Abstract
The objective of this study was to determine the ontogenetic profiles in left and right ventricle of genes implicated in cardiac growth, including mineralocorticoid (MR) and glucocorticoid (GR) receptor, 11 beta-hydroxysteroid dehydrogenase (11β-HSD) 1 and 2 and genes of the angiotensin system and insulin-like growth factor (IGF) family. Samples from left and right ventricles (LV, RV) were collected from hearts of sheep fetuses at 80, 100, 120, 130, and 145 days of gestation and from newborn lambs. Quantitative real-time PCR was performed to determine the MR, GR, 11β-HSD 1 and 2, angiotensin converting enzyme (ACE) 1 and 2, IGF1, IGF2, IGF receptors IGF-1R and IGF-2R, and IGF-binding proteins (IGFBP) 2 and 3. In the LV, MR and GR both decreased toward term. In the RV, MR and GR expression did not decrease, but both 11β-HSD 1 and 2 mRNA levels increased after birth. ACE1 expression in LV and RV sharply increases just before parturition, whereas ACE2 decreased in the LV and RV in late gestation. IGF2, IGF2R, and IGFBP2 expression levels substantially decreased in late gestation in LV and RV; IGF2R also decreased with age in LV. These patterns suggest that reduced expression of genes related to IGF and angiotensin II action occur as proliferative activity declines and terminal differentiation occurs in the late gestation fetal heart.
Keywords: Heart, Fetus, MR, GR, 11βHSD1, 11βHSD2, IGF1, IGF2, IGF1R, IGF2R, IGFBP2, ACE1, ACE2, AT1R, AT2R, Cortisol, Angiotensin, Angiotensinogen
1. Results and discussion
This study reveals ontogenetic patterns of expression of several genes implicated by other investigators to play a role in either proliferative or hypertrophic cardiac growth. There is a pronounced increase in fetal heart growth in the last third of gestation, paralleling a similar exponential growth of the fetus (Burrell et al., 2003; Jonker et al., 2007). At the same time as the heart increases in both total weight and left and right ventricle wall mass, an increasing number of myocytes terminally differentiate. This process results in decreasing numbers of mononucleate cardiomyocytes, and increasing numbers of binucleate or multinucleate myocytes which are unable to undergo further cell division (Burrell et al., 2003; Jonker et al., 2007). A similar pattern of decreasing proliferative activity near term has also been described for the human fetus (Huttenbach et al., 2001). Some differences in this pattern have been identified in the left ventricle as compared to the right ventricle; the number of proliferating myocytes in the left ventricle is approximately 50% at 100 days and decreases to 15% by 145d, whereas only 15–25% of right ventricular myocytes are proliferating over this time period (Jonker et al., 2007). In contrast the number of myocytes that are enlarged due to terminal differentiation is greater in the right ventricle than in the left ventricle, particularly from day 130 to term, and right ventricular myocytes are on average greater in volume than are those in the left ventricle. Several factors have been identified as regulators of proliferation in the fetal heart in late gestation, these include cortisol (Giraud et al., 2006), IGFs (Liu et al., 1996; Sundgren et al., 2003a), and angiotensin (Sundgren et al., 2003b). Our results show ontogenetic changes in expression of IGF2 mRNA and expression of mRNAs for the angiotensin producing enzymes; the patterns of changes in expression of these genes is consistent with the timing of changes in proliferation in late gestation, suggesting that changes in IGF2 or angiotensin in the heart are associated with fetal cardiac maturation in both left and right ventricle.
1.1. Genes related to corticosteroid action
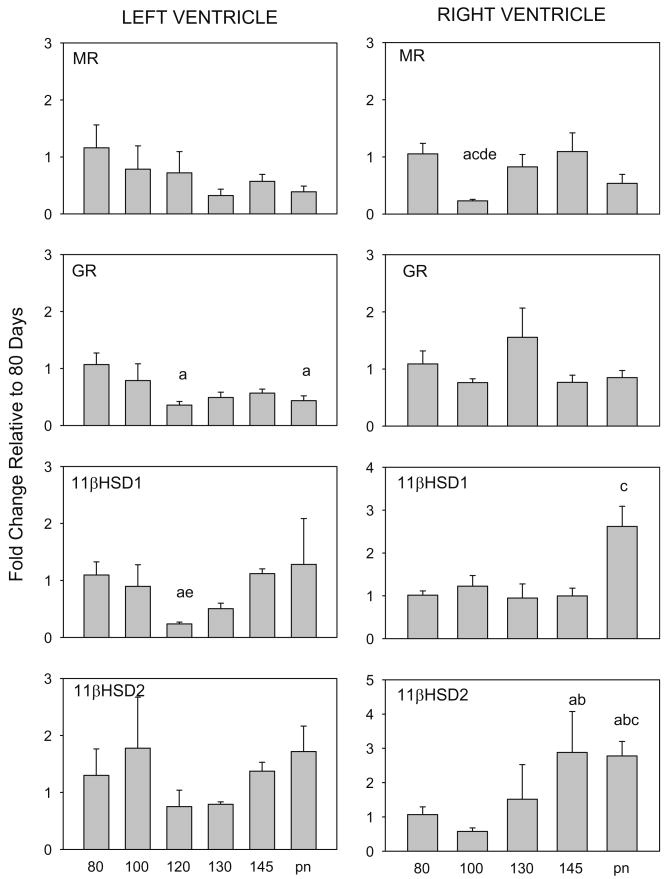
There are effects of age on expression of GR and 11β-HSD1 mRNAs in fetal LV and significant changes in MR and 11β-HSD1 and 2 in RV (Fig. 1). However the changes in 11βHSD1 in LV and MR in RV are due to small changes in expression at 100–120d, rather than a consistent change in expression pattern with maturation, so that there is no significant relation between age and expression of these genes. (Fig. 1, right; Table 1), whereas expression of MR and GR in LV significantly decreased with age and expression of 11β-HSD2 mRNA in RV significantly increased with age (Table 1). The ratio of expression of 11β-HSD1 to 11β-HSD2 was unchanged throughout the ages studied in LV, but was significantly decreased in the RV at 145d compared to 100d gestation (Table 2).
Fig. 1.
Expression of mRNA for MR, GR, 11β-HSD1, and 11β-HSD2 in left ventricles of 80, 100, 120, 130, and 145 day fetuses and postnatal lambs (pn) and in right ventricles of 80, 100, 130, 145 and postnatal lambs. Data are depicted as mRNA fold changes relative to 80d calculated using the expression 2ˆ−ΔΔCt and expressed as a mean fold change ±SEM. Letters indicate significant differences (p < 0.05) a: 80d, b: 100d, c:120d, d: 130d, f: 145d, g: newborn.
Table 1.
Results of linear regression analysis of mRNA expression vs age
| Gene | Left ventricle | Right ventricle | ||
|---|---|---|---|---|
| GR | 0.252* | − | 0.006 | + |
| MR | 0.161* | − | 0.007 | − |
| 11βHSD1 | 0.001 | − | 0.145 | + |
| 11βHSD2 | 0.031 | + | 0.623* | + |
| IGF1 | 0.067 | − | 0.061 | + |
| IGF2 | 0.685* | − | 0.597* | − |
| IGF1R | 0.237* | − | 0.001 | − |
| IGF2R | 0.643* | − | 0.651* | − |
| IGFBP2 | 0.697* | − | 0.560* | − |
| IGFBP3 | 0.001 | + | ||
| Angiotensinogen | 0.016 | − | 0.008 | + |
| AT1R | 0.255* | − | 0.012 | − |
| AT2R | 0.216* | − | 0.008 | − |
| ACE1 | 0.400* | + | 0.575* | + |
| ACE2 | 0.151* | − | 0.294* | − |
Data are expressed as coefficient of determination (r2) of the relation between age and ΔCt.
indicates p < 0.05 (n = 32 for LV, n = 20 for RV).
indicates positive relation between gene expression and age (slope of relation between age and ΔCt was negative)
indicates negative relation between gene expression and age (slope of relation between age and ΔCt was positive.
Table 2.
Expression ratio of -HSD1 to 11β-HSD2, IGF2 to IGF1, AT1R to AT2R, and ACE1 to ACE2 in LV and RV mRNA
| Age (days gestation) | 11βHSD1/11βHSD2 | IGF2/IGF1 | AT1R/AT2R | ACE1/ACE2 | ||||
|---|---|---|---|---|---|---|---|---|
| LV | RV | LV | RV | LV | RV | LV | RV | |
| 80 | 62 ± 21 | 13 ± 3 | 241 ± 62 | 978 ± 276 | 1.4 ± 0.4 | 4.8 ± 1.6 | 14 ± 4 | 2.5 ± 0.2 |
| 100 | 36 ± 11 | 16 ± 4*f | 433 ± 116 | 949 ± 265 | 1.7 ± 0.4 | 1.5 ± 0.3*ag | 23 ± 2.8 | 6.0 ± 0.7*a |
| 120 | 25 ± 6.4 | nm | 151 ± 11*b | nm | 0.9 ± 0.1 | nm | 64 ± 6.0*a | nm |
| 130 | 33 ± 4.5 | 6.1 ± 1.8 | 176 ± 25 | 268 ± 37*ab | 1.8 ± 0.4 | 1.3 ± 0.5*afg | 78 ± 31*a | 9.6 ± 1.6*a |
| 145 | 45 ± 5.0 | 5.7 ± 2.1 | 88 ± 10*ab | 146 ± 48*ab | 2.1 ± 0.5 | 3.3 ± 1.1 | 212 ± 66*abd | 31 ± 6*abd |
| Postnatal | 31 ± 9.0 | 6.7 ± 0.5 | 41 ± 7*abcdf | 105 ± 4*abd | 1.4 ± 0.2 | 3.8 ± 0.3 | 133 ± 41*ab | 37 ± 6*abd |
Ratio of -HSD1 to 11β-HSD2, IGF2 to IGF1, AT1R to AT2R, and ACE1 to ACE2 in left ventricles of 80, 100, 120, 130, and 145 day fetuses and postnatal lambs (pn) and in right ventricles of 80, 100, 130, 145 and postnatal lambs. Ratios were calculated as 2ˆ−ΔΔCt from mRNA fold changes relative to its counterpart at the same age and are expressed as mean fold change ±SEM. Letters indicate significant differences (p < 0.05) a: vs 80d, b: vs 100d, c: vs120d, d: vs 130d, f: vs 145d, g: vs postnatal lambs; nm, not measured.
Fetal secretion of cortisol increases exponentially before birth in humans and in sheep (Liggins, 1974). Previous studies have suggested that even small increases in fetal cortisol can alter heart mass (Jensen et al., 2005; Jensen et al., 2002), suggesting an action of cortisol at MR and/or GR in fetal myocytes. The ability of cortisol to bind at MR and/or GR, however, depends in large part on the activity of 11β-HSD1 relative to that of the cortisol inactivating enzyme 11β-HSD2 (Mihailidou and Funder, 2005; Seckl and Walker, 2001). The maintenance of high 11β-HSD1 expression relative to 11β-HSD2 within both ventricles of the heart throughout all of late gestation indicates a potential role of cortisol within the heart in the late gestation fetus. However the decrease in MR and GR expression in LV with advancing age suggests that as plasma cortisol concentrations are increasing in vivo, proliferative effects of cortisol in LV may be reduced.
1.2. Genes relating to the IGF system
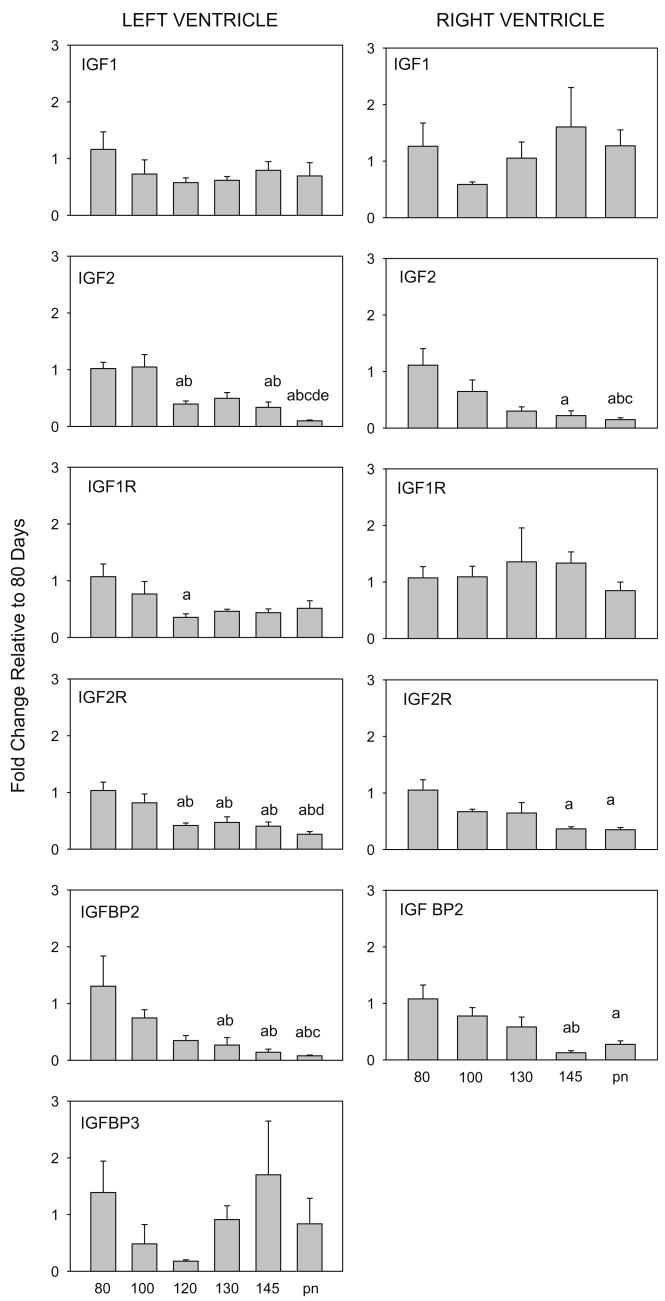
There was an effect of age on expression of IGF2, IGF1R, IGF2R, and IGFBP-2 mRNAs in the LV and on IGF2, IGF2R, and IGFBP2 mRNAs in the RV (Fig. 2). Expression of all of these genes was significantly decreased with advancing age, reaching low levels of expression near birth (Table 1). In contrast, IGF1 did not change in either RV or LV, and IGF1R did not change with age in RV. The observed decrease in expression of IGF2 in both LV and RV agrees with previous observations by others (Cheung et al., 1996; Delhanty and Han, 1993). However in these studies, investigators also found decreased expression of IGF1 in the left ventricle from 100d gestation toward term, whereas the decrease we observed after 80d was not significant.
Fig. 2.
Expression of mRNA for IGF1, IGF2, IGF1R, IGF2R and IGFBP2 from left and right ventricles of fetal and newborn lambs. IGFBP3 were measured in left ventricle only. Ages and significance are as indicated in legend to (Fig. 1).
The pattern of decreased IGF1R in LV parallels the reduced number of LV myocytes entering the cell cycle in late gestation after 120 days, whereas the decrease in IGF2 parallels the reduction in mononuclear myocytes in both ventricles (Jonker et al., 2007). IGF2 and IGF1 both appear to stimulate myocyte proliferation in vitro. IGF2 stimulated an increase in proliferation of myocytes in cultures of prenatal, but not neonatal, rat myocytes (Liu et al., 1996). In vivo infusion of an IGF1 analog to fetal sheep resulted in decreased numbers of binucleated cells, but increased percentages of monucleated myocytes; IGF1 administration in cultured fetal cardiomyocytes stimulated proliferation of the myocytes (Sundgren et al., 2003a).
As a result of the decrease in IGF2 mRNA, the ratio of IGF2 to IGF1 mRNAs decreased near term (Table 2). This change in ratio of IGF2 to IGF1 expression is consistent with the hypothesis that IGF2 is less important to postnatal growth than to prenatal growth. We speculate that IGF2 may play a role in mononuclear myocyte proliferation, accounting for the gradual decrease in proliferation observed throughout the last third of gestation as IGF2 expression within the heart decreases. Alternatively, the decrease in expression of IGF2 may reflect the decrease in mononuclear myocytes in both LV an RV as terminal differentiation proceeds.
The biological actions of IGFs are in part regulated by IGF-binding proteins 1–6 in vivo, which function to prolong the half-life of IGFs in plasma. IGFBPs have the ability to modulate the actions of IGF through regulating transport, turnover, and tissue distribution (Jones and Clemmons, 1995). IGFBP2 expression in both LV and RV was decreased after 100d with more dramatic decreases at 145d and in newborns. IGFBP3 expression in LV, on the other hand, was not significantly changed with age (Fig. 2). Previous studies have shown IGFBP2 and IGFBP3 play roles in fetal development, however although over-expression of IGFBP2 (Hoeflich et al., 1999) and IGFBP3 (Modric et al., 2001) in mice leads to decreased body weight, there is no change in heart weight. Thus the role of IGF-binding proteins in heart growth and in mediating changes in cardiac myocyte proliferation is not clear, although the decrease in IGFBP2 in both LV and RV suggest it might play a role in modulating effects of IGF1 in the heart in late gestation.
1.3. Genes related to the angiotensin
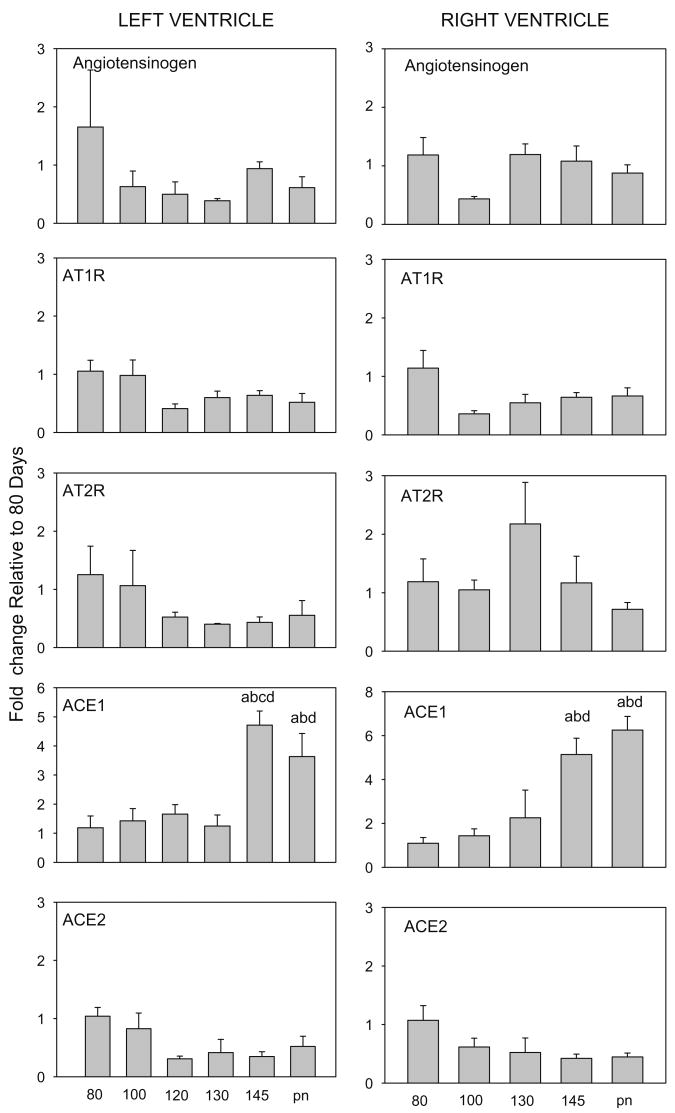
This study indicates that changes in the expression of mRNAs related to angiotensin activity correspond to changes in proliferative activity and differentiation in the maturing heart. Angiotensin has been implicated in fetal cardiac growth (Reini and S.A., 2006; Segar et al., 2001), hyperplasia of fetal cardiomyocytes (Sundgren et al., 2003b), and in cardiac hypertrophy in response to increased blood pressure in the fetus (Lumbers et al., 2005). In the adult heart, the local production of angiotensin has been implicated in playing a major role in cardiac hypertrophy and fibrosis (Xu et al., 2007). Although high doses of cortisol stimulate expression of angiotensinogen in hearts of fetal sheep (Lumbers et al., 2005), there was no increase in angiotensinogen expression at the time of the normal physiologic increase in cortisol that occurs at term in fetal sheep and there was no significant overall pattern of increase in angiotensinogen mRNA in LV or RV with increasing fetal age (Fig. 3 and Table 1). In contrast, the enzymes determining production of angiotensin II were significantly changed in late gestation. ACE1, which converts angiotensin I to angiotensin II, increased ∼ 5-fold in the LV and RV at term (Fig. 3), resulting in an overall progressive increase with age (Table 1). ACE1 is known to augment cardiac hypertrophy in rat hearts when over-expressed (Tian et al., 2004), and the pattern of expression in late gestation is consistent with expression in terminally differentiated myocytes. ACE2, which converts angiotensin I into angiotensin 1–9 and angiotensin II into angiotensin 1–7, limits the amount of angiotensin II that is produced and is thought to be cardio-protective (Danilczyk and Penninger, 2006). In this study, ACE2 expression was significantly related to age (Table 1) in both RV and LV. Thus the ACE1 to ACE2 ratio increased ∼15-fold by 145d gestation compared to 80d in both the LV and RV (Table 2). This increase in the ratio of expression suggests that local angiotensin II production may be associated with the terminal maturation of the myocytes, and suggests that during normal ontogeny changes in cardiac angiotensin II production are related to decreased ACE2 and increased ACE 1 expression, rather than by a local increase in transcription of the gene for the precursor protein.
Fig. 3.
Expression of mRNA for Angiotensinogen, AT1R, AT2R, ACE1 and ACE2 in left and right ventricles of fetal and newborn lambs. Ages and significance are as indicated in legend to (Fig. 1).
There was an overall decrease in both AT1 and AT2R in LV that was age-related, but no significant change in AT1R or AT2R in RV in late gestation (Fig. 3, Table 1). Others have previously found a decrease in AT2R protein in the heart in late gestation (Burrell et al., 2001). In the RV, the ratio of AT1R to AT2R mRNAs (Table 1) was greater at 80d and in the newborn than in 100d or 130d fetuses, and in 145d fetuses than in 130d fetuses (Table 1). There was no significant change in this ratio in LV as expression of both was similarly decreased with fetal maturation. However, the decrease in expression of AT1R in the LV coincides with the increase in ACE1 expression, suggesting that the decreased expression of the AT1R may be in response to an increase in local angiotensin II production. In the adult heart, the AT1Rs are thought to be responsible for hypertrophic effects, while actions at AT2R are hypothesized to counteract the AT1R (Booz, 2004; Zhu et al., 2003). In the RV the daily increase in ventricular mass is primarily due to enlargement with terminal differentiation (Jonker et al., 2007); the greater increase in AT1R to AT2R ratio in RV appears to correlate to this, suggesting a greater stimulation of myocyte volume is associated with greater relative AT1R expression in RV.
In conclusion, our results suggest that changes in gene expression in the RV and LV occur is associated with the changes in proliferative activity of mononuclear monocytes, and with terminal differentiation as binucleate monocytes increase near birth.
2. Experimental procedures
RNA was extracted from left ventricles (LV) and right ventricles (RV) from time-dated pregnant ewes at 80 (n = 4), 100 (n = 4), 120 (n = 4), 130 (n = 4), and 145 (143–146) (n = 5) days of gestation and from newborn lambs (days 1, 2 and 7) (n = 8 for LV: 5 at 1–2 days and 3 at 7 days of postnatal age, n = 4 for RV: 1 at 1 day, 3 at 7d). RV was not extracted from the 120 day heart, and additional left ventricular samples were also included for 3 fetuses of ewes in labor (term). RNA was extracted using Trizol (GIBCO/BRL, Grand Island, NY) according to the manufacturer's directions. LV RNA samples were checked for genomic DNA contamination using real-time PCR with the RNA as a template in place of cDNA and using probes and primers for GR (which produces a product within exon 2). LV samples did not contain genomic DNA contamination. Genomic DNA was removed from RV samples using RNeasy Plus Mini Kit (Qiagen Inc., Valencia Ca). Total RNA was measured spectrophotometrically to measure the quantity and quality of RNA. Reverse transcription of the RNA into cDNA was then performed using a high capacity cDNA archive kit (Applied Biosystems; Foster City, CA) and aliquots for cDNA were stored at −20 °C until used.
Qrt PCR was utilized to measured gene expression using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). The genes analyzed in this study for the LV were MR, GR, 11β-HSD1 and 2, IGF-I and II, IGF-1R and 2R, IGF-binding proteins 2 and 3 (IGFBP2 and, IGFBP3), angiotensinogen, ATR1 and 2, and angiotensin converting enzymes (ACE1 and ACE2). The same genes were studied in RNA from RV except for IGFBP3. Probe and primer sequences were based on previously published sequences: MR and GR (Keller-Wood et al., 2005), IGF-I (Meinel et al., 2003), angiotensinogen (Burrell et al., 2003), 11βHSD2, AT1R and AT2R (Dodic et al., 2002), IGFBP2 and IGFBP3 (Bloomfield et al., 2006), and for 11βHSD1, IGF-II, IGF-1R, IGF-2R, ACE1 and ACE2 (Reini and S.A., 2006). For ACE1 and ACE2, SYBR Green (Bio-Rad) was used instead of Taqman probes (FAM-TAMRA purchased from Applied Biosystems). Reactions were carried out using 20 or 100 ng of template cDNA, except in the case of ribosomal RNA, for which 1 ng of template was used. All analyses were performed in triplicate and samples for the same gene in each tissue (RV or LV) were run on the same plate. All genes were normalized to 18s ribosomal RNA, and data were analyzed using the ΔCt method (Livak and Schmittgen, 2001). 18s expression was unchanged between all the groups within each tissue and the slope of the relation between cDNA quantity and Ct for all genes and 18S demonstrated similar efficiencies of amplification (average efficiency for the 15 genes studied was 98.2% ± 2.2%).
2.1. Data analysis
Changes in gene and expression among groups were analyzed by one way analysis of variance (ANOVA) using the delta Ct values. When data were not normally distributed, the Kruskal–Wallis one way analysis of variance on ranks was utilized. Newman–Keul's studentized range test or Dunn's test were used as appropriate for comparing differences between ages. P < 0.05 was used as the standard for significance in all statistical tests. For graphical purposes, fold changes of the genes in the heart ontogeny study were calculated using the expression 2ˆ−ΔΔCt with respect to the mean value of delta Ct in the 80d fetal group. Data were also analyzed using linear regression analysis using the exact fetal or postnatal age to determine overall trends for increasing or decreasing gene expression as a function of age; for this analysis the term fetuses were analyzed as 148d regardless of the age at labor, whereas postnatal fetuses were analyzed using days after birth relative to 148 days, the normalized day of labor and delivery.
Acknowledgments
This research was funded by DK62080 to M.K.-W. and by an American Heart Association Florida – Puerto Rico Affiliate Predoctoral fellowship award to S.A.R.
References
- Bloomfield FH, van Zijl PL, Bauer MK, Phua HH, Harding JE. Effect of pulsatile growth hormone administration to the growth-restricted fetal sheep on somatotrophic axis gene expression in fetal and placental tissues. Am J Physiol Endocrinol Metab. 2006;291:E333–E339. doi: 10.1152/ajpendo.00045.2006. [DOI] [PubMed] [Google Scholar]
- Booz GW. Cardiac angiotensin AT2 receptor: what exactly does it do? Hypertension. 2004;43:1162–1163. doi: 10.1161/01.HYP.0000128531.39964.c0. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:952–961. doi: 10.1002/ar.a.10110. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Hegarty BD, McMullen JR, Lumbers ER. Effects of gestation on ovine fetal and maternal angiotensin receptor subtypes in the heart and major blood vessels. Exp Physiol. 2001;86:71–82. doi: 10.1113/eph8602075. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Johnson DD, Reyes V. Ontogeny of insulin-like growth factor-I and -II gene expression in ovine fetal heart. J Soc Gynecol Investig. 1996;3:309–315. [PubMed] [Google Scholar]
- Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, Han VK. The expression of insulin-like growth factor (IGF)-binding protein-2 and IGF-II genes in the tissues of the developing ovine fetus. Endocrinology. 1993;132:41–52. doi: 10.1210/endo.132.1.7678219. [DOI] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology. 2006;147:3643–3649. doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Wu M, Mohan S, Foll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–5496. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- Huttenbach Y, Ostrowski ML, Thaller D, Kim HS. Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc Pathol. 2001;10:119–123. doi: 10.1016/s1054-8807(01)00065-5. [DOI] [PubMed] [Google Scholar]
- Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol. 2005;288:R54–R61. doi: 10.1152/ajpregu.00149.2004. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endocrinol. 2002;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrinol Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Wood CE, Hua Y, Zhang D. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. J Soc Gynecol Investig. 2005;12:84–91. doi: 10.1016/j.jsgi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Parturition in the sheep and the human. In: Coutinho EM, Fuchs F, editors. Physiology and Genetics of Reproduction. Plenum Press; New York: 1974. pp. 423–443. [Google Scholar]
- Liu Q, Yan H, Dawes NJ, Mottino GA, Frank JS, Zhu H. Insulin-like growth factor II induces DNA synthesis in fetal ventricular myocytes in vitro. Circ Res. 1996;79:716–726. doi: 10.1161/01.res.79.4.716. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;288:R567–R574. doi: 10.1152/ajpregu.00556.2004. [DOI] [PubMed] [Google Scholar]
- Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, Schneider R, Gander B, Luginbuehl V, Bettschart-Wolfisberger R, Illi OE, Merkle HP, von R B. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- Mihailidou AS, Funder JW. Nongenomic effects of mineralocorticoid receptor activation in the cardiovascular system. Steroids. 2005;70:347–351. doi: 10.1016/j.steroids.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Modric T, Silha JV, Shi Z, Gui Y, Suwanichkul A, Durham SK, Powell DR, Murphy LJ. Phenotypic manifestations of insulin-like growth factorbinding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–1967. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late gestation ewes decreases fetal cardiac expression of 11{beta}-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00294.2006. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1 – a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- Segar JL, Dalshaug GB, Bedell KA, Smith OM, Scholz TD. Angiotensin II in cardiac pressure-overload hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2037–R2047. doi: 10.1152/ajpregu.2001.281.6.R2037. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol. 2003b;548:881–891. doi: 10.1113/jphysiol.2003.038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XL, Pinto YM, Costerousse O, Franz WM, Lippoldt A, Hoffmann S, Unger T, Paul M. Over-expression of angiotensin converting enzyme-1 augments cardiac hypertrophy in transgenic rats. Hum Mol Genet. 2004;13:1441–1450. doi: 10.1093/hmg/ddh147. [DOI] [PubMed] [Google Scholar]
- Xu J, Carretero OA, Lin CX, Cavasin MA, Shesely EG, Yang JJ, Reudelhuber TL, Yang XP. Role of cardiac overexpression of ANG II in the regulation of cardiac function and remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H1900–H1907. doi: 10.1152/ajpheart.00379.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YC, Zhu YZ, Lu N, Wang MJ, Wang YX, Yao T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin Exp Pharmacol Physiol. 2003;30:911–918. doi: 10.1111/j.1440-1681.2003.03942.x. [DOI] [PubMed] [Google Scholar]