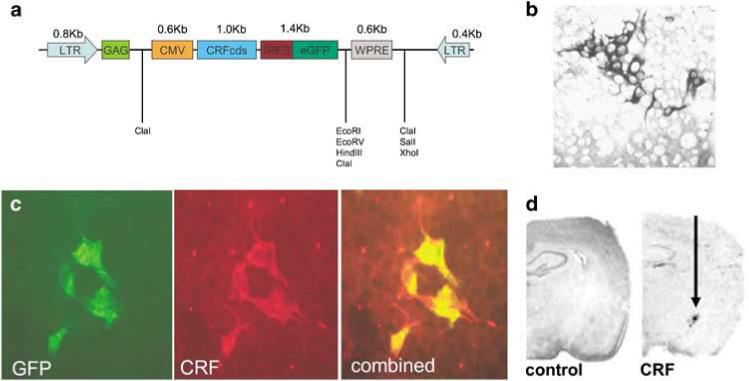
Constitutive CRF overexpression. (a) Schematic diagram of the DNA plasmid encoding the lentiviral (LV) construct for constitutive corticotropin-releasing hormone expression; CMV: cytomegalovirus; CRFcds: corticotropin-releasing hormone cDNA; IRES: internal ribosomal entry site; eGFP: enhanced green fluorescent protein; LTR: long terminal repeat. (b) In vitro functional assay. Immunocytochemistry with anti-CRF antibody demonstrates corticotropin-releasing hormone (CRF) protein production in HEK 293 cells visualized with 3,3 diaminobenzidine (DAB). (c) Coexpression of GFP and CRF in Lenti-CRF infected HEK293 cells is verified with double-immunofluorescent staining with both anti-GFP antibodies (green), anti-CRF antibody (red) and combined overlay. (d) In vivo functional assay. LV-CMV-CRF-induced increased CRF transcript expression in rat central nucleus of the amygdala (CeA; left) versus control virus (right) demonstrated via in situ hybridization.
Preparation of viral stocks
Virus was generated by transient cotransfection of the expression plasmid (20 μg), VSV-G pseudotyping construct (10 μg) and the packaging construct pCMVDR8.91 (20 μg) into a 150 mm plate of 90% confluent 293 T cells as previously described.37–39 Medium was collected 48 and 72 h post transfection, cleared of debris by low-speed centrifugation and filtered through 0.45-μm filters. High-titer stocks were prepared by an initial ultracentrifugation for 1 h at 23 000 r.p.m. (SW-28 rotor), and a secondary tabletop centrifugation at 13 000 r.p.m. for 30 min. Viral pellet was resuspended in 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS), and stored at 80 °C. Viral titers were determined by infection of 293 T cells. GFP positive cells were visualized by fluorescent microscopy. CRF positive cells were visualized by immunocytochemistry (ICC) as described separately, with anti-CRF dilutions of 1:1000−1:10 000.
Animals and housing
Adult intact female Sprague–Dawley rats (age 40 days; n = 12; 125−150 g) from Harlan Laboratories (Indianapolis, IN, USA) were single housed, on a 12:12 h light/dark cycle (lights on at 0700 hours). These intact animals were used to monitor disruption of estrous cycles, glucocorticoid negative feedback, measures of emotionality, and they provided tissue of immunohistochemical analysis. One female assigned to the group receiving the Lenti-CMV-CRF treatment died during surgery, resulting in five animals in this group and six in the GFP-injected controls. A second set of adult ovariectomized (OVX) Sprague–Dawley rats (n = 12; 150−175 g, Harlan Laboratories) were single housed, on a 12:12 h reverse light/dark cycle (with lights out starting at 0700 hours), and were used to assess sexual behavior. Both the GFP- and Lenti-CMV-CRF-injected groups had six animals each. All animals were provided with phytoestrogen-free diet (Harlan Diet no. 2016) and water ad libitum. The lentivirus would express CRF or GFP for the life of the animal. All procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Surgical procedures
Animals were anesthetized with isoflurane and placed in a stereotaxic apparatus (Model 900; Kopf Instruments, Tujunga, CA, USA). A 10 μl Hamilton microsyringe (22 gauge beveled-tip needle), previously coated with 1% BSA, was lowered to the target region. Injection coordinates relative to bregma were CeA: AP −2.3; ML 3.7; DV −8.0. Animals received 1 μl of virus per region at a rate of 0.2 μl min−1 (UltramicropumpII; World Precision Instruments, Sarasota, FL, USA). The needle was left in place for 5 min after the injection and slowly removed over a 5-min period. The skin was closed using a 6−0 Vicryl suture (Ethicon; Johnson & Johnson, Piscataway, NJ, USA). Animals were allowed 2 weeks for recovery and sufficient time for the virus to infect cells at the locus and induce them to start producing CRF or GFP. The lentivirus will express CRF or GFP for the life of the animal. Following completion of the tests and assessments described below, animals were killed at ∼7 months of age.
Monitoring estrous cycles
Vaginal smears were taken daily between 1000 and 1300 hours for 6 weeks. Several drops of sterile water were inserted into the vagina via a glass medicine dropper and were withdrawn. The fluid was placed onto a microscope slide. Slides were examined whereas they were wet at ×20 under a light microscope. The phase of estrous cycle was determined based on the predominant cell type present on each day according to standard criteria.40,41 Stages of the estrous cycle were: (1) large clumps of round, nucleated epithelial cells, a few cornified cells and no leukocytes—proestrus, (2) clumps of cornified cells, little or no round nucleated epithelial cells, no leukocytes—estrus, (3) some round nucleated epithelial cells, some cornified cells and some leukocytes and mucus—metestrus and (4) mostly leukocytes, some round nucleated epithelial cells—diestrus.40,41 At the conclusion of these 6 weeks, behavioral testing was initiated with one test paradigm a week.
Dexamethasone suppression test
The dexamethasone (DEX) suppression test was conducted to assess the consequences of increased CRF release from CeA on glucocorticoid negative feedback. DEX administration was timed to suppress the zenith of diurnal corticosterone rhythm. At 1100 hours or 4 h after lights came on, a baseline plasma sample was obtained followed by a DEX injection (30 mg kg−1 given i.p.). Animals were returned to their home cages and remained undisturbed for the next 6 h. Subsequent plasma samples were obtained at 1500 and 1700 hours. All plasma samples (0.2 ml) were obtained by venipuncture of the saphenous vein whereas the animals were briefly anesthetized with isoflurane. All samples were obtained within 2 min of removing the animal from its homeroom to minimize corticosterone release in response to environment change and handling.42 Corticosterone levels were analyzed by radioimmunoassay using a commercially available kit (Diagnostic Products Corporation, Los Angeles, CA, USA). The assay has a sensitivity of 5 ng ml−1, assaying 50 μl of plasma. The inter- and intra-assay coefficients of variation were 5.88% and 1.31%, respectively.
Behavioral tests
Forced swim test
The Porsolt methodology was followed to assess depressive-like behavior: time spent struggling versus time spent immobile.43 Briefly, the rats were placed individually in a translucent container (40 × 24 × 60 cm) during the light phase of their light cycle. This apparatus was filled with water (∼24 °C) to a depth of approximately 22 cm so that the animals could not rest on the bottom nor reach the top of the container. A conditioning trial was given to animals the day before test day comprising of a 15 min swim, after which animals were toweled off with paper towels and returned to their home cage. Twenty-four hours later, animals were given a 5-min swim test that was video recorded for later scoring. An observer blind to experimental condition assessed time spent passively floating, barely moving so as to keep nose above the water, and time spent actively coping, struggling to get out of the water. Immobility time is a measure of depressive-like behavior that responds to the administration of antidepressant drugs.43
Baseline acoustic startle test
Animals were tested in 8 × 15 × 15 cm wire mesh cages where the floor consisted of four 6.0-mm-diameter stainless steel bars spaced 18 mm apart.44 The cages are suspended between compression springs in a steel frame within a sound-attenuating, ventilated chamber (inside dimensions, 56 × 56 × 81 cm; Industrial Acoustics, Bronx, NY, USA). A General Radio (Concord, MA, USA) type 1390-B noise generator provided background noise (60 dB; wideband) that was delivered by high-frequency speakers (Supertweeter; Radio Shack, Tandy, Fort Worth, TX, USA) that were positioned 5 cm from the front of the cage. A Bruel & Kjaer (Marlborough, MA, USA) model 2235 sound-level meter (A scale; random input) was used to measure sound levels with the microphone (type 4176) located 7 cm from the center of the speaker. This distance approximated the distance between the rat's ear and the speaker during testing. Baseline startle responses were evoked by 50 ms white-noise bursts (5 ms rise-decay) generated by a Macintosh G3 computer sound file (0−22 kHz) that were run through a Radio Shack amplifier (100 watt; model MPA-2000) and played through the same speakers used for background noise. The amplitude of startle responses was measured using an Endevco (San Juan Capistrano, CA, USA) 2217E accelerometer. The cage movements produced by the startle response of the individual rat results in the displacement of the accelerometer, the output of which was integrated to produce a voltage proportional to the velocity of the cage movement. An Endevco model 104 amplifier was used to amplify the output signal, which then was digitized by an InstruNET device (model 100B; GW Instruments, Somerville, MA, USA) interfaced with a Macintosh G3 computer, on a 0−2500 unit scale. The startle amplitudes were defined as the maximal peak-to-peak voltage that occurred within the first 200 ms after the onset of the startle-eliciting noise. Rats were given two baseline startle tests 24 h apart, during the light period of their light/dark cycle. Startle measures from both tests were averaged together.
Sexual behavior tests
Following 2 weeks of recovery from neurosurgery, OVX females received standard hormonal priming (2.5 μg of estradiol benzoate in 100 ml of oil 72 h before test, 10 μg of estradiol benzoate in 100 μl of oil 48 h before test and 500 μg of progesterone in 100 μl of oil 4−6 h before the test) and were tested for proceptivity and receptivity in a paced mating chamber for 10 min.45 All testing was done in the first several hours of the dark phase under red light. Lordosis frequency and lordosis quotients (number of lordoses/number of mounts) were calculated to assess sexual receptivity, and hops and darts were tabulated to assess proceptivity.46 Animals were tested on two separate occasions 2 weeks apart.
Immunohistochemistry
Animals were given an overdose of 4% chlorohydrate and perfused transcardially with 250 ml of 0.9% sodium chloride containing 0.1% sodium nitrite, followed by 250 ml of 4% paraformaldehyde in 0.1 m phosphate buffer containing 2.5% acrolein. Control females were killed in diestrus. As Lenti-CMV-CRF-injected females were not cycling normally, these females were killed on a day most closely resembling diestrus. Brains were removed and placed into 4% paraformaldehyde for postfixation. Brains were serially sectioned at 30mm on a microtome and processed for immunohistochemistry. Parallel series were processed for CRF, GFP, GnRH and AVP immunoreactivity. Free-floating sections were rinsed in potassium PBS (KPBS; 0.1 m, pH 7.4) and then washed for 30 min in 0.5% hydrogen peroxide. Sections were washed again with KPBS and then incubated in primary antibody solution containing 0.4% Triton X at room temperature for an hour and transferred to 4 °C. Incubation times with primary antibodies varied according to protein being targeted: CRF (a kind gift from Dr Silverman at Columbia University) at a concentration of 1:100 000 for 48 h, GFP (Invitrogen, Carlsbad, CA, USA; A11120) at a concentration of 1:10 000 for 48 h, GnRH (Santa Cruz, Santa Cruz, CA, USA; HU11B) at a concentration of 1:10 000 for 96 h, and AVP (Phoenix Pharmaceuticals, Burlingame, CA, USA; H-065−07) at a concentration of 1:100 00 for 48 h. Sections were again thoroughly washed with KPBS and incubated at room temperature for 1 h in biotinylated goat anti-rabbit immunoglobulin G antibody (Vectastain Elite RTU ABC kit; Vector Laboratories, Burlingame, CA, USA). This was followed by more KPBS washing and a 1-h incubation in avidinbiotin-peroxidase complex solution (Vectastain Elite RTU ABC kit; Vector Laboratories). Following this incubation, sections were washed in KPBS and then sodium acetate (0.175 m) for 15 min. Visualization of immunoreactivity was accomplished through a 3,3′-diaminobenzedine (0.2 mg ml−1) and 3% hydrogen peroxide (83 μl ml−1) reaction in a sodium acetate solution. The reaction was terminated after 10−15 min with thorough sodium acetate rinsing, followed by KPBS washes. Sections were mounted out of KPBS onto Superfrost plus slides (Fisher Scientific, Pittsburgh, PA, USA), air dried overnight and dehydrated through a series of graded ethanol, cleared in Histoclear (Fisher Scientific) and coverslipped using permount. Immunopositive cells were quantified by eye by the same researcher. All sections for each protein stain were run in the same reaction as to minimize interassay variability.
In situ hybridization
Lentiviral-vector induced CRF expression was examined using in situ hybridization. The rat prepro-CRF plasmid (K. Mayo, Northwestern University, Evanson, IL, USA) was linearized with PvuII and transcribed with SP6 polymerase to generate a 593-base 35S-UTP labeled riboprobes. Prehybridization slides were brought to room temperature, postfixed in 4% paraformaldehyde, pH 7.5, and rinsed in PBS. The remaining steps included proteinase K treatment, acetylation, dehydration, overnight hybridization in a humidified chamber (50 °C), RNase A digestion and washes to a final stringency of 0.1% standard saline citrate,47 0.1% dithiothreitol, 60 °C for 30 min, and these were performed as previously described.48 After being stringently washed, slides were dried and placed against Kodak (Rochester, NY, USA) MR autoradiography film for at least 18 h.
Results
Design of the Lenti-CMV-CRF vector and verification of CRF constitutive expression
A Lenti-CMV vector coexpressing CRF and GFP was made as described in Methods and illustrated in Figure 1a. The virus was titered in 293 T cells by infecting equally confluent wells with serial dilutions of the virus and staining for CRF peptide expression using ICC (Figure 1b). We then confirmed that cells that expressed high levels of GFP also coexpressed high levels of CRF using double-fluorescence ICC (Figure 1c). Finally, to test the infectivity of the virus in vivo, it was injected into CeA of adult male Sprague–Dawley rats weighing approximately 300 g at the time of the surgery using a sham injection as the control. At least 10 days following surgery, rats were killed and lentiviral-vector-induced CRF expression was examined using in situ hybridization (Figure 1d-CRF) demonstrating enhanced CRF mRNA expression compared to control (Figure 1d-control).
Once we had demonstrated that the virus could successfully infect cells in vivo and cause cells to produce CRF, we proceeded to verify that we could get site-specific protein expression in CeA in female rats using stereotaxic placement with coordinates from Paxinos and Watson49 and immunohistochemistry for CRF. Control animals in all these studies were injected with a control viral vector expressing only GFP and we verified that only animals with Lenti-CMV-CRF injected in CeA showed increased CRF protein in CeA (Figures 2a–d; t9 = 4.82, P < 0.01). Once we had demonstrated that Lenti-CMV-CRF injection yielded sitespecific increases in CRF in CeA in our female rats, we then proceeded to perform the physiological and behavioral experiments.
Figure 2.
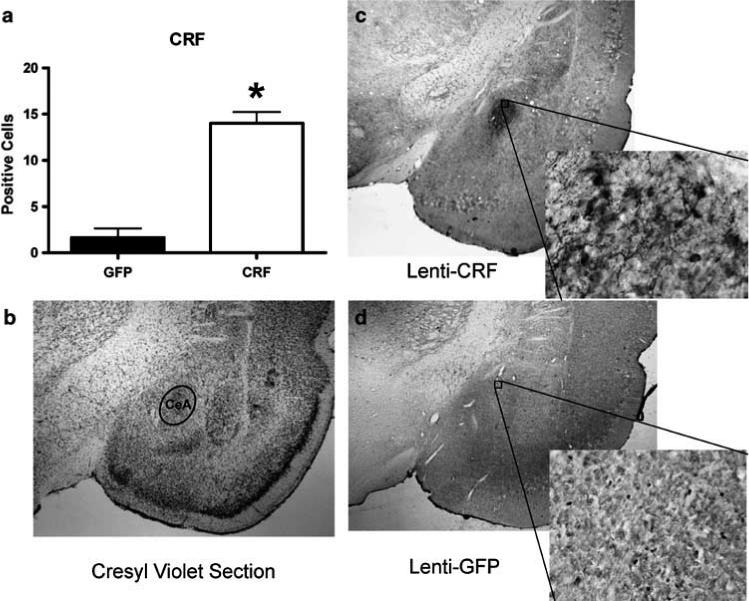
Lenti-CMV-CRF injection into the central nucleus of the amygdala (CeA) significantly increased corticotropin-releasing hormone (CRF) protein production site-specifically. (a) Mean±s.e.m. number of positively labeled CRF cells in CeA of Lenti-CMV-GFP (open bars) and Lenti-CMV-CRF treated female rats (closed bars) determined by immunohistochemistry. *P < 0.05. (b) A cresyl violet stained section representing the section used to quantify the number of CRF positive neurons in CeA. (c) A representative section at ×2 magnification with an additional ×20 magnification inset showing the effects of Lenti-CMV-CRF injection into CeA on the number of positively labeled CRF neurons. (d) A representative section at ×2 magnification with an additional ×20 magnification inset showing the amount of staining observed in the control, Lenti-CMV-GFP-treated female rats.
Dysregulation of HPA axis
CeA Lenti-CMV-CRF-injected females showed a significant increase in CRF (Figures 3a and b; t9 = 6.53, P < 0.01) and in AVP (Figures 3d and e; t9 = 2.88, P = 0.02) in PVN. To examine the effect of increased CRF synthesis from Lenti-CMV-CRF in CeA on HPA negative feedback, we performed a DEX suppression test (Figure 4). The response to DEX varied significantly by treatment over time (F2,18 = 14.32, P < 0.01). Although DEX suppressed plasma corticosterone similarly in control and Lenti-CMV-CRF-injected females at 4 h post injection, corticosterone levels were significantly elevated in Lenti-CMV-CRF-infected females compared to controls by 6 h post injection (t9 = 5.67, P < 0.01), indicating that the Lenti-CMV-CRF animals had escaped from glucocorticoid negative feedback.
Figure 3.
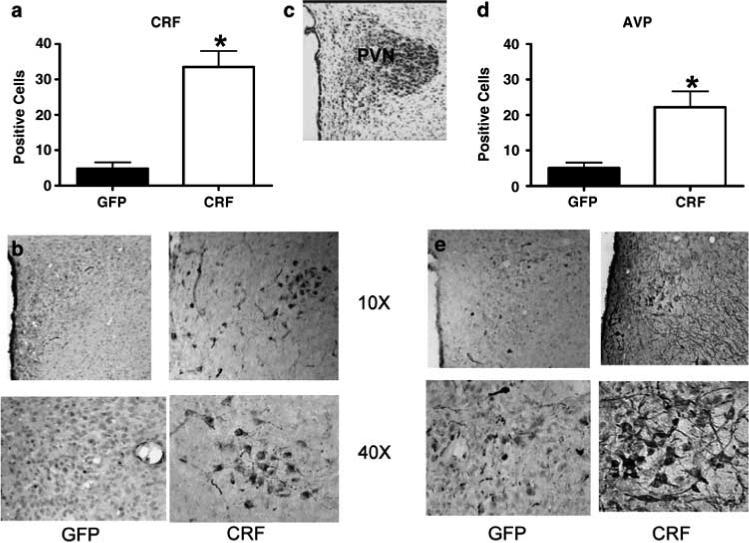
Lenti-CMV-CRF injection increased corticotropin-releasing hormone (CRF) and arginine vasopressin (AVP) protein expression in paraventricular nucleus of the hypothalamus (PVN). (a) Mean±s.e.m. number of positively labeled CRF cells in PVN of Lenti-CMV-GFP (open bars) and Lenti-CMV-CRF-treated female rats (closed bars) determined by immunohistochemistry. *P < 0.05. (b) Representative coronal sections at ×10 and ×40 magnification showing the effects of Lenti-CMV-CRF injection into the central nucleus of the amygdala (CeA) on the number of positively labeled CRF neurons in PVN. (c) Cresyl violet stained coronal section representative of the section used to quantify and number of CRF and AVP positive cells in each animal. (d) Number of positively labeled AVP cells in PVN of Lenti-CMV-GFP and Lenti-CMV-CRF treated female rats via immunohistochemistry. (e) Representative coronal sections at ×10 and ×20 magnification showing the effects of Lenti-CMV-CRF injection into CeA on the number of positively labeled AVP neurons in PVN.
Figure 4.
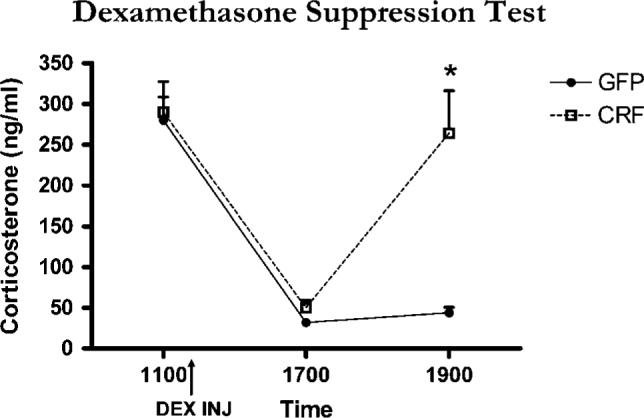
Effect of Lenti-CMV-CRF injection on negative feedback regulation of the hypothalamic–pituitary–adrenal (HPA) axis as assessed by the dexamethasone suppression test. Mean±s.e.m. corticosterone levels before and following a dexamethasone injection (shown by arrow) for green fluorescent protein (GFP)-injected control (open symbol) and Lenti-CMV-CRF-injected females (closed symbol). *P < 0.05.
Effects on emotionality
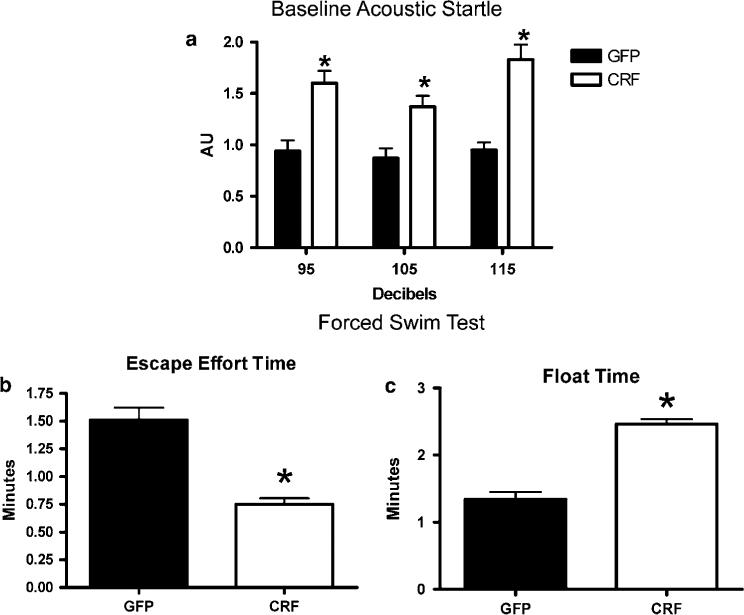
Locomotor activity, measured just before the first acoustic startle test, was not significantly different between Lenti-CMV-CRF-injected (0.214±0.040) and GFP-injected females (0.192±0.047; P > 0.05). The acoustic startle response for each individual was averaged across the 3-dB intensities and is shown in Figure 5a. As can be seen, the baseline acoustic startle response was significantly greater in Lenti-CMV-CRF-injected females compared with GFP-injected females (t9 = 2.22, P = 0.05), suggesting that basal levels of anxiety are increased in Lenti-CMV-CRF-injected females. In the forced swim test Lenti-CMV-CRF-injected females displayed increases in depression-related behavior in that they spent significantly less time attempting to escape (t9 = 2.38, P = 0.04) and significantly more time floating (t9 = 3.39, P < 0.01) than control females (Figures 5b and c).
Figure 5.
Effect of increased corticotropin-releasing hormone (CRF) production in the central nucleus of the amygdala (CeA) on anxiety- and depression-related behaviors. Mean±s.e.m. measures of emotionality for green fluorescent protein (GFP)-injected control (open bars) and Lenti-CMV-CRF-injected females (closed bars). Shown is baseline acoustic startle response (a), and amount of time animals spent actively trying to escape (b) and time spent floating (c) in the forced swim test. *P <0.05.
Adverse consequences on reproductive parameters and sexual behavior
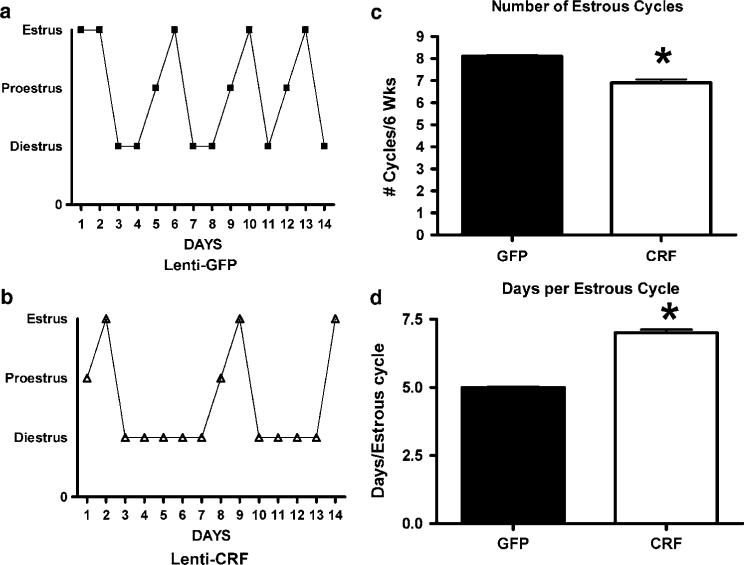
Lenti-CMV-CRF-injected females spent more days in diestrous (Figures 6a and b) and showed significantly fewer estrous cycles (Figure 6c; t9 = 3.83, P < 0.01) with more days per cycle (Figure 6d; t = 2.36, P = 0.04), indicating that CRF continuously expressed in CeA disrupts reproductive function. Comparison of the numbers of GnRH positive cells in the medial preoptic area (MPOA) between Lenti-CMV-CRF-injected and control animals (Figures 7a–d) shows that CRF treated animals had significantly fewer GnRH positive neurons (t9 = 3.15, P = 0.01) than control animals, suggesting that CRF from CeA is involved in the control of GnRH expression in this region.
Figure 6.
Increased corticotropin-releasing hormone (CRF) production in the central nucleus of the amygdala (CeA) produces disturbances in the rat estrous cycle as determined by daily examination of vaginal cytology. Representative cycles of rats injected with (a) Lenti-CMV-GFP and (b) Lenti-CMV-CRF. Also shown are mean±s.e.m. number of cycles (c) and days per cycle (d) in green fluorescent protein (GFP)-injected control (open bars) and Lenti-CMV-CRF-injected females (closed bars). *P < 0.05.
Figure 7.
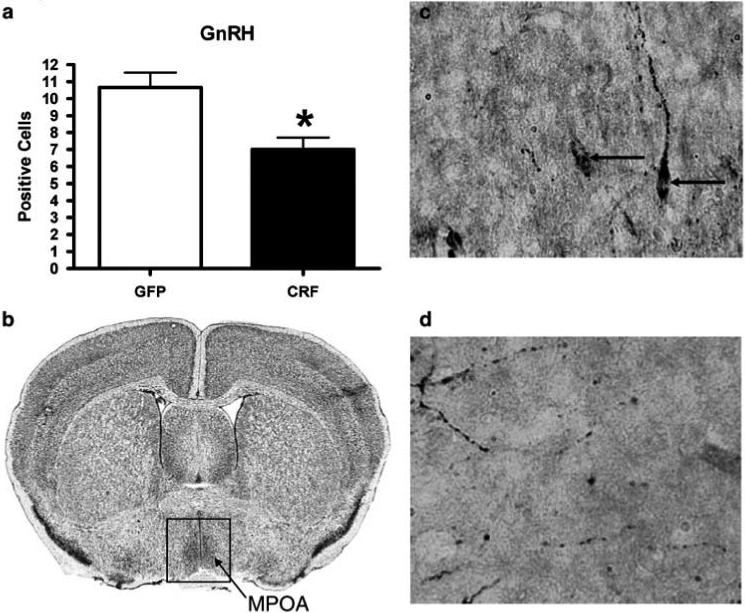
Increased CRF protein in the central nucleus of the amygdala (CeA) decreases the number of positively stained GnRH cells. (a) Mean±s.e.m. number of positively labeled GnRH cells in MPOA of Lenti-CMV-GFP (open bars) and Lenti-CMV-CRF treated female (closed bars) rats determined by immunohistochemistry. (b) Cresyl violet stained coronal section representative of the section used to quantify and number of GnRH positive cells in MPOA in each animal. Representative coronal sections of (c) Lenti-CMV-GFP and (d) Lenti-CMV-CRF treated animals at χ40 magnification. GnRH: gonadotropin-releasing hormone, GFP: green fluorescent protein, CRF: corticotropin-releasing hormone, MPOA: medial preoptic area. *P < 0.05.
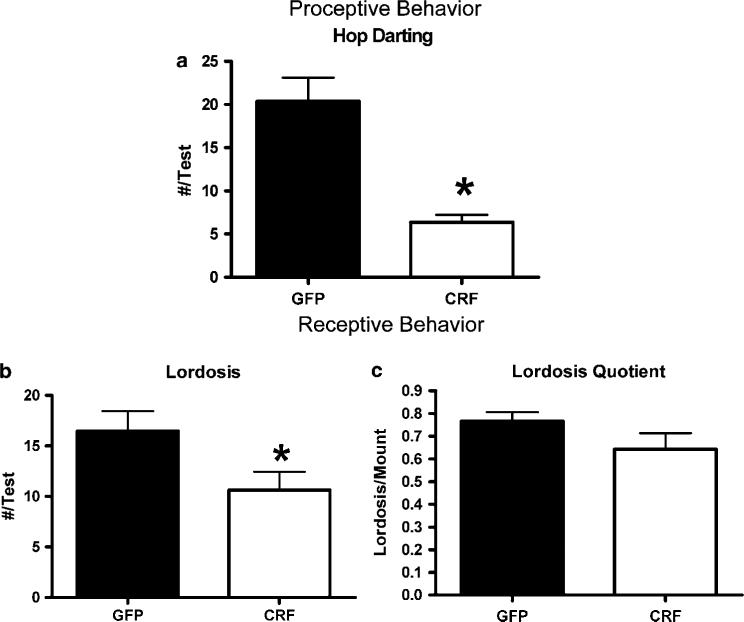
Sexual behavior was assessed at two time points separated by 2 weeks following estradiol and progesterone priming to OVX females. As illustrated in Figure 8a, Lenti-CMV-CRF-injected females showed significantly fewer proceptive behaviors (hops and darts) compared to GFP-treated females (F1,10 = 32. 59, P < 0.01) and the differences in the frequency of these behaviors were significantly greater in the second test (F1,10 = 6.97, P = 0.03). In contrast, the frequency of lordosis behavior (Figure 8b; F1,10 = 0.03, P = 0.87) and mounts received from males (data not shown; F1,10 = 0.08, P = 0.88) was not significantly different between Lenti-CMV-CRF injected and GFP females. Consequently, lordosis quotients50 were not significantly different between Lenti-CMV-CRF and GFP-treated females (Figure 8c; F1,10 = 1.00, P = 0.34).
Figure 8.
Effect of Lenti-CMV-CRF injection into the central nucleus of the amygdala (CeA) on sexual behavior in ovariectomized rats. The consequences of continuous corticotropin-releasing hormone (CRF) production in CeA of ovariectomized estradiol and progesterone primed green fluorescent protein (GFP)-injected (open bars) and Lenti-CMV-CRF-injected female rats (closed bars) at two time points 1 week apart on frequency (mean±s.e.m.) of (a) proceptive behavior measured by hop darting; (b) receptive behaviors measured by the lordosis and (c) the lordosis quotient. *P < 0.05.
Discussion
This study demonstrates that lentiviral-induced continuous expression of CRF in CeA of female rats causes an upregulation of CRF and AVP in PVN, an impairment of feedback inhibition of the HPA axis, increased acoustic startle and anxiety behavior, and an impairment of reproductive physiology and behavior. Although a single report failed to find differences in CeA CRF concentrations between suicide victims and controls,51 the data from this study suggest that disrupted control over the upregulation of CRF, which is known to take place in CeA under conditions of chronic stress, produces many of the physiological and behavioral changes observed in stress-related pathologies.6,52–54
Results here show increased concentrations of both CRF and AVP peptide in PVN of Lenti-CMV-CRF-injected females. The increase in AVP in PVN is a well-established consequence of chronic stress.23–26 However, CRF synthesis in PVN following chronic stress has been shown to remain at basal levels.55 Moreover, exposure to chronic stress downregulates the production of CRF1 receptors in PVN.56 Thus, the restriction of CRF synthesis and activity in PVN after stress is believed to be of critical importance in limiting the stress response and preventing pathologies associated with excess CRF levels.25,26,57 However, the observation that increased CRF peptide in PVN of CRF-injected females suggests that continuous expression of CRF from CeA counteracts the reduction of hypothalamic CRF that usually follows stress. Indeed, ICV administration of CRF increases the expression of CRF1 and increases CRF transcription within PVN.58 Thus, continuous expression of CRF in CeA may be constitutively upregulating CRF1 receptors at the level of PVN and result in the continuous transcription of CRF in this hypothalamic region.
Immunoreactive CRF and AVP in PVN were very low in control animals, likely due to the stage of the estrous cycle when tissue was obtained and not to any effect of the Lenti-CMV-GFP injection itself. As stated in Methods, control, Lenti-CMV-GFP-injected animals were killed on in diestrus. As Lenti-CMV-CRF-injected animals were generally acyclic, every attempt was made to kill these animals when their vaginal cytology was most like diestrus. Studies describing estrous cycle changes in neuropeptide immunoreactivity are lacking, as most studies of hypothalamic levels of CRF and AVP levels use male rats. However, several reports show that limbic-HPA activity fluctuates over the cycle and is greater at proestrus when estradiol levels are elevated relative to those observed in diestrus.59,60 Most importantly, under nonstressful, basal conditions, estradiol increases hypothalamic CRF mRNA and peptide content in monkeys61,62 and rodents63–65 as well as AVP-ir in PVN of rats.66 Future studies will compare Lenti-CMV-GFP and Lenti-CMV-CRF treatments during both OVX and OVX plus estradiol conditions to determine how estradiol differentially affects neuropeptide levels in PVN in the presence and absence of continuous expression of GFP or CRF in CeA.
A decrease in HPA inhibition is present in several psychiatric disorders, most notably major depression,67 and is believed to reflect decreases in central glucocorticoid receptor (GR) number, binding affinity or activation.68–72 Transgenic mice overexpressing CRF also exhibit HPA dysregulation.73 However, this approach likely produces developmental compensation within the system that may not mimic the consequences of region-specific increased CRF expression resulting from chronic stress.74 In this study, the increase in CeA CRF expression induced by the lentiviral vector significantly attenuated glucocorticoid negative feedback, suggesting mechanisms responsible for glucocorticoid negative feedback are compromised in these animals. Although this could reflect a change in central GRs, other data suggest that the HPA axis is regulated by glucocorticoid-independent inhibition from a number of brain regions including the lateral and dorsomedial hypothalamus, BNST and MPOA, all of which send γ-aminobutyric acid projections to PVN.75–78 The degree to which these pathways are stimulated directly or indirectly by GR activation is difficult to discern; however, adrenalectomized rats show inhibition of ACTH release, indicating that nonglucocorticoid mechanisms can curtail the stress response.79 Therefore, continuous expression of CRF in CeA may be exerting a disruptive effect on one or more of the inhibitory neuronal pathways that regulate the HPA axis.
Of these aforementioned regions, BNST is likely the intermediate nexus wherein limbic and cortical inputs involved in regulating the stress response are integrated and relayed to PVN.80,81 BNST receives afferents from cortical and limbic regions involved in the control of the HPA axis including the medial and central amygdala, the hippocampus and the prefrontal cortex.76,82,83 Moreover, BNST contains site-specific areas that activate (the anterior/lateral region) or attenuate (the posterior/medial region) the hormonal stress response.82,84–86 Furthermore, these regions are preferentially innervated by either amygdaloid afferents, in the former case, or cortical/hippocampal afferents, in the latter.76,80,87,88 Thus, afferent input to the anterior/lateral BNST is from a site governing emotional activation,89 and inputs to the posterior/medial BNST are from brain regions involved in negative feedback of the HPA response.90
The data from this study demonstrate that heightened CRF expression in CeA produces a significant elevation in acoustic startle. The acoustic startle response is a short latency (8 ms) reflex mediated by a simple neural pathway that is modulated by emotion. Acoustic startle is elevated in fear and anxiety states and this is manifest in psychopathologies like PTSD.70,91 It is well established that CRF infused into the ventricles of the brain or intra-BNST enhances acoustic startle,92,93 an effect mediated by activation of the lateral region of BNST, as reviewed recently.94 Anatomical studies show the existence of an efferent projection of CRF-expressing neurons from CeA to BNST.16,88,95 Results here suggest a functional connection between CRF from CeA and lateral BNST-dependent CRF-enhanced startle. Perhaps the persistent activation of the lateral BNST by CRF from CeA that is producing enhanced acoustic startle is also responsible for the escape from glucocorticoid feedback inhibition in CRF-injected females.
As acoustic startle is elevated by fear and anxiety and is reduced by anxiolytics,96 the elevated startle response observed in CRF-injected females implies that baseline anxiety is increased in these animals. This conclusion is substantiated by results from the forced swim test showing that CRF-injected females exhibit significantly less escape effort and significantly more immobility than control females, behaviors thought to represent depression-like states.97 These differences cannot be attributed to CRF-induced effects on locomotion, because motor activity was not increased in CRF-injected animals before the initiation of the first day of acoustic startle testing.
In addition, results from this study indicate that CRF from CeA negatively impacts reproductive physiology in female rats by lengthening the diestrus phase of the estrus cycle and significantly decreasing GnRH in MPOA. Numerous studies have shown that activation of the stress axis inhibits reproduction. For example, macaques experiencing psychosocial stress associated with subordination stress have reduced fertility,98 secondary to an increased incidence of anovulation.99 Furthermore, macaque females that are stress sensitive have decreased GnRH positive neurons in the hypothalamus.2 In rats, females subjected to chronic mild stress show a 40% lengthening in estrous cycle and decreased hypothalamic GnRH,100 effects attributed to a stress-induced increase in CRF.101 For example, CRF decreases GnRH production in the hypothalamus102 and luteinizing hormone and follicle-stimulating hormone production by the pituitary.103 Although it has been hypothesized that limbic circuitry, including CeA and BNST, is involved in the suppression of reproduction by stress,104 this is the first study tying CRF expression in CeA directly to this inhibition. Hence, these data suggest that a constitutive increase of CRF in CeA may be a causal factor in stress-related reproductive disorders like functional hypothalamic anovulation.
In addition to disrupting reproductive physiology, the continuous expression of CRF in CeA also reduced the frequency of sexually motivated behavior. OVX, steroid-primed Lenti-CMV-CRF-injected females showed significantly lower rates of proceptive behaviors compared to steroid primed controls. In contrast, lordosis behavior, even when expressed as the lordosis quotient, was not significantly different between GFP- and Lenti-CMV-CRF-treated females. It is not surprising that the lordosis behavior is not different, as male mounts were also similar between the two groups of females. We believe these data are highly significant because they indicate the more reflexive behavior, lordosis, that occurs in response to male stimulation105 is unaffected by continuous CRF from CeA whereas sexually motivated proceptive behavior that serves to communicate a female's willingness to copulate with the male is attenuated. The neurobiology of these sexually motivated behaviors is complex,106 and likely involve limbic and reward pathways.105,107,108 The results of this study indicate that CRF expression in CeA disrupts these circuits; a result consistent with observations of decreased libido or sexual desire characteristic of affective disorders.109
A synthesis of all data garnered throughout the course of this study shows that uncontrolled CRF synthesis within CeA produces dysregulation of the stress axis, increases emotional behavior and disrupts reproduction. These results imply that CeA mediates of all of these functions and suggest that increased expression of CRF within this particular limbic structure can lead to many of the maladaptive processes observed during psychopathology. Indeed, although there have been a substantial number of studies showing enhanced amygdala activity in both depressed people110–112 and those suffering from anxiety disorders,110,113 and central activation or infusion of CRF has been consistently associated with increased fear- and anxiety-related behaviors in animals,114–118 this study is, to the best of our knowledge, the first to tie endogenous synthesis of CRF in the amygdala with all of these outcomes.
In conclusion, findings in this study demonstrate that continuous expression of CRF in CeA of female rats results in a host of behavioral and neuroendocrine alterations that resemble the changes observed in several stress-induced pathologies including PTSD, anxiety and depression. Although the administration of glucocorticoids directly to CeA increases amygdalar CRF expression in a fashion similar to that achieved with the lentiviral infection used in this study,20 the results here show that the consequences of this increased expression of CRF affect multiple neurobiological targets and behavioral systems. Given the higher incidence of affective disorders in women compared with men,5 our model provides a valuable tool to assess sex differences on a number of behavioral and physiological endpoints in response to CRF overexpression in CeA. Finally, it could be argued that the results of this study were due to seizure activity induced by high concentrations of CRF.119 However, this is unlikely as rats expressing CRF had an increase in startle and seizures are associated with a marked decrease in startle.120 Therefore, lentiviral vector-induced constitutive expression of CRF in CeA constitutes a valuable new model for examining the neurobiological mechanisms underlying the dysregulation of the HPA and HPG axes, and may help to clarify a number of processes involved in the development of stress-related illness in women.
Acknowledgments
We thank Ruth Connelly, Laura Canepa, Valencia Coston and Teal Pelish for their expert technical assistance. We also thank Dr. Gloria Hoffman and Dr Andrea Gore for their immunohistochemistry expertise. This project was supported by a pilot grant from the Center for Behavioral Neuroscience as a part of the STC Program of the National Science Foundation under Agreement No. IBN-9876754 and by NIH grants HD46501, MH-42088, MH-52899, RR00165, and 5K12-GM00680.
Footnotes
Disclosure statement
Currently, Dr Nemeroff has served on the Scientific Advisory Board for Astra-Zeneca, Johnson & Johnson, Pharma Neuroboost, Forest laboratories, Quintiles and NARSAD. He is a grant recipient from NIH, NovaDel Pharmaceuticals, Mt. Cook Pharma, Inc. and the George West Mental Health Foundation. He owns equity in CeNeRx and Reevax. He owns stock or stock options in Corcept and NovaDel. In the past 3 years, Dr Owens has had research grants from Pfizer, GlaxoSmithKline, Merck, Lundbeck, Cyberonics and Johnson & Johnson. He has consulted to Pfizer, Lundbeck, Sepracor, Johnson & Johnson, Sanofi-Aventis and Forest Labs, and received speaker's honaria from GlaxoSmithKline. Dr Owens has a patent entitled ‘A method to estimate transporter occupancy’.
References
- 1.Florio P, Zatelli MC, Reis FM, degli Uberti EC, Petraglia F. Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders? Front Biosci. 2007;12:551–560. doi: 10.2741/2081. [DOI] [PubMed] [Google Scholar]
- 2.Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol. 2007;19:594–604. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 3.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2007;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 6.Berga SL, Loucks TL. The diagnosis and treatment of stress-induced anovulation. Minerva Ginecol. 2005;57:45–54. [PubMed] [Google Scholar]
- 7.Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann NY Acad Sci. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 9.Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 10.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 12.Polkowska J, Przekop F. The effect of corticotropin-releasing factor (CRF) on the gonadotropin hormone releasing hormone (GnRH) hypothalamic neuronal system during preovulatory period in the ewe. Acta Neurobiol Exp (Wars) 1997;57:91–99. doi: 10.55782/ane-1997-1216. [DOI] [PubMed] [Google Scholar]
- 13.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 14.Petraglia F, Sutton S, Vale W, Plotsky P. Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology. 1987;120:1083–1088. doi: 10.1210/endo-120-3-1083. [DOI] [PubMed] [Google Scholar]
- 15.Sirinathsinghji DJ. Modulation of lordosis behaviour in the female rat by corticotropin releasing factor, beta-endorphin and gonadotropin releasing hormone in the mesencephalic central gray. Brain Res. 1985;336:45–55. doi: 10.1016/0006-8993(85)90414-7. [DOI] [PubMed] [Google Scholar]
- 16.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 17.Beyer HS, Matta SG, Sharp BM. Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology. 1988;123:2117–2123. doi: 10.1210/endo-123-4-2117. [DOI] [PubMed] [Google Scholar]
- 18.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc. 1985;44(1 Pt 2):221–227. [PubMed] [Google Scholar]
- 19.Albeck DS MC, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 21.Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- 22.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of ‘comfort food’. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 24.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 25.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 26.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 28.Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 29.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Lai Z, Brady RO. Gene transfer into the central nervous system in vivo using a recombinanat lentivirus vector. J Neurosci Res. 2002;67:363–371. doi: 10.1002/jnr.10137. [DOI] [PubMed] [Google Scholar]
- 31.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsson J, Lundberg C. Lentiviral vectors for use in the central nervous system. Mol Ther. 2006;13:484–493. doi: 10.1016/j.ymthe.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element Enhances Expression of Transgenes Delivered by Retroviral Vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. PNAS. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long JA, Evans HM. The Oestrous Cycle in the Rat and its Associated Phenomena. University of California Press; Berkeley: 1922. pp. 1–48. [Google Scholar]
- 41.Everett JW. Neurobiology of Reproduction in the Female Rat. Vol. 32. Springer-Verlag; Berlin, Germany: 1989. pp. 1–133. [PubMed] [Google Scholar]
- 42.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–459. doi: 10.1016/s0306-4530(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 43.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 44.Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uphouse L, Selvamani A, Lincoln C, Morales L, Comeaux D. Mild restraint reduces the time hormonally primed rats spend with sexually active males. Behav Brain Res. 2005;157:343–350. doi: 10.1016/j.bbr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm Behav. 2004;45:270–277. doi: 10.1016/j.yhbeh.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 48.Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 49.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- 50.Pike AC, Brzozowski AM, Walton J, Hubbard RE, Bonn T, Gustafsson JA, et al. Structural aspects of agonism and antagonism in the oestrogen receptor. Biochem Soc Trans. 2000;28:396–400. [PubMed] [Google Scholar]
- 51.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 53.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 54.de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- 55.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 56.Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol. 1998;275(5 Part 2):R1438–R1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- 57.Aguilera G, Kiss A, Liu Y, Kamitakahara A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress. 2007;10:153–161. doi: 10.1080/10253890701391192. [DOI] [PubMed] [Google Scholar]
- 58.Mansi JA, Rivest S, Drolet G. Regulation of corticotropin-releasing factor type 1 (CRF1) receptor messenger ribonucleic acid in the paraventricular nucleus of rat hypothalamus by exogenous CRF. Endocrinology. 1996;137:4619–4629. doi: 10.1210/endo.137.11.8895325. [DOI] [PubMed] [Google Scholar]
- 59.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 60.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 61.Kerdelhue B, Jones GS, Gordon K, Seltman H, Lenoir V, Melik Parsadaniantz S, et al. Activation of the hypothalamo-anterior pituitary corticotropin-releasing hormone, adrenocorticotropin hormone and beta-endorphin systems during the estradiol 17 beta-induced plasma LH surge in the ovariectomized monkey. J Neurosci Res. 1995;42:228–235. doi: 10.1002/jnr.490420210. [DOI] [PubMed] [Google Scholar]
- 62.Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140:2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- 63.Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O'Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol. 2003;15:468–476. doi: 10.1046/j.1365-2826.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- 64.Paulmyer-Lacroix O, Hery M, Pugeat M, Grino M. The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: role of the adrenal gland. J Neuroendocrinol. 1996;8:515–519. doi: 10.1046/j.1365-2826.1996.04835.x. [DOI] [PubMed] [Google Scholar]
- 65.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 66.Greer ER, Caldwell JD, Johnson MF, Prange AJ, Jr, Pedersen CA. Variations in concentration of oxytocin and vasopressin in the paraventricular nucleus of the hypothalamus during the estrous cycle in rats. Life Sci. 1986;38:2311–2318. doi: 10.1016/0024-3205(86)90638-7. [DOI] [PubMed] [Google Scholar]
- 67.Heuser I. Anna-Monika-Prize paper. The hypothalamic-pituitary-adrenal system in depression. Pharmacopsychiatry. 1998;31:10–13. doi: 10.1055/s-2007-979288. [DOI] [PubMed] [Google Scholar]
- 68.Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 70.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry. 1996;39:703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 72.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, van der Gugten J, et al. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry. 2002;51:875–881. doi: 10.1016/s0006-3223(02)01334-3. [DOI] [PubMed] [Google Scholar]
- 74.Peeters PJ, Fierens FL, van den Wyngaert I, Goehlmann HW, Swagemakers SM, Kass SU, et al. Gene expression profiles highlight adaptive brain mechanisms in corticotropin releasing factor overexpressing mice. Brain Res Mol Brain Res. 2004;129:135–150. doi: 10.1016/j.molbrainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 75.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 76.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 77.Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 78.Herman JP, Cullinan WE, Young EA, Akil H, Watson SJ. Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA expression. Brain Res. 1992;592:228–238. doi: 10.1016/0006-8993(92)91680-d. [DOI] [PubMed] [Google Scholar]
- 79.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 80.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–185. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 81.Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- 82.Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysio-tropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. J Comp Neurol. 2003;467:232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- 83.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 84.Dunn JD. Differential plasma corticosterone responses to electrical stimulation of the medial and lateral septal nuclei. Neuroendocrinology. 1987;46:406–411. doi: 10.1159/000124853. [DOI] [PubMed] [Google Scholar]
- 85.Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 86.Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- 87.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 88.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 89.Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 91.Orr SP, Metzger LJ, Pitman RK. Psychophysiology of posttraumatic stress disorder. Psychiatr Clin North Am. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 92.Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9−41). Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- 93.Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 95.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 96.Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 97.Kitada Y, Miyauchi T, Satoh A, Satoh S. Effects of antidepressants in the rat forced swimming test. Eur J Pharmacol. 1981;72:145–152. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- 98.Wilson ME, Gordon TP, Bernstein IS. Timing of births and reproductive success in rhesus monkey social groups. J Med Primatol. 1978;7:202–212. doi: 10.1159/000459880. [DOI] [PubMed] [Google Scholar]
- 99.Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- 100.Baker SL, Kentner AC, Konkle AT, Santa-Maria Barbagallo L, Bielajew C. Behavioral and physiological effects of chronic mild stress in female rats. Physiol Behav. 2006;87:314–322. doi: 10.1016/j.physbeh.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 102.Rivest S, Plotsky PM, Rivier C. CRF alters the infundibular LHRH secretory system from the medial preoptic area of female rats: possible involvement of opioid receptors. Neuroendocrinology. 1993;57:236–246. doi: 10.1159/000126365. [DOI] [PubMed] [Google Scholar]
- 103.Ortega E, Ruiz E, Rodriguez E, Frias J. Effect of corticotropin releasing factor (CRF) in the median eminence on gonadotropins in ovariectomized rats with or without steroid priming: dose-response study. Neurochem Res. 1994;19:1225–1230. doi: 10.1007/BF01006810. [DOI] [PubMed] [Google Scholar]
- 104.Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. J Reprod Immunol. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Frohlich J, Ogawa S, Morgan M, Burton L, Pfaff D. Hormones, genes and the structure of sexual arousal. Behav Brain Res. 1999;105:5–27. doi: 10.1016/s0166-4328(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 106.Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neurosci Biobehav Rev. 1990;14:233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- 107.Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- 108.Kato A, Sakuma Y. Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Res. 2000;862:90–102. doi: 10.1016/s0006-8993(00)02076-x. [DOI] [PubMed] [Google Scholar]
- 109.Green B. Post-traumatic stress disorder: symptom profiles in men and women. Curr Med Res Opin. 2003;19:200–204. doi: 10.1185/030079903125001604. [DOI] [PubMed] [Google Scholar]
- 110.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 111.Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- 112.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 113.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 114.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- 117.Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res. 1990;509:80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- 118.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, et al. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. discussion 290–275. [DOI] [PubMed] [Google Scholar]
- 119.Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gallager DW, Kehne JH, Wakeman EA, Davis M. Development changes in pharmacological responsivity of the acoustic startle reflex: effects of picrotoxin. Psychopharmacology (Berl) 1983;79:87–93. doi: 10.1007/BF00427790. [DOI] [PubMed] [Google Scholar]