Abstract
Isovaleric acidemia (IVA) is an autosomal recessive inborn error of leucine metabolism caused by a deficiency of the mitochondrial enzyme isovaleryl-CoA dehydrogenase (IVD) resulting in the accumulation of derivatives of isovaleryl-CoA. It was the first organic acidemia recognized in humans and can cause significant morbidity and mortality. Early diagnosis and treatment with a protein restricted diet and supplementation with carnitine and glycine are effective in promoting normal development in severely affected individuals. Both intra- and inter-familial variability have been recognized. Initially, two phenotypes with either an acute neonatal or a chronic intermittent presentation were described. More recently, a third group of individuals with mild biochemical abnormalities who can be asymptomatic have been identified through newborn screening of blood spots by tandem mass spectrometry. IVD is a flavoenzyme that catalyzes the conversion of isovaleryl-CoA to 3-methylcrotonyl-CoA and transfers electrons to the electron transfer flavoprotein. Human IVD has been purified from tissue and recombinant sources and its biochemical and physical properties have been extensively studied. Molecular analysis of the IVD gene from patients with IVA has allowed characterization of different types of mutations in this gene. One missense mutation, 932C>T (A282V), is particularly common in patients identified through newborn screening with mild metabolite elevations and who have remained asymptomatic to date. This mutation leads to a partially active enzyme with altered catalytic properties; however, its effects on clinical outcome and the necessity of therapy are still unknown. A better understanding of the heterogeneity of this disease and the relevance of genotype/phenotype correlations to clinical management of patients are among the challenges remaining in the study of this disorder in the coming years.
Keywords: Isovaleric acidemia, organic acidemia, flavoenzyme, leucine metabolism, acyl-CoA dehydrogenase
INTRODUCTION
Isovaleric acidemia (IVA) was the first of the organic acidemias to be described and its nature was elucidated due to a combination of astute clinical acumen and new technology. The two original patients were 4 and 2½ years old, respectively, and had similar histories of recurrent episodes of vomiting and lethargy that resolved with supportive therapy including glucose infusions [Budd et al., 1967; Efron, 1967; Tanaka et al., 1966]. A “smell specialist” suggested that the unusual odor associated with their acute episodes was likely a short chain fatty acid, and isovaleric acid was identified in patient plasma through the then novel approach of gas chromatography followed by mass spectrometry [Tanaka, 1990]. Urine was subsequently shown to contain isovalerylglycine and 3-hydroxyisovaleric acid as well as other metabolites [Tanaka and Isselbacher, 1967; Tanaka et al., 1968]. Oxidation of labeled isovaleric acid by patient white blood cells was reduced compared to control cells, and a defect of leucine metabolism was postulated [Hyman and Tanaka, 1986; Rhead and Tanaka, 1980; Shih et al., 1973; Yoshida et al., 1985]. It must be remembered that until the characterization of these patients, the oxidation of isovaleryl-CoA was assumed to be catalyzed by the newly identified short chain acyl-CoA dehydrogenase [Crane et al., 1956; Engel and Massey, 1971; Frerman et al., 1980; McKean et al., 1979]. Over the next 20 years IVD was separated from the other acyl-CoA dehydrogenases, purified, cloned, and the molecular nature of the defects responsible for loss of IVD activity elucidated. The use of gas chromatography/mass spectrometry (GC-MS) ultimately became the mainstay of the identification and routine clinical diagnosis of a new class of inborn errors of metabolism resulting in the abnormal accumulation of organic acids in urine, and it remains a valuable tool for biochemical geneticists today [Burke et al., 1983; Tanaka et al., 1980]. The more recent application of tandem mass spectrometry (MS/MS) for analysis of the cylcarnitine profile of blood spots from newborn screening filter paper cards has allowed a significant expansion of the recognition of mildly affected and potentially asymptomatic individuals with IVD deficiency through newborn screening [Ensenauer et al., 2004].
DISEASE SPECTRUM
Early literature on IVA, an autosomal recessive disorder, emphasized two apparent phenotypes [Tanaka, 1990]. The first was an acute, neonatal presentation with patients becoming symptomatic within the first two weeks of life [Budd et al., 1967; Efron, 1967; Elsas and Naglak, 1988; Levy et al., 1973; Lott et al., 1972; Tanaka et al., 1966]. Patients appeared initially well, then developed vomiting and lethargy, progressing to coma. The second group presented with relatively non-specific failure to thrive and/or developmental delay (chronic intermittent presentation) [Berry et al., 1988; Elsas and Naglak, 1988; Levy et al., 1973; Mehta et al., 1996; Shih et al., 1984]. Patients who survived an early acute presentation subsequently were indistinguishable from those with the chronic phenotype, and both groups of patients were prone to intermittent acute episodes of decompensation with minor illnesses [Tanaka, 1990]. In reality it is now apparent that patients can fall anywhere on the spectrum of acute to chronic presentation and that there is probably little predictive value to the initial presentation. Moreover, with the application of MS/MS in newborn screening, potentially asymptomatic patients with one recurring IVD gene mutation and a mild biochemical phenotype are being identified in increasing numbers, representing an additional phenotype of IVA [Ensenauer et al., 2004]. This type may represent a biochemical phenotype only without the expression of any clinical symptoms (such as in benign hyperphenylalaninemia) and therefore needs to be differentiated from the classic forms of IVA. For practical purposes, we suggest classifying patients with IVA as “metabolically severe” and “metabolically mild or intermediate”, giving consideration to the broader spectrum of IVA detectable by newborn screening.
CLINICAL PRESENTATION
Nearly all published clinical information on patients with IVA is retrospective [Sweetman and Williams, 2001], and thus the following discussion of clinical symptoms is limited to the classic presentations of IVA prior to newborn screening, i.e. manifestation in the neonatal period versus later in childhood. In one summary of 37 patients compiled from different publications, 28 presented in the first 2 weeks of life, 7 between 2 weeks and 1 year of age, and the remaining 2 after 1 year of age [Tanaka, 1990]. Sixteen of the patients were deceased, and of those still alive, 7 were reported to have mild to moderate mental retardation.
Neonatal symptoms are non-specific and include poor feeding, vomiting, decreased level of consciousness, and seizures [Budd et al., 1967; Efron, 1967; Elsas and Naglak, 1988; Levy et al., 1973; Lott et al., 1972; Spirer et al., 1975; Tanaka et al., 1966]. Infants may develop hypothermia and appear to be dehydrated. A characteristic smell of “dirty socks” may be present when the patient is acutely sick though, unlike other organic acidemias, the urine has no odor since the unconjugated isovaleric acid responsible for the odor is not excreted in urine in appreciable quantity [Tanaka, 1990; Tanaka et al., 1966]. The odor may be best appreciated in body sweat or cerumen from the ear. Acidosis with an unexplained anion gap is characteristic, and hyperammonemia, hyper- or hypoglycemia and hypocalcemia may be present [Budd et al., 1967; Levy et al., 1973; Lott et al., 1972; Mendiola et al., 1984; Tanaka, 1990; Tanaka et al., 1966; Worthen et al., 1994; Yoshino et al., 1982]. Secondary hyperammonemia is presumed to be due to inhibition of N-acetylglutamate synthetase by isovaleryl-CoA and/or intracellular depletion of acetyl-CoA leading to reduced N-acetylglutamate synthesis and impairment of the urea cycle [Coude et al., 1979; Stewart and Walser, 1980]. Pancytopenia as well as isolated neutropenia and thrombocytopenia can occur due to bone marrow suppression [Kelleher et al., 1980]. Left untreated, patients may progress to coma and death often due to cerebral edema or hemorrhage [Fischer et al., 1981]. Overall, the clinical picture overlaps other organic acidemias including the β-oxidation defects as well as the urea cycle disorders and other primary causes of hyperammonemia, all of which must therefore be considered in the differential diagnosis. Patients who survive a neonatal crisis may be clinically indistinguishable from children diagnosed later in life [Tanaka, 1990].
Children diagnosed outside the newborn period may present with more chronic, relatively non-specific findings of failure to thrive and/or developmental delay or mental retardation [Tanaka, 1990]. Minus the “sweaty feet odor” of isovaleric acid, which is not present when a patient is otherwise well, there is little to suggest a specific diagnosis in these children and thus, it must be considered in all patients with this clinical picture. They also are at risk of episodes of acute acidosis and metabolic decompensation, usually due to intercurrent illnesses or other physiologic stress including fasting [Berry et al., 1988; Tanaka, 1990]. Acute episodes may be misdiagnosed as diabetic ketoacidosis due to hyperglycemia, acidosis and the apparent presence of blood and urinary ketones [Attia et al., 1996]. Acute pancreatitis, myeloproliferative syndrome, Fanconi syndrome, and cardiac arrhythmias have been reported [Arnold et al., 1986; Gilbert-Barness and Barness, 1999; Kahler et al., 1994; Weinberg et al., 1997]; abnormalities of the globus pallidus can be seen [Sogut et al., 2004]. The variability of this disorder is highlighted by the diagnosis of IVA in a previously well 18-year-old man who developed acute nausea, vomiting, and mental status changes during basic training camp for the United States Air Force [Feinstein and O'Brien, 2003].
There have been reports of successful pregnancies in women with IVA resulting in apparently well infants [Shih et al., 1984; Spinty et al., 2002].
BIOCHEMICAL DIAGONOSIS AND FOLLOW UP
The majority of patients with IVA today are diagnosed pre-symptomatically through newborn screening by use of MS/MS which reveals elevations of the marker metabolite C5 acylcarnitine in dried blood spots. Because C5 acylcarnitine represents a mixture of isomers (isovalerylcarnitine, 2-methylbutyrylcarnitine, and pivaloylcarnitine), further diagnostic evaluation is required (TableI). Elevations of 2-methylbutyrylcarnitine are seen in patients with 2-methylbutyrylglycinuria caused by a deficiency of short/branched-chain acyl-CoA dehydrogenase (SBCAD), an inborn error of isoleucine catabolism [Andresen et al., 2000; Gibson et al., 2000], whereas pivaloylcarnitine is derived from pivalic acid, a component of several antibiotics [Abdenur et al., 1998]. Isovaleryl-CoA intermediates can also be seen in deficiencies of the electron transfer flavoprotein and its dehydrogenase (glutaric aciduria type 2).
Table I.
Initial diagnostic evaluation for isovaleric acidemia
| Evaluation following abnormal newborn screening with an elevated C5 acylcarnitine concentration | |
|---|---|
| Test | Determination of |
| Urine organic acid analysis | Multiple abnormal metabolites; isovalerylglycine concentration |
| Plasma acylcarnitine analysis | Isovalerylcarnitine concentration |
| Plasma carnitine analysis | Free carnitine concentration |
| Molecular genetic analysis | Common 932C>T (A282V) IVD gene mutation associated with a mild biochemical phenotype; otherwise heterogeneous mutations |
| Enzymatic analysis optional (fibroblasts, lymphocytes) |
Residual enzyme activity |
A long list of other isovaleryl-CoA derived metabolites has been reported in blood and urine from patients with IVA and can assist in confirmation of the disorder [Burke et al., 1983; Dorland et al., 1983; Hine and Tanaka, 1984; Lehnert, 1981a; Lehnert, 1981b; Lehnert, 1983; Lehnert and Niederhoff, 1981; Loots et al., 2005; Poorthuis et al., 1993]. Isovaleryl conjugates of multiple amino acids have also been detected in urine, as have free 3- and 4-hydroxyisovaleric acids [Lehnert and Niederhoff, 1981; Loots et al., 2005; Tanaka et al., 1968]. Free isovaleric acid in blood during episodes of acute metabolic decompensation can reach several hundred times normal values but is not readily seen in blood and urine due to its rapid conjugation to other compounds. Thus, isovalerylcarnitine and isovalerylglycine are the hallmarks of this disorder in plasma and urine, respectively, and are elevated regardless of a patient’s metabolic condition. Quantification of both conjugates has suggested a correlation of the metabolite concentrations with genotype, differentiating between groups of patients with a metabolically severe phenotype associated with heterogeneous IVD gene mutations and patients with a metabolically mild or intermediate phenotype associated with one recurring mutation [Ensenauer et al., 2004]. Disease-specific metabolites also accumulate in amniotic fluid during pregnancy with an affected fetus and provide the opportunity for prenatal diagnosis [Hine et al., 1986; Jakobs et al., 1984; Shigematsu et al., 1991].
Several direct and indirect methods to assay IVD activity have been published and, in addition to molecular genetic analysis, can be used to confirm a diagnosis of IVA [Hyman and Tanaka, 1986; Mohsen et al., 1998; Rhead and Tanaka, 1980; Shih et al., 1973; Tajima et al., 2005; Vockley et al., 1991; Yoshida et al., 1985]. Fibroblasts, lymphocytes, and amniocytes all have measurable amounts of IVD activity and serve as ready sources of tissue for this purpose [Ensenauer et al., 2004; Kleij et al., 1995; Mohsen et al., 1998; Vockley et al., 1991]. While significant residual activity blunts the level of abnormal metabolites, correlation between clinical presentation and enzyme activity has been poor [Hyman and Tanaka, 1986; Ikeda et al., 1985b; Vockley et al., 1991].
Regarding routine follow up visits, there is no established laboratory marker for monitoring therapeutic control or disease state. Weight gain, growth and development should be age-appropriate and thus, body measurements are key parameters to follow on a routine basis. Specifically, protein malnutrition must be avoided if the patient is protein restricted. Analysis of amino acids, albumin, and prealbumin in plasma is recommended to monitor this. Plasma levels of leucine are not elevated in IVA, even if untreated, due to the irreversible oxidative decarboxylation earlier in the leucine degradation pathway [Sweetman and Williams, 2001]. Plasma free carnitine concentrations may be helpful for determining necessity and monitoring of carnitine supplementation.
MOLECULAR FINDINGS
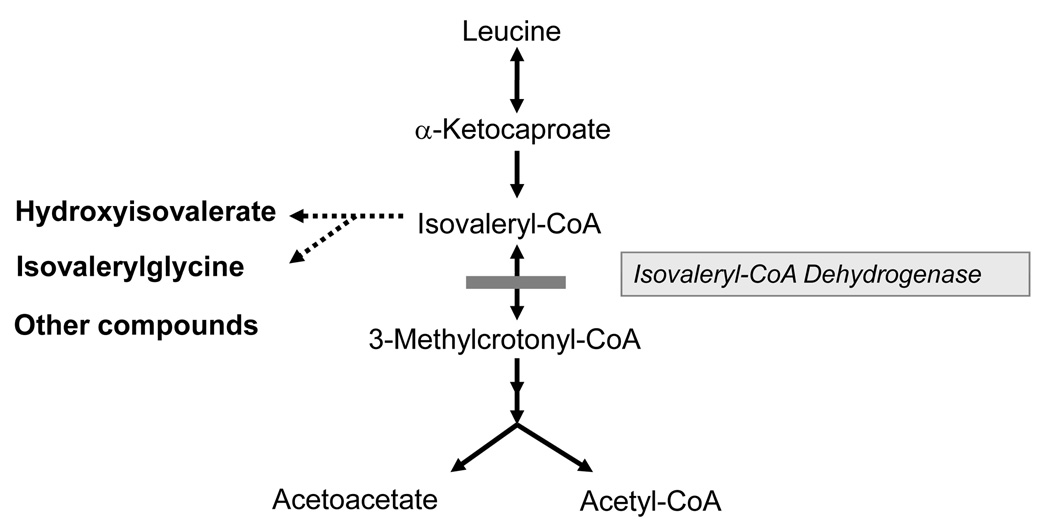
IVD is an intramitochondrial homotetrameric flavoenzyme that catalyzes the α,β-dehydrogenation of isovaleryl-CoA resulting in 3-methylcrotonyl-CoA (Fig. 1) and transfers the reducing equivalents to the electron transfer flavoprotein [Ikeda et al., 1983; Ikeda and Tanaka, 1988; Mohsen and Vockley 1995]. It is encoded in the nuclear genome as a precursor protein, but is active in the mitochondrial matrix [Ikeda et al., 1987; Parimoo and Tanaka, 1993; Vockley et al., 1991]. Following transcription in the nucleus and translation in the cytoplasm, it is held in a partially folded state by chaperonin proteins, targeted to mitochondria by a characteristic amino terminal peptide sequence and imported into mitochondria. The target peptide is cleaved in the mitochondrial matrix, folding of the monomer is completed, and the final active tetramer is assembled. The FAD cofactor is probably added after import into the mitochondria, but the exact timing and mechanism of this reaction is poorly characterized [Nagao and Tanaka, 1992]. The reaction catalyzed by IVD is initiated upon acyl-CoA substrate binding. In the reductive half-reaction, the formation of a hydrogen bond between the acyl carbonyl oxygen and the 2′–hydroxyl hydrogen of the FAD ribityl moiety is crucial for activation of the acyl moiety [Ghisla, 1992; Miura et al., 1996; Nishina et al., 1995]. The Cα and Cβ hydrogens of the acyl moiety are then removed in concert as a proton and a hydride, respectively, forming a stable charge-transfer complex. The electron transfer flavoprotein (ETF), considered a second substrate, extracts the reducing equivalents from the charge-transfer complex in the oxidative half-reaction during which electrons are transferred to the latter and the enoyl-CoA product is released [Ghisla and Massey, 1989; Gorelick et al., 1985; Ikeda et al., 1985a]. The molecular mechanism of the oxidative half-reaction and the order of binding of the substrates have not been elucidated.
Figure 1.
The catabolic pathway of leucine. Isovaleryl-CoA dehydrogenase catalyzes the conversion of isovaleryl-CoA to 3-methylcrotonyl-CoA. Enzyme deficiency results in the accumulation of isovaleryl-CoA derivatives.
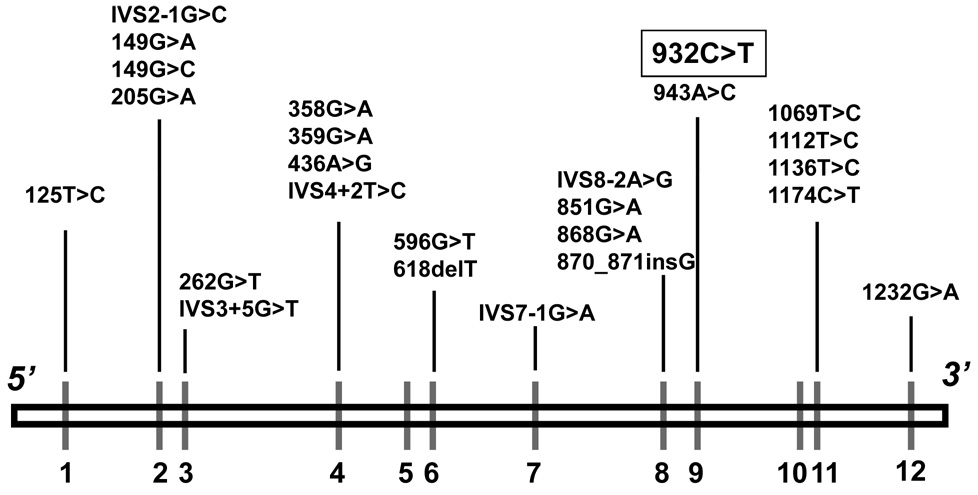
Molecular cloning of the IVD gene showed it to be located on chromosome 15q14–15, consisting of 12 exons that span ~15 kb of genomic DNA [Parimoo and Tanaka, 1993]. Molecular analysis of the IVD gene in patients with symptomatic IVA has identified numerous point mutations in the protein coding region that lead to the production of an inactive or unstable protein (Fig. 2) [Ikeda et al., 1985b; Mohsen et al., 1998; Vockley et al., 1992a; Vockley et al., 1992b; Vockley et al., 1991; Vockley et al., 2000]. Some of these have been shown to cause only a mild alteration in enzyme function, correlating to some extent with a mild clinical phenotype in patients [Mohsen et al., 1998; Nasser et al., 2004]. A significant proportion of the mutant alleles lead to abnormal splicing of the IVD RNA and subsequent complete lack of IVD protein [Vockley et al., 1991; Vockley et al., 2000]. Studies of the bioprocessing of IVD protein in fibroblasts from patients with IVA have reflected the effects of these mutations but have not provided insight into the clinical variability seen in the disorder [Ikeda et al., 1985b; Vockley et al., 1991]. In general, genotype and phenotype have not been well correlated.
Figure 2.
Molecular defects in the IVD gene on chromosome 15q14–15 [Mohsen et al., 1998; Ensenauer et al., 2004]. The nucleotide change 932C>T (A282V) in exon 9 has been identified in a significant proportion of individuals diagnosed by newborn screening [Ensenauer et al., 2004].
NEWBORN SCREENING AND ISOVALERIC ACIDEMIA
With the advent of the use of MS/MS to screen newborn blood spots for acylcarnitine concentrations, most if not all patients with IVA should be identified as newborns prior to the development of symptoms. One unexpected finding to arise from newborn screening studies is the identification of individuals with only mild elevations of isovaleryl-CoA related metabolites in plasma and urine, orders of magnitude lower than in the classic forms of IVA, and apparently only partial reduction in IVD activity (Table II) [Ensenauer et al., 2004]. Nearly half of the mutant IVD alleles sequenced from infants diagnosed by newborn screening have been found to contain a common recurring missense mutation [Ensenauer et al., 2005] (932C>T; A282V; Fig. 2). This specific mutation was present in approximately two thirds of newborns in this cohort, mostly in a compound heterozygous fashion. All of the affected newborns carrying the common mutation have remained asymptomatic with mild or no dietary protein restriction and carnitine supplementation if necessary over a maximum duration of follow up of up to 5 years of age. Subsequently, asymptomatic siblings of patients identified through newborn screening (ages 3 to 11 years at the time of diagnosis) have been found to be homozygous or compound heterozygous for the same mutation with a similarly mild biochemical phenotype [Ensenauer et al., 2004]. They have remained without symptoms during episodes of febrile illnesses.
Table II.
Recommendations for therapy in isovaleric acidemia
| Therapy | Biochemical phenotype | |
|---|---|---|
| Metabolically mild or intermediate1 |
Metabolically severe2 | |
| Prevention of metabolic crisis |
Close clinical observation; promote anabolism during illness | |
| Diet | None | Protein restriction |
| Medication | Carnitine (30–50 mg/kg per day) if plasma free carnitine concentration is low |
Carnitine (100 mg/kg per day) |
| None | Glycine (150–250 mg/kg per day) | |
Metabolically mild or intermediate: NBS blood spot C5 acylcarnitine concentration 0.8 to 6 µmol/L; urine isovalerylglycine concentration 15 to 195 mmol/mol creatinine.
Metabolically severe: NBS blood spot C5 acylcarnitine concentration up to 21.7 µmol/L; urine isovalerylglycine concentration up to 3300 mmol/mol creatinine.Values derived from Ensenauer et al., 2004.
Prior to newborn screening, this mutation was identified only in a single patient with mild IVD deficiency originally evaluated for a tic disorder and slight developmental delay [Mohsen et al., 1998; Vockley et al., 1992b]. The mutant A282V protein is stable in vitro but is kinetically impaired, exhibiting an increased Km and a reduced catalytic efficiency, and has diminished thermal stability [Mohsen et al., 1998; Nasser et al., 2004]. It is clear that the newborn screening patients who carry the common mutation either in a homozygous or compound heterozygous state and their sibs skew the spectrum of IVA with more than half of individuals representing a new mild phenotype and potentially remaining asymptomatic. This is an expansion of our view of the natural history of IVA prior to the newborn screening era and leads to significant implications for management and genetic counseling. As opposed to the classic forms of IVA, it is still uncertain whether individuals with the latter type have a disease, a risk of clinical manifestation, or simply express a clinically insignificant biochemical phenotype. While these individuals may have normal leucine homeostasis under physiological conditions, their risk of metabolic decompensation under stress conditions remains to be elucidated.
THERAPY
There are three goals for therapy of IVA (Table II). The first is prevention of metabolic decompensation by careful clinical observation of the patient regardless of the type of IVA. During times of metabolic stress (including illness and fasting) endogenous leucine from protein catabolism adds significantly to the production of isovaleryl-CoA [Collins et al., 1987; Millington et al., 1987; Pollitt, 1987]. Achieving anabolism is the main therapeutic approach. Thus, sick day precautions for patients with IVA should include increased caloric intake in addition to decreased leucine intake. This is most easily accomplished with oral solutions containing simple sugars and leucine free metabolic formulae or powders. IV glucose infusions need to be added if oral intake is interrupted. Leucine intake should be decreased to approximately 50% of the patient’s usual daily minimum, but returned to normal after 24 hours in order to promote protein anabolism.
The second goal is long term reduction of the production of isovaleryl-CoA from leucine catabolism through dietary manipulation [Berry et al., 1988; Levy et al., 1973; Lott et al., 1972; Sweetman and Williams, 2001]. Total protein and caloric intake must be adequate to support normal growth in children and maintain an anabolic state, and thus monitoring of weight, length and head circumference is essential at follow up. In many cases, it may be sufficient to moderately lower protein intake with natural foods to approximately 1.5 gm/kg per day. In patients with recurrent clinical symptoms, leucine restriction in excess of total natural protein may also be necessary [Sweetman and Williams, 2001]. Natural protein necessary to reach the recommended age-appropriate daily requirement must then be provided with leucine-free amino acids. Because of the specific role of leucine in promoting protein synthesis, however, there is a potential for adverse side effects of rigorous leucine restriction including muscle wasting [Harris et al., 2004].
Acute episodes of metabolic decompensation can present with emesis, lethargy and signs of overwhelming acidosis. Under these circumstances, immediate hospitalization is required so that IV access can be established and glucose administered. Glucose infusion should be calculated to give at least 8 mg/kg per min with concomitant use of IV insulin if necessary to maintain euglycemia. Reintroduction of oral intake including catabolism-sparing levels of protein (0.5 gm/kg per day) with leucine should occur as soon as it can be tolerated, otherwise parenteral amino acids should be provided. If present, hyperammonemia will reverse with correction of the primary metabolic derangement; alternative ammonia conjugating agents such as sodium benzoate or phenylbutyrate are generally not indicated.
The third goal of therapy in patients with IVA is to prevent the accumulation of toxic metabolites by shunting isovaleryl-CoA towards reactions that produce metabolites presumed to be non-toxic and that can readily be excreted. Recognition of isovalerylglycine in urine in the initial patients with IVA first led to the use of glycine to achieve this end [Cohn et al., 1978; Elsas and Naglak, 1988; Krieger and Tanaka, 1976; Yudkoff et al., 1978]. Isovaleryl-CoA is enzymatically conjugated to glycine, a reaction that can be augmented by supplementation with exogenous glycine to supra-physiologic levels. Such supplementation prevents or reduces the accumulation of isovaleric acid in blood following a leucine load, and the length and severity of symptoms during intercurrent illnesses [Krieger and Tanaka, 1976; Shigematsu et al., 1982; Yudkoff et al., 1978]. Doses of 150 to 600 mg/kg per day given orally and divided in three or four equal doses of body weight have been proposed, but the optimum dose has not been determined. Patients exhibit a dose sensitive increase in excretion of isovaleryglycine, but at least in one report an increase in the glycine dose from 300 to 600 mg/kg of body weight led to a decrease in the excretion of isovalerylglycine, presumably due to inhibition of glycine-N-acylase by glycine [Elsas and Naglak, 1988]. Thus, initial dosing in the range of 150 to 250 mg/kg per day is reasonable in patients with a metabolically severe type of IVA (Table II). Concern has been raised about the potential for glycine toxicity, though no reports of such an occurrence have been published.
The identification of isovalerylcarnitine in blood and urine along with the frequent observation of a secondary deficiency of free carnitine in patients with IVA has prompted treatment with carnitine. A dose of 100 mg/kg body weight per day has generally been used (Table II) and has been shown to increase the excretion of isovalerylcarnitine in urine [Fries et al., 1996; Mayatepek et al., 1991]. Combined therapy with carnitine and glycine has been shown to maximize the total excretion of isovaleryl-CoA conjugates, but the clinical benefit of combined versus single therapy has not been established through controlled studies [Fries et al., 1996; Itoh et al., 1996]. The relative merits of the two therapies either singly or together in patients with more severe presentations including recurrent crises remains a matter of debate.
The necessity of any treatment for individuals diagnosed by newborn screening and carrying the common 932C>T (A282V) mutation is unclear. Specifically, the potential for metabolic decompensation under stress conditions remains to be elucidated. It appears reasonable to observe these individuals clinically, particularly when exposed to metabolic stressors such as febrile illnesses or fasting (e.g. when undergoing surgery). Additional recommendations include low-dose carnitine supplementation if the plasma free carnitine concentration is reduced (Table II).
SUMMARY
IVA was originally viewed as a relatively rare, life threatening inborn error of metabolism with both acute and chronic manifestations. Recent data from newborn screening studies and additional molecular and cellular laboratory investigations have revealed a far more heterogeneous condition with a potential for normal growth and development. Prospective long term follow-up of newborns identified with IVA and clinical trials of carnitine and glycine therapy will be critical to optimization of outcome in these patients.
Acknowledgments
This review is dedicated to the memory of Professor Kay Tanaka who originally identified isovaleric acidemia. Dr. Tanaka died on August 21, 2005 at the age of 75. JV was supported in part by NIH grant RO1-DK45482 and the Pennsylvania Tobacco Settlement Fund. RE was supported by the “Hochschul- und Wissenschaftsprogramm” of the Ludwig-Maximilians University Munich, Germany.
Biographies
Jerry Vockley is Professor of Pediatrics and Human Genetics at the University of Pittsburgh School of Medicine and Graduate School of Public Health, and Chief of Medical Genetics at the Children's Hospital of Pittsburgh. He has extensively researched the molecular and biochemical basis of isovaleric acidemia.
Regina Ensenauer is a pediatrician and biochemical geneticist at the Children’s Hospital of the Ludwig-Maximilians University in Munich, Germany. She currently holds an academic career research scholarship. Her research interests are in the field of branched-chain amino acid catabolism and fatty acid oxidation.
REFERENCES
- Abdenur JE, Chamoles NA, Guinle AE, Schenone AB, Fuertes AN. Diagnosis of isovaleric acidaemia by tandem mass spectrometry: false positive result due to pivaloylcarnitine in a newborn screening programme. J Inherit Metab Dis. 1998;21:624–630. doi: 10.1023/a:1005424331822. [DOI] [PubMed] [Google Scholar]
- Andresen B, Christensen E, Corydon T, Bross P, Pilgaard B, Wanders R, Ruiter J, Simonsen H, Winter V, Knudsen I, Schroeder LD, Gregersen N, Skovby F. Isolated 2-methylbutyrylglycinuria caused short/branched-chain acyl-CoA dehydrogenase deficiency: Identification of a new enzyme defect, resolution of its molecular basis, and evidence for distinct acyl-CoA dehydrogenases in isoleucine and valine metabolism. Am J Hum Gen. 2000;67:1095–1103. doi: 10.1086/303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold WC, Brewster M, Byrne WJ, Booth B. Fanconi syndrome in a patient with a variant of isovaleric acidemia. Intl J Pediatr Nephr. 1986;7:95–98. [PubMed] [Google Scholar]
- Attia N, Sakati N, al Ashwal A, al Saif R, Rashed M, Ozand PT. Isovaleric academia appearing as diabetic ketoacidosis. J Inherited Metab Disease. 1996;19:85–86. doi: 10.1007/BF01799353. [DOI] [PubMed] [Google Scholar]
- Berry GT, Yudkoff M, Segal S. Isovaleric acidemia: medical and neurodevelopmental effects of long-term therapy. J Pediatr. 1988;113:58–64. doi: 10.1016/s0022-3476(88)80528-6. [DOI] [PubMed] [Google Scholar]
- Budd MA, Tanaka K, Holmes LB, Efron ML, Crawford ML, Isselbacher KJ. Isovaleric acidemia: clinical features of a new genetic defect of leucine metabolism. NEJ Med. 1967;277:321–327. doi: 10.1056/NEJM196708172770701. [DOI] [PubMed] [Google Scholar]
- Burke DG, Halpern B, Malegan D, McCairns E, Danks D, Schlesingerx P, Wilken B. Profiles of urinary volatiles from metabolic disorders characterized by unusual odors. Clinical Chemistry. 1983;29:1834–1838. [PubMed] [Google Scholar]
- Cohn RM, Yudkoff M, Rothman R, Segal S. Isovaleric acidemia: use of glycine therapy in neonates. NEJ Med. 1978;299:996–999. doi: 10.1056/NEJM197811022991807. [DOI] [PubMed] [Google Scholar]
- Collins JE, Umpleby AM, Boroujerdi MA, Leonard JV, Sonksen PH. Effect of insulin on leucine kinetics in maple syrup urine disease. Pediatr Res. 1987;21:10–13. doi: 10.1203/00006450-198701000-00004. [DOI] [PubMed] [Google Scholar]
- Coude FX, Sweetman L, Nyhan WL. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J Clin Inv. 1979;64:1544–1551. doi: 10.1172/JCI109614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane FL, Mii S, Hauge JG, Green DE, Beinert H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. I. The general fatty acyl coenzyme A dehydrogenase. J Biol Chem. 1956;218:701–706. [PubMed] [Google Scholar]
- Dorland L, Duran M, Wadman SK, Niederwieser A, Bruinvisx L, Ketting D. Isovalerylglucuronide, a new urinary metabolite in isovaleric acidemia. Identification problems due to rearrangement reactions. Clinica Chimica Acta. 1983;134:77–83. doi: 10.1016/0009-8981(83)90186-9. [DOI] [PubMed] [Google Scholar]
- Efron ML. Isovaleric acidemia. Am J Disease of Children. 1967;113:74–76. doi: 10.1001/archpedi.1967.02090160124015. [DOI] [PubMed] [Google Scholar]
- Elsas LJ, 2nd, Naglak M. Acute and chronic-intermittent isovaleric acidemia: diagnosis and glycine therapy. Acta Paediatrica Japonica. 1988;30:442–451. doi: 10.1111/j.1442-200x.1988.tb02535.x. [DOI] [PubMed] [Google Scholar]
- Engel PC, Massey V. Green butyryl-coenzyme A dehydrogenase. An enzyme-acyl-coenzyme A complex. Bioch Journal. 1971;125:889–902. doi: 10.1042/bj1250889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensenauer R, He M, Willard JM, Goetzman ES, Corydon TJ, Vandahl BB, Mohsen AW, Isaya G, Vockley J. Human acyl-CoA dehydrogenase-9 plays a novel role in the mitochondrial beta-oxidation of unsaturated fatty acids. J Biol Chem. 2005;280:32309–32316. doi: 10.1074/jbc.M504460200. [DOI] [PubMed] [Google Scholar]
- Ensenauer R, Vockley J, Willard JM, Huey JC, Sass JO, Edland SD, Burton BK, Berry SA, Santer R, Grunert S, Koch HG, Marquardt I, Rinaldo P, Hahn S, Matern D. A common mutation is associated with a mild, potentially asymptomatic phenotype in patients with isovaleric acidemia diagnosed by newborn screening. Am J Hum Genet. 2004;75:1136–1142. doi: 10.1086/426318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JA, O'Brien K. Acute metabolic decompensation in an adult patient with isovaleric acidemia. Southern Med Journal. 2003;96:500–503. doi: 10.1097/01.SMJ.0000051141.03668.1D. [DOI] [PubMed] [Google Scholar]
- Fischer AQ, Challa VR, Burton BK, McLean WT. Cerebellar hemorrhage complicating isovaleric acidemia: a case report. Neurology. 1981;31:746–748. doi: 10.1212/wnl.31.6.746. [DOI] [PubMed] [Google Scholar]
- Frerman FE, Kim JJP, Huhta K, McKean MC. Properties of the General Acyl-CoA Dehydrogenase from Pig Liver. J. Biol. Chem. 1980;255:2195–2198. [PubMed] [Google Scholar]
- Fries MH, Rinaldo P, Schmidt-Sommerfeld E, Jurecki E, Packman S. Isovaleric acidemia: response to a leucine load after three weeks of supplementation with glycine, L-carnitine, and combined glycine-carnitine therapy. J of Pediatr. 1996;129:449–452. doi: 10.1016/s0022-3476(96)70081-1. [DOI] [PubMed] [Google Scholar]
- Ghisla S, Engst S, Moll M, Bross P, Strauss AW, Kim JJP. a,b-Dehydrogenation by Acyl-CoA Dehydrogenases: Role of Functional Groups at the Active Center. Progress in Clinical and Biological Research - New Developments in Fatty Acid Oxidation. 1992;375:127–142. [PubMed] [Google Scholar]
- Ghisla S, Massey V. Mechanisms of Flavoprotein-Catalyzed Reactions. Euro J Biochem. 1989;181:1–17. doi: 10.1111/j.1432-1033.1989.tb14688.x. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Burlingame TG, Hogema B, Jakobs C, Schutgens RBH, Millington D, Roe CR, Roe DS, Sweetman L, Steiner RD, Linck L, Pohowalla P, Sacks M, Kiss D, Rinaldo P, Vockley J. 2-Methylbutyryl-coenzyme A dehydrogenase deficiency: A new inborn error of L-isoleucine metabolism. Pediatric Research. 2000;47:830–833. doi: 10.1203/00006450-200006000-00025. [DOI] [PubMed] [Google Scholar]
- Gilbert-Barness E, Barness LA. Isovaleric acidemia with promyelocytic myeloproliferative syndrome. Pediatr Dev Path. 1999;2:286–291. doi: 10.1007/s100249900125. [DOI] [PubMed] [Google Scholar]
- Gorelick RJ, Schopfer LM, Ballou DP, Massey V, Thorpe C. Interflavin oxidation-reduction reactions between pig kidney general acyl-CoA dehydrogenase and electron-transferring flavoprotein. Biochemistry. 1985;24:6830–6839. doi: 10.1021/bi00345a015. [DOI] [PubMed] [Google Scholar]
- Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313:391–396. doi: 10.1016/j.bbrc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Hine DG, Hack AM, Goodman SI, Tanaka K. Stable isotope dilution analysis of isovalerylglycine in amniotic fluid and urine and its application for the prenatal diagnosis of isovaleric acidemia. Pediatric Research. 1986;20:222–226. doi: 10.1203/00006450-198603000-00005. [DOI] [PubMed] [Google Scholar]
- Hine DG, Tanaka K. The identification and the excretion pattern of isovaleryl glucuronide in the urine of patients with isovaleric acidemia. Pediatric Research. 1984;18:508–512. doi: 10.1203/00006450-198406000-00004. [DOI] [PubMed] [Google Scholar]
- Hyman DB, Tanaka K. Isovaleryl-CoA dehydrogenase activity in isovaleric academia fibroblasts using an improved tritium release assay. Pediatric Research. 1986;20:59–61. doi: 10.1203/00006450-198601000-00017. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Dabrowski C, Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. J. Biol. Chem. 1983;258:1066–1076. [PubMed] [Google Scholar]
- Ikeda Y, Hine DG, Okamura-Ikeda K, Tanaka K. Mechanism of action of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases: Direct evidence for carbanion formation as an intermediate step using enzyme-catalyzed C-2 proton/deuteron exchange in the absence of C-3 exchange. J. Biol. Chem. 1985a;260:1326–1337. [PubMed] [Google Scholar]
- Ikeda Y, Keese S, Fenton WA, Tanaka K. Biosynthesis of four rat liver mitochondrial acyl-CoA dehydrogenases. Import into mitochondria and processing of their precursors in a cell-free system and in cultured cells. Arch. Biochem. Biophys. 1987;252:662–674. doi: 10.1016/0003-9861(87)90072-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Keese S, Tanaka K. Molecular heterogeneity of variant isovaleryl acyl-CoA dehydrogenase from cultured isovaleric acidemia fibroblasts. Proc. Natl. Acad. Sci. USA. 1985b;82:7081–7085. doi: 10.1073/pnas.82.20.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Tanaka K. Isovaleryl-CoA dehydrogenase from rat liver. Methods Enzymol. 1988;166:374–389. doi: 10.1016/s0076-6879(88)66049-6. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ito T, Ohba S, Sugiyama N, Mizuguchi K, Yamaguchi S, Kidouchi K. Effect of carnitine administration on glycine metabolism in patients with isovaleric acidemia: significance of acetylcarnitine determination to estimate the proper carnitine dose. Tohoku Journal of Experimental Medicine. 1996;179:101–109. doi: 10.1620/tjem.179.101. [DOI] [PubMed] [Google Scholar]
- Jakobs C, Sweetman L, Nyhan WL. Hydroxy acid metabolites of branched-chain amino acids in amniotic fluid. Clinica Chimica Acta. 1984;140:157–166. doi: 10.1016/0009-8981(84)90340-1. [DOI] [PubMed] [Google Scholar]
- Kahler SG, Sherwood WG, Woolf D, Lawless ST, Zaritsky A, Bonham J, Taylor CJ, Clarke JT, Durie P, Leonard JV. Pancreatitis in patients with organic acidemias. J Pediatr. 1994;124:239–243. doi: 10.1016/s0022-3476(94)70311-6. [DOI] [PubMed] [Google Scholar]
- Kelleher JF, Jr, Yudkoff M, Hutchinson R, August CS, Cohn RM. The pancytopenia of isovaleric acidemia. Pediatrics. 1980;65:1023–1027. [PubMed] [Google Scholar]
- Kleij W, Van der Kraan M, Huijmans J, Van den Heuvel C, Jakobs C. Prenatal diagnosis of isovaleric acidaemia by enzyme and metabolite assay in the first and second trimesters. Prenatal Diagnosis. 1995;15:527–533. doi: 10.1002/pd.1970150605. [DOI] [PubMed] [Google Scholar]
- Krieger I, Tanaka K. Therapeutic effects of glycine in isovaleric acidemia. Pediatric Research. 1976;10:25–29. doi: 10.1203/00006450-197601000-00005. [DOI] [PubMed] [Google Scholar]
- Lehnert W. 3-Hydroxyisoheptanoic acid: a new metabolite in isovaleric acidemia. Clinica Chimica Acta. 1981a;113:101–103. doi: 10.1016/0009-8981(81)90445-9. [DOI] [PubMed] [Google Scholar]
- Lehnert W. Excretion of N-isovalerylglutamic acid in isovaleric acidemia. Clinica Chimica Acta. 1981b;116:249–252. doi: 10.1016/0009-8981(81)90030-9. [DOI] [PubMed] [Google Scholar]
- Lehnert W. N-Isovalerylalanine and N-isovalerylsarcosine: two new minor metabolites in isovaleric acidemia. Clinica Chimica Acta. 1983;134:207–212. doi: 10.1016/0009-8981(83)90198-5. [DOI] [PubMed] [Google Scholar]
- Lehnert W, Niederhoff H. 4-hydroxyisovaleric acid: a new metabolite in isovaleric acidemia. Euro J Pediatr. 1981;136:281–283. doi: 10.1007/BF00442995. [DOI] [PubMed] [Google Scholar]
- Levy HL, Erickson AM, Lott IT, Kurtz DJ. Isovaleric acidemia: results of family study and dietary treatment. Pediatrics. 1973;52:83–94. [PubMed] [Google Scholar]
- Loots DT, Erasmus E, Mienie LJ. Identification of 19 new metabolites by induced by abnormal amino acid conjugation in isovaleric acidemia. Clinical Chemistry. 2005;51:1510–1512. doi: 10.1373/clinchem.2005.048421. [DOI] [PubMed] [Google Scholar]
- Lott IT, Erickson AM, Levy HL. Dietary treatment of an infant with isovaleric acidemia. Pediatrics. 1972;49:616–618. [PubMed] [Google Scholar]
- Mayatepek E, Kurczynski TW, Hoppel CL. Long-term L-carnitine treatment in isovaleric acidemia. Pediatric Neuro. 1991;7:137–140. doi: 10.1016/0887-8994(91)90011-9. [DOI] [PubMed] [Google Scholar]
- McKean MC, Frerman FE, Mielke DM. General acyl-CoA dehydrogenase from pig liver. Kinetic and binding studies. J. Biol. Chem. 1979;254:2730–2735. [PubMed] [Google Scholar]
- Mehta KC, Zsolway K, Osterhoudt KC, Krantz I, Henretig FM, Kaplan P. Lessons from the late diagnosis of isovaleric acidemia in a five-year-old boy. J Pediatr. 1996;129:309–310. doi: 10.1016/s0022-3476(96)70261-5. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Jr, Robotham JL, Liehr JG, Williams JC. Neonatal lethargy due to isovaleric acidemia and hyperammonemia. Texas Medicine. 1984;80:52–54. [PubMed] [Google Scholar]
- Millington DS, Roe CR, Maltby DA, Inouex F. Endogenous catabolism is the major source of toxic metabolites in isovaleric acidemia. J Pediatr. 1987;110:56–60. doi: 10.1016/s0022-3476(87)80288-3. [DOI] [PubMed] [Google Scholar]
- Miura R, Nishina Y, Fujii S, Shiga K. C-13-Nmr Study On the Interaction Of Medium-Chain Acyl-Coa Dehydrogenase With Acetoacetyl-Coa. J Biochem. 1996;119:512–519. doi: 10.1093/oxfordjournals.jbchem.a021271. [DOI] [PubMed] [Google Scholar]
- Mohsen A-WA, Vockley J. Identification of the active site catalytic residue in human isovaleryl-CoA dehydrogenase. Biochemistry. 1995;34:10146–10152. doi: 10.1021/bi00032a007. [DOI] [PubMed] [Google Scholar]
- Mohsen AW, Anderson BD, Volchenboum SL, Battaile KP, Tiffany K, Roberts D, Kim JJ, Vockley J. Characterization of molecular defects in isovaleryl-CoA dehydrogenase in patients with isovaleric acidemia. Biochemistry. 1998;37:10325–10335. doi: 10.1021/bi973096r. [DOI] [PubMed] [Google Scholar]
- Nagao M, Tanaka K. FAD-Dependent regulation of transcription, translation, Post-Translational processing, and Post-Processing stability of various mitochondrial Acyl-CoA dehydrogenases and of electron transfer flavoprotein and the site of holoenzyme formation. J. Biol. Chem. 1992;267:17925–17932. [PubMed] [Google Scholar]
- Nasser I, Mohsen AW, Jelesarov I, Vockley J, Macheroux P, Ghisla S. Thermal unfolding of medium-chain acyl-CoA dehydrogenase and iso(3)valeryl-CoA dehydrogenase: study of the effect of genetic defects on enzyme stability. Biochim Biophys Acta. 2004;1690:22–32. doi: 10.1016/j.bbadis.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Nishina Y, Sato K, Hazekawa I, Shiga K. Structural modulation of 2-enoyl-CoA bound to reduced acyl-CoA dehydrogenases: a resonance Raman study of a catalytic intermediate. J Biochem. 1995;117:800–808. doi: 10.1093/oxfordjournals.jbchem.a124779. [DOI] [PubMed] [Google Scholar]
- Parimoo B, Tanaka K. Structural organization of the human Isovaleryl-CoA dehydrogenase gene. Genomics. 1993;15:582–590. doi: 10.1006/geno.1993.1111. [DOI] [PubMed] [Google Scholar]
- Pollitt RJ. Endogenous catabolism as source of toxic metabolites in isovaleric acidemia. J Pediatr. 1987;111:477–478. doi: 10.1016/s0022-3476(87)80488-2. [DOI] [PubMed] [Google Scholar]
- Poorthuis BJ, Jille-Vlckova T, Onkenhout W. Determination of acylcarnitines in urine of patients with inborn errors of metabolism using high-performance liquid chromatography after derivatization with 4′-bromophenacylbromide. Clinica Chimica Acta. 1993;216:53–61. doi: 10.1016/0009-8981(93)90138-t. [DOI] [PubMed] [Google Scholar]
- Rhead WJ, Tanaka K. Demonstration of a specific mitochondrial isovaleryl-CoA dehydrogenase deficiency in fibroblasts from patients with isovaleric acidemia. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:580–583. doi: 10.1073/pnas.77.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu Y, Kikawa Y, Sudo M, Kanaoka H, Fujioka M, Dan M. Prenatal diagnosis of isovaleric acidemia by fast atom bombardment and tandem mass spectrometry. Clinica Chimica Acta. 1991;203:369–374. doi: 10.1016/0009-8981(91)90310-9. [DOI] [PubMed] [Google Scholar]
- Shigematsu Y, Sudo M, Momoi T, Inoue Y, Suzuki Y, Kameyama J. Changing plasma and urinary organic acid levels in a patient with isovaleric acidemia during an attack. Pediatr Res. 1982;16:771–775. doi: 10.1203/00006450-198209000-00013. [DOI] [PubMed] [Google Scholar]
- Shih VE, Aubry RH, DeGrande G, Gursky SF, Tanaka K. Maternal isovaleric acidemia. J Pediatr. 1984;105:77–78. doi: 10.1016/s0022-3476(84)80367-4. [DOI] [PubMed] [Google Scholar]
- Shih VE, Mandell R, Tanaka K. Diagnosis of isovaleric acidemia in cultured fibroblasts. Clinica Chimica Acta. 1973;48:437–439. doi: 10.1016/0009-8981(73)90425-7. [DOI] [PubMed] [Google Scholar]
- Sogut A, Acun C, Aydin K, Tomsac N, Demirel F, Aktuglu C. Isovaleric acidemia: cranial CT and MRI findings. Pediatr Radiol. 2004;34:1. doi: 10.1007/s00247-003-1049-8. [DOI] [PubMed] [Google Scholar]
- Spinty S, Rogozinski H, Lealman GT, Wraith JE. Second case of a successful pregnancy in maternal isovaleric acidaemia. J Inherit Metab Dis. 2002;25:697–698. doi: 10.1023/a:1022837416232. [DOI] [PubMed] [Google Scholar]
- Spirer Z, Swirsky-Fein S, Zakut V, Legum C, Bogair N, Charles R, Gil-Av E. Acute neonatal isovaleric acidemia. A report of two cases. Israel J of Med Sci. 1975;11:1005–1010. [PubMed] [Google Scholar]
- Stewart PM, Walser M. Failure of the normal ureagenic response to amino acids in organic acid-loaded rats. Proposed mechanism for the hyperammonemia of propionic and methylmalonic acidemia. J Clin Invest. 1980;66:484–492. doi: 10.1172/JCI109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L, Williams JD. Branched chain organic acidurias. In: Scriver C, Beaudet AL, Sly W, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. 8th edition. New York: McGraw-Hill; 2001. pp. 2125–2164. [Google Scholar]
- Tajima G, Sakura N, Yofune H, Dwi Bahagia Febriani A, Nishimura Y, Sakamoto A, Ono H, Shigematsu Y, Kobayashi M. Establishment of a practical enzymatic assay method for determination of isovaleryl-CoA dehydrogenase activity using high-performance liquid chromatography. Clinica Chimica Acta. 2005;353:193–199. doi: 10.1016/j.cccn.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Isovaleric acidemia: personal history, clinical survey and study of the molecular basis. Progress in Clinical & Biological Research. 1990;321:273–290. [PubMed] [Google Scholar]
- Tanaka K, Budd MA, Efron ML, Isselbacher KJ. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc. Natl. Acad. Sci. USA. 1966;56:236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hine DG, West-Dull A, Lynn TB. Gas-chromatographic method of analysis for urinary organic acids. I. Retention indices of 155 metabolically important compounds. Clinical Chemistry. 1980;26:1839–1846. [PubMed] [Google Scholar]
- Tanaka K, Isselbacher KJ. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J. Biol. Chem. 1967;242:2966–2972. [PubMed] [Google Scholar]
- Tanaka K, Orr JC, Isselbacher KJ. Identification of beta-hydroxyisovaleric acid in the urine of a patient with isovaleric acidemia. Biochimica et Biophysica Acta. 1968;152:638–641. doi: 10.1016/0005-2760(68)90107-0. [DOI] [PubMed] [Google Scholar]
- Vockley J, Nagao M, Parimoo B, Tanaka K. The variant human isovaleryl-CoA dehydrogenase gene responsible for type-II isovaleric acidemia determines an RNA splicing error, leading to the deletion of the entire 2nd coding exon and the production of a truncated precursor protein that interacts poorly with mitochondrial import receptors. J.Biol. Chem. 1992a;267:2494–2501. [PubMed] [Google Scholar]
- Vockley J, Parimoo B, Nagao M, Tanaka K. Identification of the molecular defects responsible for the various genotypes of isovaleric acidemia. Progress in Clinical & Biological Research. 1992b;375:533–540. [PubMed] [Google Scholar]
- Vockley J, Parimoo B, Tanaka K. Molecular characterization of four different classes of mutations in the isovaleryl-CoA dehydrogenase gene responsible for isovaleric acidemia. Am J Hum Genet. 1991;40:147–157. [PMC free article] [PubMed] [Google Scholar]
- Vockley J, Rogan PK, Anderson BD, Willard J, Seelan RS, Smith DI, Liu WG. Exon skipping in IVD RNA processing in isovaleric acidemia caused by point mutations in the coding region of the IVD gene. Am J Hum Gen. 2000;66:356–367. doi: 10.1086/302751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg GL, Laurito CE, Geldner P, Pygon BH, Burton BK. Malignant ventricular dysrhythmias in a patient with isovaleric acidemia receiving general and local anesthesia for suction lipectomy. Journal of Clinical Anesthesia. 1997;9:668–670. doi: 10.1016/s0952-8180(97)00187-6. [DOI] [PubMed] [Google Scholar]
- Worthen HG, al Ashwal A, Ozand PT, Garawi S, Rahbeeni Z, al Odaib A, Subramanyam SB, Rashed M. Comparative frequency and severity of hypoglycemia in selected organic acidemias, branched chain amino acidemia, and disorders of fructose metabolism. Brain & Development. 1994;16 Suppl:81–85. doi: 10.1016/0387-7604(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Yoshida I, Sovik O, Sweetman L, Nyhan WL. Metabolism of leucine in fibroblasts from patients with deficiencies in each of the major catabolic enzymes: branched-chain ketoacid dehydrogenase, isovaleryl-CoA dehydrogenase, 3-methylcrotonyl-CoA carboxylase, 3-methylglutaconyl-CoA hydratase, and 3-hydroxy-3-methylglutaryl-CoA lyase. J Neurogen. 1985;2:413–424. doi: 10.3109/01677068509101427. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Yoshida I, Yamashita F, Mori M, Uchiyama C, Tatibana M. Neonatal isovaleric acidemia associated with hyperammonemia. Advances in Experimental Medicine & Biology. 1982;153:141–146. doi: 10.1007/978-1-4757-6903-6_19. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Cohn RM, Puschak R, Rothman R, Segal S. Glycine therapy in isovaleric acidemia. J Pediatr. 1978;92:813–817. doi: 10.1016/s0022-3476(78)80164-4. [DOI] [PubMed] [Google Scholar]