Summary
The nondestructive chemical analysis of biological processes in the crowded intracellular environment, at cellular membranes, and between cells with a spatial resolution well beyond the diffraction limit is made possible through Nano-Biophotonics. A number of sophisticated schemes employing nanoparticles, nano-apertures, or shaping of the probe volume in the far field have significantly extended our knowledge about lipid rafts, macromolecular complexes, i.e. chromatin, vesicles, and cellular organelles, and their interactions and trafficking within the cell. Here, I review some of the most recent developments in Nano-Biophotonics that already are or soon will become relevant to the analysis of intracellular processes. The pros and cons of the various techniques will be discussed and an outlook of their prospects for the near future will be provided.
Introduction
Arguably, the origins of Nano-Biophotonics are closely tied in with the first experimental realization of near-field optics, which enabled optical imaging beyond the diffraction limit. Biophotonics is the use of light to image, probe, and manipulate biological materials. Nano-Biophotonics intends to bridge the gap between the light microscope and the electron microscope, but it goes beyond just imaging and also includes sensing and manipulation at the nanoscale. For example, it includes the use of nanostructures to probe biomaterials with higher sensitivity, or higher specificity. The particular strength of Nano-Biophotonics is that in the ideal case it retains the non-invasive nature of light and permits live cell sensing and imaging with near molecular resolution and sensitivity. The area of Nano-Biophotonics is too broad to possibly capture all aspects and developments and ensure completeness in a single review article. Thus, here I will highlight those recent key developments that have particular value or have demonstrated great promise for applications for nano-analytics in chemical biology within the last two years.
Imaging and analysis beyond the diffraction limit
The primary idea behind nanoscale optical imaging and analysis is that in order to overcome the hurdles imposed by Abbe's diffraction limit, a nanoscale light source has to be brought in close proximity with the sample to probe a subset of the sample. By raster-scanning the source across the sample, a map of the sample on the sub-wavelength scale can then be created, which bridges the gap between conventional optics and electron-beam or X-ray based analyses. Imaging, local probing, and manipulation of biological targets on the nanoscale or the use of optically active nanoscale objects is then exploited to either obtain higher spatial resolution or to achieve higher sensitivity and e.g. obtain information about individual events even in the crowded molecular environment inside a cell. To be of particular value for chemical analysis, these nano-structures should ideally not simply function as optical tags, but also actively report on changes in their local chemical environment similar to fluorescent indicator dyes.
Even though the initially most successful realization of near-field optics, which was also successfully commercialized, has seen a number of interesting applications in materials science, its use with biological materials is rather limited. This is mostly due to the fact, that these early near-field optical probes were made by pulling optical glass fibers to very fine tips that then are coated with a thin film of aluminum to prevent leakage of light except for the very end of the probe tip. This results in optical apertures acting as light sources with a diameter of 50 – 100 nm on average. The concept of and an example for such near-field optical probe tips is shown in Figure 1a. These tips are rather fragile and tedious to handle, which makes their use in aqueous environments particularly difficult. Some of the more interesting biological applications involving such tips, such as e.g. the subwavelength-scale imaging of the colocalization of fluorescently labeled membrane-associated proteins in cells, or intra-cellular probing of analytes have thus already been demonstrated a number of years ago. Nonetheless, these applications have demonstrated the limitations and the potential of near-field optical techniques and have sparked a large number of follow-on developments, such as tip-enhanced spectroscopies, the development of novel probes, the use of single molecule fluorescence in biology, and a number of novel concepts for optical super-resolution microscopies that do not require the use of tips or nanoparticles.
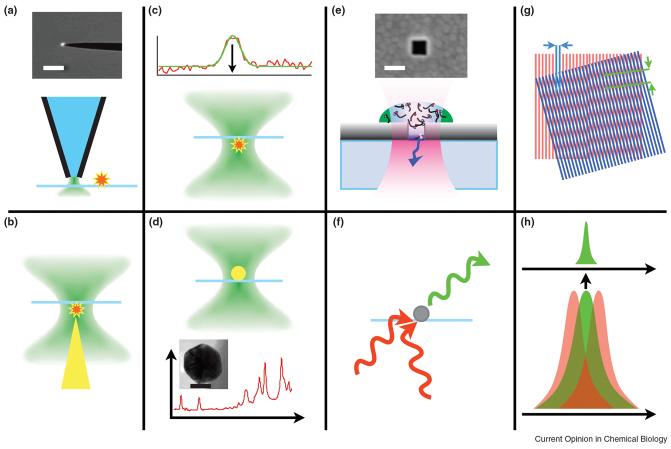
Figure 1.
Schematics of different Nano-Biophotonics techniques. A) Near-field optical microscopy based on tapered, metal-coated fiber probes. Typical aperture diameters range from 50 – 100 nm. The photograph shows emission from an actual near-field tip. The white scale bar is 100 µm. B) Tip-enhanced spectroscopy uses a confocal laser spot in which a gold or silver metal tip is positioned and which enhances the electromagnetic field between tip and sample. C) Single molecule localization: the intensity distribution of a single molecule can be fit with a Gaussian fit function to achieve localization of the centroid to approximately 1.5 nm. D) Plasmon-resonant particles can be detected by dark-field white light scattering, or if they are coated with Raman active molecules, by their surface-enhanced Raman response (shown in the spectrum for Rhodamine 6G molecules attached to a 60 nm silver particle). The scale bar in the electron micrograph of a gold nanoparticle is 25 μm. E) Nano-apertures restrict the probe volume of a confocal laser spot and permit single molecule analysis at micromolar concentration. The scale bar in the electron micrograph of a square nano-aperture is 50 nm. F) SHG or upconverting particles can act as nanoscale light sources. G) Principle of structured illumination microscopy. Sample features that are smaller than the wavelength of light become visible as beat frequencies in a pattern with known periodicity. H) Principle of stimulated emission depletion microscopy. Red-shifted laser beams that overlap with the emission spectrum of the excited fluorophore deplete the emission except for the very center of the excitation beam (green).
Even though the dynamic registration of single fluorescent molecules in aqueous media was achieved a number of years earlier, near-field optics also marked the beginnings of single molecule fluorescence microscopy. Although most single molecule experiments are now conducted with confocal fluorescence microscopes, a number of highly sensitive and quantitative analysis techniques have recently been developed, that include variations of fluorescence correlation spectroscopy, and more quantitative ways to analyze fluorescence resonance energy transfer (FRET) and determine distances on the molecular scale that have proven useful to the analysis of protein folding and RNA dynamics, or protein-protein and protein-DNA interactions. Of particular value are pulsed interleaved or alternating laser excitation schemes that probe both donor and acceptor fluorescence in these complexes and enable the isolation of true FRET events or the determination of the stoichiometry of molecular complexes [1]. Similarly, fluorescence photon antibunching enables the counting of a discrete number of fluorophores in complexes [2,3]. The recent implementation of fluorescence detection from two confocal laser spots in close proximity to each other by Dertinger et al. [4] also provides significantly more precise values for molecular diffusion and reaction rates than conventional fluorescence correlation spectroscopy which uses a single confocal laser spot. While these techniques work well in vitro, biological applications in the crowded environment of cells are difficult to achieve, because single molecule detection with conventional optics results in diffraction-limited laser spots on the order of a few hundred nanometer and is thus limited to nanomolar molecular concentrations to ensure that only a single molecule is probed at any given point in time. Typical association constants of biological molecules, in particular proteins, however, are in the micromolar concentration range. This requires that one focuses either on rare events, e.g. low copy number gene expression [5,6], or sparse labeling of the molecules of interest, where only approximately every thousandth molecule is fluorescently labeled [7]. This problem represents a target of particular value for the use of nano-structures and will be discussed in more detail further below. If, however, biological targets, e.g. motor proteins, are labeled with single fluorescent molecules, and if they can operate under single molecule conditions, then their position can be located with very high precision [8]. The point spread function (PSF) of a microscope objective with high numerical aperture can be determined very accurately and, in fact, the emission pattern of a single fluorescent molecule typically represents exactly the PSF. Typical PSFs in the lateral dimensions of a microscope can be fitted with high accuracy by two-dimensional Gaussian fit functions. Localization of the position of a single molecule to within 1.5 nm has been demonstrated [8] (see the schematics in Figure 1c). This is sufficient to determine even small steps during enzymatic turnovers or the translational movement of molecular motors. If labeling with brighter probes is possible, e.g. through attachment of multiple fluorophores, or fluorescent nanoparticles, this can even be extended to the cellular analysis of vesicular trafficking by motor proteins [9] or virus-cell interactions [10].
Tip-enhanced spectroscopies
Partly because of the tedious handling and fragility of near-field optic fiber tips, a number of other approaches have been developed to create nanoscopic light sources that can operate in the near-field of a sample. These typically use modifications of the more robust silicon and silicon nitride tips used in scanning probe microscopy. One of the main mechanisms for these tip-enhanced spectroscopies is the interaction of the light field of a tightly focused laser beam with surface plasmons in gold or silver-coated tips (see the schematics in Figure 1b). Here, typically, special excitation modes with an on-axis polarization component are required to optimize excitation of the surface plasmons along the axis of the tip. In this case, the local field enhancement underneath the tip is sufficient to locally excite one or two-photon fluorescence in the sample. The same concept can also be used to obtain surface-enhanced Raman signatures from the sample. Since Raman scattering is based on the inelastic scattering of photons by molecular bonds, no exogenous probes, i.e. fluorophores, are needed to produce a signal in the sample. A limitation of this technique is, however, that any organic molecule that adheres to the tip will produce a permanent signal that will no longer depend on variations in the sample, thus it is easily affected by contaminations with other organic molecules, e.g. albumin from serum or similar proteins that are not typically of interest and which are always present in a biological environment. A particular problem with all tip-enhanced spectroscopies is that they depend significantly on geometry factors, such as the shape of the very end of the tip or the morphology of the gold or silver coating, which are not easy to control. Therefore, reproducibility from tip to tip is problematic. To overcome this problem, the concept of optical antennas has recently been introduced [11,12]. These are optically resonant metallic nano-structures that can be incorporated to tips through nanofabrication techniques (e.g. electron beam lithography or focused ion beam milling) and significantly improve the performance and reproducibility of tip-enhanced near-field microscopy. Hoppener et al., for example, have recently demonstrated the use of optical antenna tips in imaging transmembrane proteins [13]. Other approaches to create near-field sources on a tip include the attachment of organic fluorophores or quantum dots to force microscopy tips to result in nanoscopic light sources that can be freely positioned on the sample [14]. Ultimately, all processes involving fluorescence, however, are limited by the fairly short lifespan of fluorescent probes due to photobleaching.
Fluorescent quantum dots as sensors
The shortcomings of most fluorescent probes (especially under the intense illumination conditions that enable single particle detection) have lead to a continued search for alternative probes. One of the most prominent potential replacements for fluorescent probes are inorganic quantum dots [15]. These are now commercially available through a number of sources and exhibit narrow spectral fluorescence emission, higher robustness against photobleaching than organic fluorophores, and multiple dots with a wide range of emission spectra can be excited by a single blue source (e.g. a diode laser at 405 nm), i.e. emission with Stokes shifts of >100 nm can be observed. In many cases, these particles do, however, still have a considerably larger size than traditional fluorophores (up to 40 nm diameter, depending on the source), typically because thick shell are required to render them water-soluble and for bioconjugation. There also remain concerns about their toxicity, which greatly limit in vivo applications. Nonetheless, in vivo detection has been demonstrated [15,16], and efforts to create peptide-coated quantum dots that are water soluble, highly specific, and retain a small size are very successful [15]. For analytical applications, though, quantum dots have mostly been used as pure tags, and have only recently been extended to become active probes, for example as part of a FRET pair with organic fluorophores [17], or coupled to quenchers [18]. These efforts are complicated mostly by the requirement for shells surrounding the dots, which increase the distance between the active core and its partner in a FRET pair.
Light-scattering particles as alternatives to fluorophores
Another interesting alternative to fluorophores are plasmon-resonant nanoparticles. These scatter light based on their surface plasmon resonance which can be tuned by varying their size, shape, the coupling between particles, or their core-shell ratio [19,20]. In particular, the effects of aggregation, i.e. shifts in the plasmon resonance or the occurrence of new resonant wavelengths due to interparticle plasmon coupling have been used recently to create functional nanostructures with “on-off” reporter behavior and even to create molecular rulers [21-24].
When illuminated in darkfield geometries, these particles can also be localized very accurately and very quickly due to their extremely bright optical response [19,23]. To overcome the typically large size of plasmon-resonant particles, other detection schemes such as interferometry and photothermal deflection have enabled the detection of gold nanoparticles as small as 5 nm diameter, which could potentially make them interesting probes for use in intracellular sensing [25,26]. Even smaller particles consisting of just a few gold or silver atoms have been created and characterized to produce fluorescence and enhanced Raman responses as shown by Dickson and colleagues, but their use and performance under conditions found in biological samples remain to still be characterized [27]. Typically, however, Raman-enhancing nanoparticles require a plasmon resonance at visible or near-infrared wavelengths, which limits their size to between 30 – 100 nm diameter (see Figure 1d). In particular, nano-engineered geometries, such as e.g. nanocrescents, nanoshells, or bow-tie structures have demonstrated excellent Raman-enhancing capabilities [12,28,29]. These, and coupled nanoparticle structures, or nanoparticle dimers will likely generate a great amount of data in biological applications in the near future. Such engineered structures have significant potential to lead us from the current qualitative understanding of the SERS effect to true quantitative measurements and applications.
A particularly interesting aspect of SERS nanoparticles is that the Raman spectrum is very specific to the probe molecules attached to the particle surface and individual Raman peaks are very narrow, typically about 1 nm wide on the wavelength scale in the visible. The Stokes-shifted Raman emission can be detected similarly to fluorescence with standard fluorescence microscopes and filters, but SERS active particles exhibit significantly stronger emission than organic fluorophores and even quantum dots. The narrow, characteristic peaks, their spectral distribution and relative intensity ratios open up a potential for multiplexed detection that exceeds the possibilities of fluorescence-based probes by far. Also, the ability to wavelength-tune their response, especially when moved into the near-infrared part of the optical spectrum enables applications, such as in vivo sensing and imaging (in live animals) that are difficult to achieve by other means. This has recently been demonstrated by Qian et al. [30] and Keren et al. [31]. Lastly, if such probes are functionalized with reporter molecules, i.e. molecules that respond to changes in pH, ion concentration, reactive oxygen, etc., these probes can become active chemical sensors of their local environment [29].
Nano-apertures enhance the dynamic range of single molecule techniques
As mentioned before, single molecule analysis techniques are usually limited by the fact that very low molecular concentrations outside the physiologically relevant range are required to isolate individual molecules. The tiny apertures provided by near-field optical fiber tips would in principle make excellent pinholes that reduce the probe volume to isolate single molecules even at micromolar concentrations. Since these tips are cumbersome to handle and our ability to create these tips in a well-reproducible fashion is rather poor, lithographically produced isolated apertures in an extended metal film were found to work just as well for analyzing molecules in solution (see Figure 1e). Levene et al. have demonstrated the use of such “zero-mode waveguides” for DNA sequencing by immobilizing a DNA polymerase molecule in a nano-aperture and following the incorporation of fluorescently labeled DNA bases during replication [32]. Other applications that extent fluorescence correlation spectroscopy into the crowded cellular environment include e.g. the observation of lateral diffusion of membrane proteins in cells grown on top of a metal film with apertures [33,34], or the extension of FRET and pulsed interleaved laser excitation to DNA-DNA [35], and in the future also DNA-protein, and protein-protein interactions.
Alternatively, an inverse structure to nano-apertures that would permit diffusion measurements of molecules at micromolar concentration inside a cell could be achieved if a local light source or otherwise enhancing structure with nanometer dimensions could be introduced into a cell. In principle, quantum dots should work well for this application, but are somewhat limited by photobleaching and the presence of an extended protective shell surrounding commercially available q-dots that makes interactions with e.g. fluorescent proteins difficult. For these applications, other, robust light sources, such as second harmonic generating (SHG) nanoparticles [36] or upconverting phosphorescent particles might prove more useful [37] (see Figure 1f for a schematic of SHG by a nanoparticle).
Superresolution imaging techniques
Super-resolution microscopy techniques have seen a dramatic increase in activity in recent years. In essence, all these techniques rely on schemes that make use of our ability to control molecular emission properties in the far-field. They can be roughly separated into approaches that either use our ability to locate individual fluorescent molecules with high precision (see Figure 1c), or approaches that reduce the probe volume by exploiting specific molecular processes. The most prominent of these techniques, such as photoactivated localization microscopy (PALM) [38,39] or stochastic optical reconstruction microscopy (STORM) [40,41] use photoswitching of molecular fluorescence emission to identify individual molecules, the coordinates of which are then located on the nanometer scale. Typically, PALM and STORM require the acquisition of large amounts of image data, each of which have to be fitted to the known PSF of the microscope system, and the final images have to be reconstructed from the large set of individual snapshots. Special fluorescent probes with photoswitchable emission are often required for this purpose. These techniques work well in the lateral dimensions, but extension to the third dimension at high resolution remains somewhat challenging, and it seems unlikely that the extensive number of images that have to be acquired together with the high rate of sample exposure, and the computational effort needed for reconstructing images will permit rapid live cell imaging anytime soon.
Other approaches to high-resolution microscopy with applications in chemical biology make use of modifications to the probe volume to improve spatial resolution. Structured illumination microscopy acquires high-resolution images of fluorescent samples by exciting them with a sinusoidal illumination pattern rather than uniform illumination [42]. No special fluorescent probes are required for this type of superresolution microscopy, but they might be beneficial in enhancing its ultimately achievable resolution even further. In structured illumination microscopy, a periodic illumination pattern is created in the focal plane and then moved across the sample laterally and at different angles. Typically about 15 images have to be acquired in order to reconstruct a high-resolution image per vertical plane. This scheme works because sample features with higher spatial frequency than the illumination pattern are modulated by the pattern and result in beat frequencies that fall within the transfer function of the microscope (see the schematics in Figure 1g). Since the periodicity of the illumination pattern is known, its effects on the image can be calculated and high-resolution images can be reconstructed. By moving the sample through the illumination pattern in the vertical direction, high-resolution images of the entire sample in all three dimensions are obtained. Since structured illumination is a widefield imaging technique, special attention has to be paid to index-match the mounting medium and the immersion oil in order to avoid detrimental effects from spherical aberration. Typically, a spatial resolution of approximately 100 nm laterally and 200 nm vertically can be achieved, but if nonlinear illumination conditions are used, e.g. by saturating the fluorescence emission, this form of super-resolution microscopy is virtually unlimited in its resolving power [43].
Lastly, stimulated emission depletion (STED) microscopy is of particular interest for nano-analytics, because not only is it a direct super-resolution imaging technique, requiring no further image processing or mathematical deconvolution, but it is also directly compatible with most other confocal optical analysis techniques [44,45]. STED makes use of the fact that fluorescence can be depleted by stimulated emission - the same mechanism that is e.g. used to achieve tunable emission in dye lasers. In STED, a shaped laser beam with a wavelength within the emission spectrum of the fluorescent probes in the sample is overlapped with the excitation beam (see Figure 1h). The shape of this STED beam is typically a donut mode, which depletes fluorescence in those parts of the sample where both beams overlap, while the central hole continues to spontaneously emit fluorescence photons. The resolution of this technique is in principle only limited by the specific depletion pattern used and by the amount of overlap that can be achieved between the two beams [46]. As a super-resolution microscopy technique, STED has been demonstrated to produce images with near 20 nm resolution in lateral directions and, when combined with counter-propagating beams (4pi illumination), even in the vertical direction. In fluorescence correlation spectroscopy mode, where the excitation and STED beams are focused into a solution of probe molecules, a reduction of the excitation volume by a factor of 5 has been demonstrated - avoiding the need for nanoapertures, where the physical constraint might modify the diffusion behavior of molecules [47]. This same experiment also demonstrated the single molecule fluorescence sensitivity of STED. More recently, fluorescence lifetime imaging was achieved with STED by combining a broadband supercontinuum-generating pulsed laser as excitation source together with single photon counting [48]. Other, important aspects that have recently been demonstrated using STED include the implementation of multi-color detection [49], and video-rate imaging of synaptic vesicle movement [50]. It should also be noted that no special probes are needed for STED, thus samples prepared for regular fluorescence microscopy are fully compatible with STED microscopy.
Conclusions
The phrase “Nano-Biophotonics” is used to describe a collection of powerful analysis tools and techniques for analyzing cellular biochemistry at micromolar concentrations and macromolecular length scales, such as macromolecular interactions, diffusion behavior, and nanoscopic imaging. Here, I have discussed some of the most recent developments in this area that either use near-field optical probes, tip-enhanced spectroscopies, nanoparticles, nano-apertures, or single molecule localization and probe volume engineering to achieve optical super-resolution. Many of these techniques have been implemented in commercial devices and demonstrated valuable new insights into cellular processes, such as inter- and intracellular signaling, replication, and vesicular trafficking. During the next few years, these areas are expected to grow exponentially in the number of applications in chemical biology and the number of publications resulting from these applications. Many of the different areas of research described here will also begin to be merged to take advantage of their individual strengths. For example, localization techniques can be considerably improved in their data acquisition speed and accuracy if robust and bright probes other than fluorescent molecules are used. Some of the probes that I have discussed in this review, i.e. plasmon-resonant particles, SERS probes, or upconverting nanoparticles are readily extendable to high-resolution localization approaches, which has already been demonstrated in a few select cases. Techniques that engineer the probe volume, such as structured illumination and STED could also be readily extended to other probes, or even label-free analysis techniques, such as Raman spectroscopy. If appropriate switching schemes can be found, the principle behind STED could even be applied to label-free microscopies such as Raman scattering, and even nonlinear imaging, such as for example SHG or coherent Anti-Stokes Raman spectroscopy. Many of the schemes discussed here are either already commercially available (e.g. fluorescent quantum dots, plasmon-resonant particles, nanoapertures) or are at the brink of becoming commercially available (structured illumination, STED), which will make them readily accessible to the wider biology community and further spur applications. The future looks indeed very bright for Nano-Biophotonics.
Acknowledgements
I would like to thank my current and former colleagues for many illuminating discussions on the topic of Nano-Biophotonics over the last couple of years. Specifically, I would like to thank Samantha Fore, James Chan, Chad Talley, Christopher Hollars, Adam Schwartzberg, Stephen Lane, Heiko Winhold, Ted Laurence, Tammy Olson, Christine Orme, John Rutledge, Juliana Sampson, Gregory McNerney, Tyler Weeks, Sonny Ly, Iwan Schie, Yin Yuen, and Lambertus Hesselink. This work was supported in part by funding from the National Science Foundation. The Center for Biophotonics, an NSF Science and Technology Center, is managed by the University of California, Davis, under Cooperative Agreement No. PHY 0120999. Support is also acknowledged from the Clinical Translational Science Center under grant number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kapanidis AN, Laurence TA, Lee NK, Margeat E, Kong XX, Weiss S. Alternating-laser excitation of single molecules. Accounts of Chemical Research. 2005;38:523–533. doi: 10.1021/ar0401348. [DOI] [PubMed] [Google Scholar]
- 2.Sykora J, Kaiser K, Gregor I, Bonigk W, Schmalzing G, Enderlein J. Exploring fluorescence antibunching in solution to determine the stoichiometry of molecular complexes. Analytical Chemistry. 2007;79:4040–4049. doi: 10.1021/ac062024f. [DOI] [PubMed] [Google Scholar]
- 3.Fore S, Laurence TA, Hollars CW, Huser T. Counting Constituents in Molecular Complexes by Fluorescence Photon Antibunching. IEEE Journal of Selected Topics in Quantum Electronics. 2007;13:996–1005. [Google Scholar]
- *4.Dertinger T, Pacheco V, von der Hocht I, Hartmann R, Gregor I, Enderlein J. Two-focus fluorescence correlation spectroscopy: A new tool for accurate and absolute diffusion measurements. Chemphyschem. 2007;8:433–443. doi: 10.1002/cphc.200600638. The application of two-focus FCS is demonstrated. This paper uses the acquisition of data from two closely overlapping laser spots to significantly boost the capabilities of FCS in e.g. distinguishing diffusion rates of proteins in different conformational states. It also overcomes some of the other problems of FCS, such as sensitivity to aberrations that limited the reproducibility of FCS. [DOI] [PubMed] [Google Scholar]
- **5.Yu J, Xiao J, Ren XJ, Lao KQ, Xie XS. Probing gene expression in live cells, one protein molecule at a time. Science. 2006;311:1600–1603. doi: 10.1126/science.1119623. This is the first in a series of recent papers that demonstrated the capability to follow low-level gene expression and which showed that proteins are expressed in bursts. [DOI] [PubMed] [Google Scholar]
- 6.Elf J, Li GW, Xie XS. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007;316:1191–1194. doi: 10.1126/science.1141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AE, Fischer AJ, Laurence TA, Hollars CW, Saykally RJ, Lagarias JC, Huser T. Single-molecule dynamics of phytochrome-bound fluorophores probed by fluorescence correlation spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11136–11141. doi: 10.1073/pnas.0604724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Myosin V Walks Hand-Over-Hand: Single Fluorophore Imaging with 1.5-nm Localization. Science. 2003;300:2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- **9.Kural C, Serpinskaya AS, Chou Y-H, Goldman RD, Gelfand VI, Selvin PR. Tracking melanosomes inside a cell to study molecular motors and their interaction. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5378–5382. doi: 10.1073/pnas.0700145104. This paper extends the principles of high resolution localization in intra-cellular tracking of motor proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandenburg B, Zhuang XW. Virus trafficking - learning from single-virus tracking. Nature Reviews Microbiology. 2007;5:197–208. doi: 10.1038/nrmicro1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlschlegel P, Eisler HJ, Martin OJF, Hecht B, Pohl DW. Resonant optical antennas. Science. 2005;308:1607–1609. doi: 10.1126/science.1111886. [DOI] [PubMed] [Google Scholar]
- 12.Jackel F, Kinkhabwala AA, Moerner WE. Gold bowtie nanoantennas for surface-enhanced Raman scattering under controlled electrochemical potential. Chemical Physics Letters. 2007;446:339–343. [Google Scholar]
- 13.Hoppener C, Novotny L. Antenna-based optical imaging of single Ca2+ transmembrane proteins in liquids. Nano Letters. 2008;8:642–646. doi: 10.1021/nl073057t. A single gold nanoparticle attached to the tip of a scanning probe microscope is used to locally enhance the emission of fluorescently labeled membrane proteins. [DOI] [PubMed] [Google Scholar]
- 14.Farahani JN, Pohl DW, Eisler HJ, Hecht B. Single quantum dot coupled to a scanning optical antenna: A tunable superemitter. Physical Review Letters. 2005;95 doi: 10.1103/PhysRevLett.95.017402. [DOI] [PubMed] [Google Scholar]
- 15.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Medicine. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 17.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE, Mattoussi H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nature Materials. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 18.Pons T, Medintz IL, Sapsford KE, Higashiya S, Grimes AF, English DS, Mattoussi H. On the quenching of semiconductor quantum dot photoluminescence by proximal gold nanoparticles. Nano Letters. 2007;7:3157–3164. doi: 10.1021/nl071729+. [DOI] [PubMed] [Google Scholar]
- 19.Schultz S, Smith DR, Mock JJ, Schultz DA. Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:996–1001. doi: 10.1073/pnas.97.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loo C, Hirsch L, Lee MH, Chang E, West J, Halas N, Drezek R. Gold nanoshell bioconjugates for molecular imaging in living cells. Optics Letters. 2005;30:1012–1014. doi: 10.1364/ol.30.001012. [DOI] [PubMed] [Google Scholar]
- *21.Li HX, Nelson E, Pentland A, Van Buskirk J, Rothberg L. Assays based on differential adsorption of single-stranded and double-stranded DNA on unfunctionalized gold nanoparticles in a colloidal suspension. Plasmonics. 2007;2:165–171. A simple, colorimetric assay for DNA detection with a sensitivity similar to ELISA is demonstrated. The simplicity, universality, and sensitivity of this approach should make it widely applicable - particularly for forensics. [Google Scholar]
- 22.Sonnichsen C, Reinhard BM, Liphardt J, Alivisatos AP. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nature Biotechnology. 2005;23:741–745. doi: 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]
- **23.Reinhard BM, Sheikholeslami S, Mastroianni A, Alivisatos AP, Liphardt J. Use of plasmon coupling to reveal the dynamics of DNA bending and cleavage by single EcoRV restriction enzymes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2667–2672. doi: 10.1073/pnas.0607826104. Modifications of the plasmon coupling between pairs of gold and silver nanoparticles are used to bridge the gap in our ability to measure length scales of single molecules in the 1-100 nm length scale. This mechanism provides unlimited observation times with high sensitivity that provide new insights into the action of enzymes on DNA substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GL, Yin YD, Kunchakarra S, Mukherjee B, Gerion D, Jett SD, Bear DG, Gray JW, Alivisatos AP, Lee LP, et al. A nanoplasmonic molecular ruler for measuring nuclease activity and DNA footprinting. Nature Nanotechnology. 2006;1:47–52. doi: 10.1038/nnano.2006.51. [DOI] [PubMed] [Google Scholar]
- **25.Lasne D, Blab GA, Berciaud S, Heine M, Groc L, Choquet D, Cognet L, Lounis B. Single nanoparticle photothermal tracking (SNaPT) of 5-nm gold beads in live cells. Biophysical Journal. 2006;91:4598–4604. doi: 10.1529/biophysj.106.089771. Small gold nanoparticles are used as in vivo tags and detected by photothermal deflection. This enables extended observation times of molecular events at high speed (video rate). The authors demonstrated the potential of this technique by acquiring AMPA receptor trajectories on the plasma membrane of live neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen V, Stoller P, Brunner C, Vogel V, Sandoghdar V. Interferometric optical detection and tracking of very small gold nanoparticles at a water-glass interface. Optics Express. 2006;14:405–414. doi: 10.1364/opex.14.000405. [DOI] [PubMed] [Google Scholar]
- 27.Peyser-Capadona L, Zheng J, Gonzalez JI, Lee TH, Patel SA, Dickson RM. Nanoparticle-free single molecule anti-stokes Raman spectroscopy. Physical Review Letters. 2005;94 doi: 10.1103/PhysRevLett.94.058301. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Liu GL, Kim J, Mejia YX, Lee LP. Nanophotonic crescent moon structures with sharp edge for ultrasensitive biomolecular detection by local electromagnetic field enhancement effect. Nano Letters. 2005;5:119–124. doi: 10.1021/nl048232+. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzberg AM, Oshiro TY, Zhang JZ, Huser T, Talley CE. Improving nanoprobes using surface-enhanced Raman scattering from 30-nm hollow gold particles. Analytical Chemistry. 2006;78:4732–4736. doi: 10.1021/ac060220g. [DOI] [PubMed] [Google Scholar]
- **30.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnology. 2008;26:83–90. doi: 10.1038/nbt1377. Raman-enhancing nanoparticles are demonstrated to act as highly sensitive tags for in vivo sensing that exceed the sensitivity obtained by other optical probes with significant potential for in vivo tumor targeting and high multiplexing. [DOI] [PubMed] [Google Scholar]
- **31.Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5844–5849. doi: 10.1073/pnas.0710575105. Similar to the previous paper, SERS tags are demonstrated for in vivo tumor targeting and imaging, but the authors demonstrated that other Raman-active probes, such as carbon nanotubes can extend this approach and extended the range of available probes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–686. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- 33.Danelon C, Perez JB, Santschi C, Brugger J, Vogel H. Cell membranes suspended across nanoaperture arrays. Langmuir. 2006;22:22–25. doi: 10.1021/la052387v. [DOI] [PubMed] [Google Scholar]
- 34.Wenger J, Conchonaud F, Dintinger J, Wawrezinieck L, Ebbesen TW, Rigneault H, Marguet D, Lenne PF. Diffusion analysis within single nanometric apertures reveals the ultrafine cell membrane organization. Biophysical Journal. 2007;92:913–919. doi: 10.1529/biophysj.106.096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Fore S, Yuen Y, Hesselink L, Huser T. Pulsed-interleaved excitation FRET measurements on single duplex DNA molecules inside C-shaped nanoapertures. Nano Letters. 2007;7:1749–1756. doi: 10.1021/nl070822v. The principle of nano-apertures is extended beyond simply detecting the diffusion of molecules at high concentration and the authors showed that they can also apply other popular single molecules techniques, such as fluorescence resonance energy transfer (FRET), multi-color, and pulsed interleaved excitation. [DOI] [PubMed] [Google Scholar]
- 36.Sandeau N, Le Xuan L, Chauvat D, Zhou C, Roch JF, Brasselet S. Defocused imaging of second harmonic generation from a single nanocrystal. Optics Express. 2007;15:16051–16060. doi: 10.1364/oe.15.016051. [DOI] [PubMed] [Google Scholar]
- 37.Lim SF, Riehn R, Ryu WS, Khanarian N, Tung CK, Tank D, Austin RH. In vivo and scanning electron microscopy imaging of upconverting nanophosphors in Caenorhabditis elegans. Nano Letters. 2006;6:169–174. doi: 10.1021/nl0519175. [DOI] [PubMed] [Google Scholar]
- 38.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nature Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- **39.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. This paper was the first to demonstrate high resolution optical microscopy based on single molecule localization and has spurred significant activity in this area. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Wang WQ, Bates M, Zhuang XW. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 2008;319:810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rust MJ, Bates M, Zhuang XW. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nature Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Gustafsson MGL, Shao L, Carlton PM, Wang CJR, Golubovskaya IN, Cande WZ, Agard DA, Sedat JW. Three-dimensional Resolution Doubling in Widefield Fluorescence Microscopy by Structured Illumination. Biophysical Journal. 2008;94:4957–4970. doi: 10.1529/biophysj.107.120345. This paper explains and demonstrates in detail the principles behind structured illumination microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson MGL. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Donnert G, Keller J, Medda R, Andrei MA, Rizzoli SO, Lurmann R, Jahn R, Eggeling C, Hell SW. Macromolecular-scale resolution in biological fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11440–11445. doi: 10.1073/pnas.0604965103. A resolution in confocal far-field fluorescence microscopy between 15-20 nm in biological samples is demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. This paper demonstrated by high-resolution stimulated emission depletion microscopy that components of synaptic vesicles stay together after synaptic endocytosis preceding the recycling of synaptic vesicles. This paper represents a landmark in demonstrating the possibilities of superresolution microscopy with biological samples. [DOI] [PubMed] [Google Scholar]
- 46.Harke B, Keller J, Ullal CK, Westphal V, Schoenle A, Hell SW. Resolution scaling in STED microscopy. Optics Express. 2008;16:4154–4162. doi: 10.1364/oe.16.004154. [DOI] [PubMed] [Google Scholar]
- 47.Kastrup L, Blom H, Eggeling C, Hell SW. Fluorescence Fluctuation Spectroscopy in Subdiffraction Focal Volumes. Physical Review Letters. 2005;94:178104. doi: 10.1103/PhysRevLett.94.178104. [DOI] [PubMed] [Google Scholar]
- 48.Auksorius E, Boruah BR, Dunsby C, Lanigan PMP, Kennedy G, Neil MAA, French PMW. Stimulated emission depletion microscopy with a supercontinuum source and fluorescence lifetime imaging. Optics Letters. 2008;33:113–115. doi: 10.1364/ol.33.000113. [DOI] [PubMed] [Google Scholar]
- 49.Donnert G, Keller J, Wurm CA, Rizzoli SO, Westphal V, Schonle A, Jahn R, Jakobs S, Eggeling C, Hell SW. Two-color far-field fluorescence nanoscopy. Biophysical Journal. 2007;92:L67–L69. doi: 10.1529/biophysj.107.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **50.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. This paper demonstrated that stimulated emission depletion can be extended to video-rate confocal microscopy. It is likely to spur developments and applications of STED microscopy, such as vesicle and protein tracking in living cells. [DOI] [PubMed] [Google Scholar]