Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is associated with adult T-cell leukemia–lymphoma (ATLL) in about 5% of infected individuals. Coinfection by Strongyloides stercoralis has been suggested to be a cofactor for development of ATLL. We describe a patient who presented with HTLV-1-associated chronic ATLL and Strongyloides infection. Studies of this patient’s viral RNA levels demonstrated stimulation of HTLV-1 replication by Strongyloides, which resolved with anti-helminthic therapy. This case provides support for the hypothesis that Strongyloides is a cofactor for ATLL via T-cell stimulation.
Introduction
Strongyloides stercoralis has been proposed as a cofactor for human T-cell leukemia Type 1 (HTLV-1)-associated adult T-cell leukemia–lymphoma (ATLL) [1]. This is based on epidemiologic association of Strongyloides and HTLV-1 infections in Brazil, the Caribbean Islands, and Southern Japan, and from small clinical series reporting more severe disease manifestations in the presence of both infections than with either one alone [2–4]. The mechanism for the synergism between these two infectious agents is unclear. The current report describes such a dual-infected patient, and the results of newly developed viral expression assays which were applied for the first time to assess the effects on HTLV-1 RNA expression of treatment of S. stercoralis infection.
Case Report
The patient is a 69-year-old Japanese-American woman from Okinawa, who was previously in excellent health. She presented with a 1-month history of abdominal discomfort, bloating, anorexia, vomiting, nonproductive cough, mild hoarseness, generalized weakness, and an 8 lb weight loss, representing 7% of her body weight. She had been breast-fed as an infant. She moved to U.S. from Okinawa 43 years ago. She had no exposure to illicit drugs. Her physical examination was remarkable for normal cardiopulmonary, neurologic, dermatologic, and lymph node examinations. She had moderate abdominal tenderness and distention, and pedal edema.
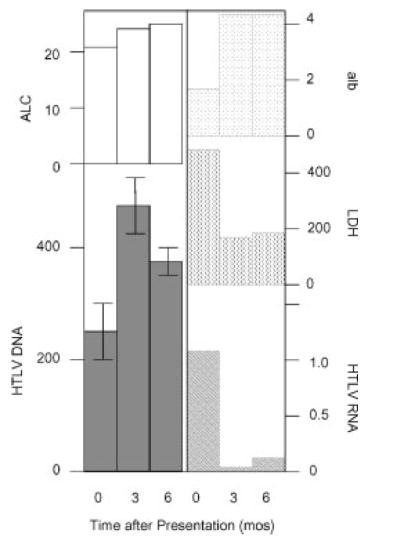
Laboratory data included a hemoglobin value of 12.3 g/dl, platelet count of 490,000/mm3, and a total leukocyte count of 48,500/mm3 with 26% lymphocytes. The absolute lymphocyte count (ALC) ranged from 14,800 to 22,700/mm3 during the first week of hospitalization (Fig. 1). The peripheral smear showed many atypical lymphocytes with cerebriform nuclei, occasional “flower” cells, and rare cells with immature chromatin (Fig. 2a–c). Chemistries revealed a calcium of 7.8 mg/dl (lower limit of normal, LLN = 8.6) and an albumin of 2.4 g/dl, which declined to 1.6 g/dl (LLN = 2.6, Fig. 1). The lactate dehydrogenase (LDH) was 477 U/l (ULN = 250, Fig. 1).
Figure 1.
Clinical course of patient. Values for absolute lymphocyte count (ALC, × 10−3), albumin (alb, g/dl), lactate dehydrogenase (LDH, U/l), HTLV DNA (copies/cell, × 10−3), HTLV RNA (copies/104 copies of hprt mRNA) are reported at presentation (0 months), and 3 and 6 months later. Bars on graph of HTLV DNA show standard errors.
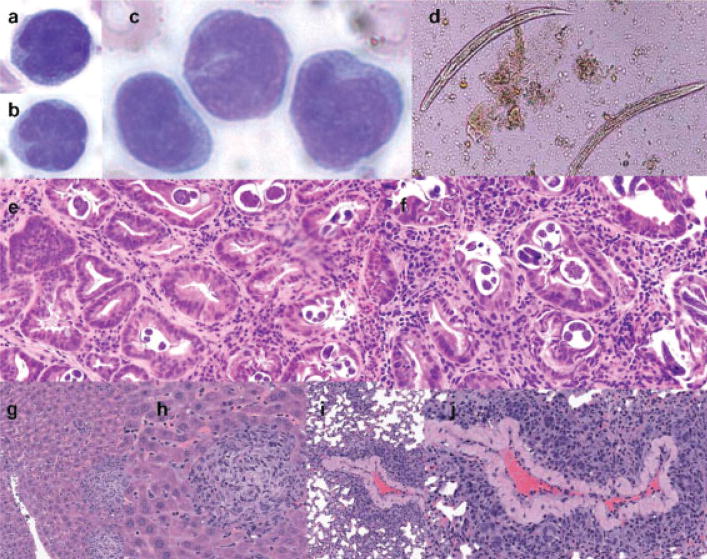
Figure 2.
Pathologic findings. (a–c) Atypical lymphocytes in peripheral blood, and (d) Strongyloides stercoralis in the stool and (e,f) gastric biopsy are shown. (g,h) Liver and (i,j) lung infiltration in NOD.Cg-PrkdcSCIDIL2rgtm1Wjl/SzJ mouse inoculated with PBMCs from the patient. Routine hematoxylin and eosin staining is shown.
Computerized tomographic scans of the chest, abdomen, pelvis, and brain showed thickening of the gastric, duodenal, and colonic walls, and a right lower lobe infiltrate. A skeletal survey showed no lytic lesions. Cerebrospinal fluid was normal. ELISA and Western blot assays for HTLV-1 were positive with detection of antibodies to Gag p19 and p24 and Env gp21 and gp46 proteins. Bone marrow biopsy showed a mature T-cell neoplasm. Flow cytometry studies showed that the T-cells expressed cytoplasmic CD3 and surface CD2, CD4, CD5, and CD25, but not surface CD3, CD7, CD8, CD10, or CD56, consistent with the diagnosis of ATLL. Stool examination and upper endoscopy and gastric and duodenal biopsies revealed a heavy infection with S. stercoralis adult worms and larvae (Fig. 2d–f).
The patient received two 5-day courses of ivermectin (100 μg/kg/d) 2 weeks apart. Repeat stool examinations performed 1, 2, and 3 months later showed that the treatment had cured the infection. Her abdominal symptoms and edema resolved within 1 month after ivermectin treatment; her appetite improved, she gained weight, and her albumin and LDH normalized (Fig. 1). The ALC gradually increased over the subsequent 9 months (Fig. 1). She developed mild peripheral adenopathy, but remained asymptomatic with no evidence of visceral disease or hypercalcemia. The patient received no chemotherapy or glucocorticoid treatment for her ATLL during the time period of this report.
Materials and Methods
HTLV-1 DNA assay
The HTLV-1 DNA assay, performed with peripheral blood mononuclear cells (PBMCs), measures the number of copies of viral genome. HTLV-I provirus load was measured using ABI PRISM 7700 sequence detector (Perkin Elmer/Applied Biosystems), as described elsewhere, using primers in pX [5]. The assay was standardized by measuring the number of copies of β-actin DNA, as described previously [5]. All samples were tested in triplicate. The amount of HTLV-I proviral DNA was calculated as copy number of HTLV-I (pX) per 100 PBMC = [(copy number of pX)/(copy number of β-actin/2)] × 100.
HTLV-1 RNA assay
The HTLV-1 RNA assay, performed with PBMCs, measured the number of copies of viral RNA that are expressed from the viral genome. RNA extraction, complementary DNA (cDNA) synthesis, and real time PCR were performed as described previously and summarized briefly below [6]. Primers were designed for the amplification of HTLV-1 tax cDNA, and the human housekeeping gene hypoxanthine ribosyl transferase (hprt) for internal calibration. Standard curves for the value of HTLV-1 tax mRNA and hprt mRNA were generated using cDNA from MT-2 cells. All standards and samples were assayed in duplicate. The thresh-old cycle (Ct) values were used to plot a standard curve in which Ct decreased in linear proportion to the log of the template copy number. The correlation values of standard curves were always more than 99%. The relative HTLV-1 tax mRNA load was calculated by the following formula: HTLV-1 tax mRNA load = (value of tax)/(value of hprt) × 10,000.
Xenotransplant studies
Xenotransplant studies were performed to assess the tumorigenicity of the patient’s ATLL cells. A female NOD.Cg-PrkdcSCIDIL2rgtm1Wjl/SzJ mouse (pathogen free), purchased from Jackson Laboratory, was inoculated intraperitoneally with 2 × 107 fresh PBMCs isolated from patient blood sample. Periodically, serum was collected via the anterior facial vein. Serum concentrations of the soluble released IL-2Rα chain (Tac) were measured using the R&D systems ELISA kit. At the time of necropsy, tissues were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin.
Results
Virus load assays were performed on samples obtained at presentation, and on samples collected 3 and 6 months later after she had completely recovered from Strongyloides infection. These included measurements of HTLV-1 DNA and RNA in peripheral blood mononuclear cells. The viral DNA level increased from 0.26 ± 0.68 copies/cell at presentation to 0.48 ± 0.68 and 0.38 ± 0.27 copies/cell, respectively, 3 and 6 months later (Fig. 1). These values reflected the levels of ALC. In contrast, the viral tax mRNA level at presentation was 1.05 copies/104 copies of control hprt mRNA, and exhibited marked decline to 0.05 and 0.18 copies/104 copies of hprt mRNA, respectively, 3 and 6 months later. These values correlated well with the levels of albumin and LDH.
To assess tumorigenicity of the patient’s PBMCs, they were also injected into a severely immunocompromised NOD.SCID.IL2rg mouse. After four and a half months, the mouse manifested marked splenomegaly, hepatomegaly, and generalized lymphadenopathy as a result of infiltration by an aggressive T-cell lymphoma (Fig. 2g–j).
Discussion
ATLL is a mature CD4+ lymphoid malignancy that occurs in 5–10% of individuals infected by HTLV-1 by breast feeding [7]. It occurs in four different subtypes, smoldering, chronic, leukemic, or lymphomatous disease [8]. The leukemic and lymphomatous subtypes account for 60–90% of cases; these are characterized by an aggressive course, with frequent skin, bone, central nervous system, and other visceral involvement, hypercalcemia, and limited responses to chemotherapy [9–12]. In contrast, chronic ATLL is characterized by lymphocytosis and minimal adenopathy or skin involvement, as manifested by our patient. This disease is more prolonged, although conversion to acute ATLL may occur. Even though this patient’s illness was consistent with chronic ATLL, her T-cells were capable of causing an aggressive lymphoma in a xenotransplant model system. This may be indicative of better immunological control of the malignancy in the patient than the immunocompromised mouse. Strongyloides infections have been reported in 5–20% of ATLL cases, and these infections are often more severe than in individuals who are not coinfected with HTLV-1 [13]. Okinawa, the birthplace of this patient, is endemic for both HTLV-1 and Strongyloides [4]. S. stercoralis is an intestinal nematode usually acquired transcutaneously. Adult worms reside in the small bowel and release larvae into the intestinal lumen that are excreted in the stool for years [14]. Autoin-fection is normally limited by the host immune system, but immunosuppressed patients can develop disseminated disease (hyperinfection syndrome). This patient had typical symptoms of a severe Strongyloidiasis, with bloating, vomiting, intestinal and peripheral edema, and weight loss, coinciding with the development of T-cell leukemia. Therapy for ATLL was not initiated because of the risk of exacerbating the parasitic infection. Ivermectin, used for treatment of this patient, has been reported to cure 97–100% of patients with Strongyloidiasis, and is more effective than albendazole and better tolerated than thiabendazole [15].
The synergistic severity of disease caused by these infections has been suggested to be due to immunocompromise resulting from HTLV-1 as a result of interferon γ expression, and shift from Th2 to Th1 responses, resulting in decreased levels of IL4, IL5, IL13, and IgE [4,16–19]. Alternatively, a Strongyloides antigen has been postulated to induce a potent polyclonal T-cell mitogenic response, and reactivation of HTLV-1 expression [1,20]. Although the patient’s ALC and viral DNA load did not decline after ivermectin treatment, the dramatic drop in viral RNA after treatment suggests that Strongyloides was a potent stimulus for virus expression, presumably through T-cell activation. Alternatively, Strongyloides may have caused general immunosuppression that permitted HTLV-1 replication and spread, which reversed after ivermectin treatment with associated weight gain and decreased intestinal inflammation. Expansion of infected T-cells may enhance genetic instability resulting in inactivation of DNA repair mechanisms, predisposing to cancer [3,21,22]. Thus, Strongyloides and HTLV-1 coinfection may lead to ATLL in a manner parallel to coinfection with malaria and Epstein–Barr virus which has been linked to Burkitt’s lymphoma [23]. Thus, this case illustrates the complexities of pathogen and host interactions that may contribute to hematologic malignancy.
Acknowledgments
Contract grant sponsor: PHS; Contract grant numbers: CA105218, CA10073, CA63417.
References
- 1.Gabet AS, Mortreux F, Talarmin A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–4960. doi: 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 2.Chiefffi PP, Chiattone CS, Feltrim EN, Alves RC, Paschoalotti MA. Coinfection by Strongyloides stercoralis in blood donors infected with human T-cell leukemia/lymphoma type 1 in Sao Paulo city, Brazil. Mem Inst Oswaldo Cruz. 2000;95:711–712. doi: 10.1590/s0074-02762000000500017. [DOI] [PubMed] [Google Scholar]
- 3.Agape P, Copin MC, Cavrois M, et al. Implication of HTLV-I infection, Strongyloidiasis, and p53 overexpression in the development, response to treatment, and evolution of non-Hodgkin’s lymphoma in an endemic area (Martiniques, West Indies) J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:394–402. doi: 10.1097/00042560-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Hirata T, Uchima N, Kishimoto K, et al. Impairment of host immune response against Strongyloides stercoralis by human T cell lymphotropic virus type 1 infection. Am J Trop Med Hyg. 2006;74:246–249. [PubMed] [Google Scholar]
- 5.Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax 11–19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myleopathy/tropical spastic paraparesis: Correlation with HTLV-I proviral load. J Infect Dis. 2001;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- 6.Yamano Y, Nagai M, Brennan M, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 7.Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 8.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: A report from the Lymphoma Study Group. Br J Hematol. 1991;79:426–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Tomonaga M, Fukuda H, et al. A new G-CSF supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma. Japan Clinical Oncology Group Study 9303. Br J Hematol. 2001;113:375–382. doi: 10.1046/j.1365-2141.2001.02737.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsukasaki K, Tobinai K, Shimoyama M, et al. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109) Int J Hematol. 2003;77:164–170. doi: 10.1007/BF02983215. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda H, Takatsuki K, Ohno R, et al. Treatment of adult T-cell leukaemia-lymphoma with irinotecan hydrochloride (CPT-11). CPT-11 study group on hematological malignancy. Br J Cancer. 1994;70:771–774. doi: 10.1038/bjc.1994.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taguchi H, Kinoshita KI, Takatsuki K, et al. An intensive chemotherapy of adult T-cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, rani-mustine, and mitoxantrone with granulocyte colony-stimulating factor support. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:182–186. doi: 10.1097/00042560-199606010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Plumelle Y, Gonin C, Edouard A, et al. Effect of Strongyloides stercoralis infection and eosinophilia on age at onset and prognosis of adult T-cell leukemia. Am J Clin Pathol. 1997;107:81–87. doi: 10.1093/ajcp/107.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaha O, Hirata T, Uchima N, Kinjo F, Saito A. Comparison of antihelminthic effects of two doses of ivermectin on intestinal Strongyloidiasis in patients negative or positive for anti-HTLV-1 antibody. J Infect Chemother. 2004;10:348–351. doi: 10.1007/s10156-004-0345-z. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho EM, PortoDaFonseca A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487–497. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 17.Porto AF, Santos SB, Muniz AL, et al. Helminthic infection down-regulates type 1 immune responses in human T cell lymphotropic virus type 1 (HTLV-1) carriers and is more prevalent in HTLV-1 carriers than in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2005;191:612–618. doi: 10.1086/427560. [DOI] [PubMed] [Google Scholar]
- 18.Newton RC, Kimpuangthip P, Greenberg S, Gam A, Neva FA. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. Am J Med. 1992;92:202–208. doi: 10.1016/0002-9343(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Miike T, Mizoguchi K, et al. Decreased serum levels of IgE and IgE-binding factors in individuals infected with HTLV-I. Clin Exp Immunol. 1990;81:207–211. doi: 10.1111/j.1365-2249.1990.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh M, Toma H, Sugahara K, et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)CD25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene. 2002;21:2466–2475. doi: 10.1038/sj.onc.1205329. [DOI] [PubMed] [Google Scholar]
- 21.Park HU, Jeong JH, Chung JH, Brady JN. Human T-cell leukemia virus type 1 Tax interacts with Chk1 and attenuates DNA-damage induced G2 arrest mediated by Chk1. Oncogene. 2004;23:4966–4974. doi: 10.1038/sj.onc.1207644. [DOI] [PubMed] [Google Scholar]
- 22.Franchini G, Nicot C, Johnson JM. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv Cancer Res. 2003;89:69–132. doi: 10.1016/s0065-230x(03)01003-0. [DOI] [PubMed] [Google Scholar]
- 23.Facer CA, Playfair JH. Malaria, Epstein-Barr virus, and the genesis of lymphomas. Adv Cancer Res. 1989;53:33–72. doi: 10.1016/s0065-230x(08)60278-x. [DOI] [PubMed] [Google Scholar]