Abstract
Vaccination of mice with Francisella tularensis live vaccine strain (LVS) mutants described so far have failed to induce protection in C57BL/6 mice against challenge with the virulent strain F. tularensis SchuS4. We previously have reported that a mutant of F. tularensis LVS deficient in iron superoxide dismutase (sodBFt) is hypersensitive to oxidative stress and attenuated for virulence in mice. Herein, we evaluated the efficacy of this mutant as a vaccine candidate against respiratory tularemia caused by F. tularensis SchuS4. C57BL/6 mice were vaccinated intranasally (i.n.) with the sodBFt mutant and challenged i.n. with lethal doses of F. tularensis SchuS4. The level of protection against SchuS4 challenge was higher in sodBFt vaccinated group as compared to the LVS vaccinated mice. SodBFt vaccinated mice following SchuS4 challenge exhibited significantly reduced bacterial burden in lungs, liver and spleen, regulated production of pro-inflammatory cytokines and less severe histopathological lesions compared to the LVS vaccinated mice. The sodBFt vaccination induced a potent humoral immune response and protection against SchuS4 required both CD4 and CD8 T cells in the vaccinated mice. SodBFt mutants revealed upregulated levels of chaperonine proteins DnaK, GroEL and Bfr that have been shown to be important for generation of a potent immune response against Francisella infection. Collectively, this study describes an improved live vaccine candidate against respiratory tularemia that has an attenuated virulence and enhanced protective efficacy than the LVS.
Keywords: Francisella, Vaccine, Mice, Superoxide dismutase
Introduction
Francisella tularensis, the causative agent of tularemia is a potential bioweapon due to ease of its dissemination, multiple routes of infection, high infectivity and lethality [1]. SchuS4 is a highly virulent strain of F. tularensis with a dose as low as 10 CFU can cause death in humans [2]. Attenuated F. tularensis live vaccine strain (LVS) has been used as a vaccine against tularemia for several years in the western world, and has been very efficient in reducing the incidence of natural and laboratory-acquired tularemia [3]. Despite better protective efficacy, LVS was found to be virulent for humans especially when given via aerosol and in some cases the higher accination dose required for protection resulted in tularemia [4]. In addition, availability of a limited data on safety and efficacy of LVS vaccination in humans prevented its licensing as a vaccine in the USA [5;6]. Thus, there is a dire need for the development of a prophylactic agent against tularemia that is more attenuated than LVS, retains its protective efficacy, and could be administered via aerosol for immunization. Mice serve as a valuable model for the screening of F. tularensis vaccine candidates. Previous studies have shown that vaccination with LVS provide protection in BALB/c mice but fail to protect C57BL/6 mice against both systemic or intranasal (i.n.) challenge with virulent type A strains of F. tularensis [7;8]. In addition, BALB/c but not C57BL/6 can be protected by oral immunization with LVS against an i.n. challenge with type A strains of F. tularensis [9]. The goal of the present study was to evaluate an attenuated and genetically defined mutant of F. tularensis LVS as a potential vaccine candidate against respiratory tularemia caused by F. tularensis SchuS4 in C57BL/6 mice.
Superoxide dismutases (SODs) play an important role in dismutation of superoxide radicals generated during the course of aerobic respiration or respiratory burst in phagocytic cells. Deletion of genes encoding SODs results in the loss of virulence in many bacterial pathogens [10;11]. F. tularensis possesses two SODs: an iron containing SOD (FeSOD) encoded by the sodB gene and a copper-zinc containing SOD (CuZnSOD) encoded by the sodC gene [12]. Earlier, we reported a mutant of the sodB gene in F. tularensis LVS (sodBFt) has diminished FeSOD activity, enhanced sensitivity to oxidative stress and attenuated virulence for mice [13]. In the present study we evaluated the efficacy of i.n. immunization with sodBFt to confer protection against experimental respiratory tularemia caused by highly virulent SchuS4 strain of F. tularensis. We observed that immunization with sodBFt mutant offered a highly reproducible 40–42% protection in C57BL/6 mice with a significantly extended median time to death (MTD) as compared to naïve or LVS vaccinated mice. Our results demonstrate that the sodBFt mutant is superior to LVS in providing protection in C57BL/6 mice and this study represents an important advance in the development of a live attenuated vaccine for the prevention of respiratory tularemia caused by F. tularensis SchuS4.
Materials and Methods
Bacterial strains
F. tularensis LVS (ATCC 29684; American Type Culture Collection, Rockville, MD) was kindly provided by Dr. Karen Elkins (U.S. Food and Drug Administration, Bethesda, MD). F. tularensis SchuS4, originally isolated from a human case of tularemia, was obtained from the U.S. Army Medical Research Institute for Infectious Diseases (Frederick, MD) and sodBFt was generated in our laboratory [13]. The bacteria were cultured on modified Mueller-Hinton (MH) chocolate agar plates [13;14] or in MH broth (Difco Laboratories, Lawrence, KA) supplemented with ferric pyrophosphate and Iso-Vitalex (BD Biosciences, San Jose, CA). Active mid-log phase bacteria were harvested and stored in liquid nitrogen; one ml aliquots were thawed periodically for use.
Mice
C57BL/6 mice (Taconic, Germantown, NY), C57BL/6CD4−/− and CD8−/− mice were obtained from Jackson Laboratories (Bar Harbor, Maine). The mice were maintained and bred in a specific pathogen free environment in the Animal Resource Facility at Albany Medical College. All experiments were conducted using six to eight week-old mice of both sexes and all the animal procedures conformed to the Institutional Animal Care and Use Committee guidelines.
Immunizations and challenge
Prior to i.n. inoculation, mice were deeply anesthetized via intraperitoneal injection of a cocktail of Ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and Xylazine (Phoenix Scientific, St. Joseph, MO). Mice were immunized i.n. with 5×102 or 5×103 CFU of LVS or sodBFt in a volume of 20 µl PBS (10 µl/nare). Unvaccinated mice, which served as a control, received an equal volume of PBS. Mice immunized with 5×103 CFU of either LVS or sodBFt were challenged i.n. with 1×101 CFU (10LD100) of SchuS4 on day 21 post-immunization. Mice immunized with 5×102 CFU of LVS or sodBFt received an additional booster dose of 1×103 CFU 21 days after the primary immunization. The immunized mice were then challenged i.n. with 110 1×102 CFU (100LD100) of F. tularensis SchuS4 on day 42 post-primary immunization. An identical vaccination regimen was followed for experiments conducted with CD4−/− and CD8−/− mice, and the long-term survival experiments. However, in the long-term experiments, the immunized mice were challenged with 100LD100 of F. tularensis SchuS4 after 132 days or with 1×106 CFU (100LD100) of LVS after 210 days of primary immunization, respectively. Actual numbers of bacteria were determined by plating the inoculum after each immunization and challenge, and CFU were determined. Mice were monitored closely for morbidity and mortality for a period of 21–30 days post-challenge and the MTD was calculated for each group. All mice that survived the SchuS4 challenge were sacrificed at the end of the experiment to recover bacteria from lung, liver and spleen. All SchuS4 challenge experiments were performed in the CDC-certified Animal Biosafety Level 3 (ABSL-3) facility of Albany Medical College.
For time course experiments, C57BL/6 mice were immunized with 5×103 CFU and challenged with 1×101 CFU of SchuS4 21 days after the immunization. Groups of 3–4 C57BL/6 mice were sacrificed on day one, three, six 10, 14 and 21 post-challenge. Lung, liver and spleen were collected aseptically for quantitation of bacterial burden and histological evaluation. Homogenates of the lungs were prepared to measure the tissue cytokine and antibody levels. Whole blood was collected from the challenged mice at the indicated times post-challenge and serum was used to determine humoral immune responses.
Quantification of F. tularensis SchuS4 burden and cytokine measurement
Bacterial numbers were quantified in the lung, liver and spleen of LVS and sodBFt vaccinated mice on day one, three, six, 10, 14, and 21 following the SchuS4 challenge. The lungs were inflated with sterile PBS and excised aseptically in PBS containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Liver and spleen also were excised and stored in protease inhibitor cocktail. The organs were subjected to mechanical homogenization using a Mini-Bead Beater-8™ (BioSpec Products Inc. Bartlesville, OK). The tissue homogenates were spun briefly at 1000 × g for 10 sec in a microcentrifuge to pellet tissue debris. The supernatants were diluted 10-fold in sterile PBS and 10 µl of each dilution was spotted onto MH chocolate agar plates in duplicate and incubated at 37°C for 48–72 hr in the presence of 5% CO2. The colonies on the plates were counted and expressed as CFU per organ as reported earlier [7;9;15]. The remaining tissue homogenate was spun at 14,000 × g for 20 min and the clarified supernatant was stored at −20°C and used for measurement of tissue cytokine levels. The protein content in the lung homogenates was normalized using a bicinchoninic protein assay kit (Pierce, Rockford, IL). Mouse Inflammation Cytometric Bead Array (CBA) Kits (BD Biosciences, San Jose, CA) were used for the simultaneous measurement of multiple pro-inflammatory cytokines in lung homogenates. Data were acquired on a FACS Array instrument (BD Biosciences, San Jose, CA) and analyzed using CBA software version 1.1 (BD Biosciences, San Jose, CA). The cytokine production was expressed as pg/ml.
Histopathology
The lung, liver and spleen from F. tularensis LVS or sodBFt vaccinated and SchuS4 challenged C57BL/6 mice were excised and fixed in 10% neutral buffered formalin for histological evaluation. The organs were collected on day one, three, six, 10, 14 and 21 post-challenge. Lungs were inflated via instillation of PBS into the trachea prior to fixation. Tissues were processed using standard histological procedures and 5-µm paraffin sections were stained with hematoxylin-eosin (H and E) and examined by light microscopy.
The H and E sections were analyzed blind folded using a histopathological (HSP) scoring system. The criteria used for assigning HSP scores for lung, liver and spleen are shown in Table 1. The inflammatory lesions in lungs were graded on a scale of 0–3 for peribronchiolar /bronchial and perivascular infiltration, inflammation of the lung parenchyma (terminal bronchioles, alveolar ducts, alveolar sacs, and alveoli) and given a numerical score ranging from 0 to 18 (mild to severe) as described earlier [16]. The HSP scoring was also developed for liver and spleen. Liver was assessed for degenerative/necrotic changes of hepatocytes in the hepatic lobules, degree of infiltration within the sinusoids, presence of granulomatous lesions, their nature and distribution (discrete/ diffuse), and the type of cells involved. Spleen sections were evaluated for the extent of granulomatous lesions involving white pulp, marginal zones and red pulp parenchyma, their distribution and cellular composition.
Table 1.
Histopathological scoring system for lung, liver and spleen of F. tularensis SchuS4 infected mice.
| Lung | Liver | Spleen | |
|---|---|---|---|
| A | Peribronchial and bronchial infiltrates (% of sites) | Hepatic lobules infiltration | Marginal zone thickening and inflammation |
| 0 = None | 0 = None | 0 = None | |
| 1 = Few (<25%) | 1 = Few neutrophils in sinuses | 1 = Mild | |
| 2 = Many (25–75%) | 2–3 = Many neutrophils and engorgement | 2 = Moderate | |
| 3 = All (>75%) | 3 = Severe | ||
| B | Inflammatory infiltrates | Quantity of granulomas (10 × magnification) and distribution | Perilymphoid red pulp infiltration |
| 0 = None | 0 = None | 0 = None | |
| 1 = Mild (interrupted) | 1= Few (1–5) | 1 = Mild | |
| 2 = Moderately complete (Collar <5 cells) | 2 = Moderate (5–10) | 2 = Moderate | |
| 3 = Severe (collar >5–10 cells) | 3 = Many (>10) | 3 = Severe | |
| C | Quality of infiltrate | Quality of granulomatous infiltrates | Red pulp parenchymal inflammation |
| 0 = None | 0 = None | 0 = None | |
| 1 = Mild neutrophilic | 1 = Non discrete (mild neutrophilic) | 1 = Mild | |
| 2 = Moderate neutrophilic | 2 = Discrete (more neutrophilic and lymphocytic) | 2 = Moderate | |
| 3 = macrophages and neutrophils | 3 = Severe and necrotic | 3 = Severe | |
| 4 = Macrophages only | |||
| D | Parenchymal pneumonia | Portal triad infiltration | Quality of granulomatous inflammation |
| 0 = None | 0 = None | 0 = None | |
| 1 = Minimal (patchy) | 1 = Mild neutrophilic | 1 = Mild | |
| 2 = Heavy (patchy) | 2 = Moderate neutrophilic | 2 = Moderate | |
| 3 = Heavy, confluent and necrotizing | 3 = Severe (macrophages and neutrophils) | 3 = Necrotizing | |
| E | Bronchiolar and bronchiole lumen exudates | ||
| 0 = None | |||
| 1 = Minimal (25% lumen occlusion) | |||
| 2 = Heavy (>25%) | |||
| F | Perivascular infiltrate | ||
| 0 = None | |||
| 1 = Mild | |||
| 2 = Moderate (10–50%) | |||
| 3 = Heavy (>50% blood vessels involved) | |||
| Total Score | 18 | 12 | 12 |
Measurement of antibody levels
Anti- F. tularensis antibody levels in mouse serum and lung homogenates were quantified prior to- and at days 14 and 21 post- SchuS4 challenge by enzyme linked immunosorbent assay (ELISA). To accomplish this, microtitre plates were coated with 5 × 106 CFU of SchuS4 in bicarbonate buffer for two hr at 37°C, washed three times with PBS-T (0.1% Tween-20) and blocked with 10% FBS in PBS overnight at 4°C. Two-fold dilutions of test sera or the lung homogenates (100µl/well) were added to the plates and incubated for two hr at 37°C. This was followed by the addition of biotinylated primary goat anti-mouse antibodies specific for IgA, IgG1, IgG2a or IgG2b (Caltag, Burlingam, CA). Plates were incubated for one hr at 37°C, followed by three washes with PBS-T and incubation with streptavidin-horse radish peroxidase (HRP) conjugate (Biosource, Camarillo, CA) for 30 min. The plates were washed again and peroxidase substrate solution (BCIP/NBT) (KPL, Gaithersburg, MD) was added to each well. The plates were incubated for 20 min at 37°C for color development. The reaction was stopped by adding 1.8N H2SO4 and the optical density was read at 450 nm (OD450) using the PowerWave HT microplate reader (BioTek Instruments, Winooski, VT). For quantitation of antibody levels against stress proteins bacteroferritin (Bfr) and GroEL, the microtitre plates were coated with 10µg of the purified recombinant protein per well. The antibody levels were determined in the individual serum samples collected from sodBFt and LVS vaccinated mice at day 14 post-SchuS4 challenge following the protocol described above. The results were expressed as end point dilution titers which represent the highest dilution of serum where the OD450 was 0.1 above the normal serum control.
Microarray analysis
The 70-mer oligonucleotide microarrays representing open reading frames from F. tularensis were obtained were obtained through the National Institute of Allergy and Infectious Diseases? Pathogen Functional Genomics Research Center, managed and funded by the Division of Microbiology and Infectious Diseases, NIAID, NIH, DHHS, and operated by TIGR. The microarray slides were prehybridized, washed, and dried immediately before hybridization by using the protocol recommended by TIGR (http://www.tigr.org/tdb/microarray/protocolsTIGR.shtml). RNA was isolated from four replicates of sodBFt and F. tularensis LVS cultures grown under identical conditions in MH-broth for six hr using a Nucleospin RNA II kit (BD BioSciences Palo Alto CA) and cDNA was synthesized by Superscript III first strand kit (Invitrogen, Carlsbad, CA). For hybridization, cDNA with 200 pmol Cy3 and cDNA with 200 pmol Cy5 were included in a 130-µl hybridization solution containing 25% (vol/vol) formamide, 5x SSC, 0.1% sodium dodecyl sulfate (SDS), and 100 µg/ml of sonicated salmon sperm DNA (1x SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Hybridization was performed on a TEcan hyrbridization station. Briefly, slides were incubated 2x in SSC-0.1% SDS at 50°C for 1 min, incubated in 0.1 SSC-0.1% SDS at 50°C for one min and then washed twice for 10 min in H20. Slides were then incubated in 0.1x SSC at 50°C for one min and washed twice for one min in H20. Finally slides were washed in ethanol for 30 min and dried in N2 for 10 min. Arrays were scanned using an Agilent 2505B scanner with laser power set to 100% and PMT gains set to auto. GenePix Pro 6 was used to grid arrays. Lossless image files were stored for later analysis. The microarray data was analyzed using the Limma module of the Bioconductor package for the R statistical environment [17]. The "normexp" method was used for background correction, followed by print tip loess normalization and between-array normalization of intensities. The microarray data for each gene were fitted to a linear model, and statistics were generated using the lmFit and eBayes functions [18]. The P values displayed were adjusted for multiple testing using the Benjamini and Hochberg method within Limma. Genes with P values of <0.05 were considered differentially regulated. Annotations for microarray data were derived from TIGR gal files.
Reverse Transcriptase (RT) and quantitative real time PCR (qRt-PCR)
The cDNA prepared from F. tularensis LVS and sodBFt mutant as described above was amplified using GroEL (Forward CTCAACCATATCACCATAAGTATC; Reverse TGCGGCTGTAGAAGAAGG) and DnaK (Forward GTAATGAGATCACTTGAGCCTTG; Reverse CTAATACACCACCTTGAATAGCC) primers. The amplified products were run on a 1.5% agarose gel and stained with ethidium bromide to visualize the amplified bands. 16S 230 rRNA gene (Forward ACGGTAACAGGTCTTAGGATG; Reverse GATATTATGCGTATTAACAGTCG) of F. tularensis was used as a loading control. The qRT PCR was run on a IQ5 real time PCR machine (Bio Rad, Hercules CA) for quantitation of GroEL and DnaK transcripts using IQ SYBR green supermix (Bio-Rad, Hercules CA). 16S rRNA gene was used for normalization of the copy numbers. The data was analyzed on a Bio-Rad IQ5 software and expressed as fold change over LVS.
Western blot analysis
Generation of antibodies against the immunogenic stress proteins DnaK, GroEL and Bfr in vaccinated and SchuS4 challenged mice was determined by western blot analysis. F. tularensis SchuS4 lysates prepared by repeated freeze thawing were resolved by SDS-PAGE on a NuPAGE 4–12% Bis-Tris gels using NuPAGE MES-SDS running buffer (Bio-Rad Laboratories, Hercules, CA) and transferred to Immobilon-P nylon membrane (Millipore, Billerica, MA) for western blot analysis. Membranes were probed with the pooled serum from LVS or sodBFt vaccinated mice collected at day 14 post- SchuS4 challenge. This was followed by the addition of a 1:10,000 dilution of goat anti-mouse antibody conjugated to HRP (Southern Biotechnology Associates, Birmingham, AL). Blots were developed using the Pierce West Pico chemiluminescent substrate (Pierce Biotechnology Inc., Rockford, IL) and images were captured using a Fluorchem 8000 Imaging System (Alpha Innotech Inc., San Leandro, CA). To facilitate the identification of DnaK, the membranes were stripped for 30 min at 60°C in buffer containing 60 mM Tris pH 6.8, 2% SDS and 100 mM β-mercaptoethanol, washed twice in PBS with 0.1% Tween 20 and developed to confirm the stripping. The stripped membranes were re-probed with anti- DnaK monoclonal antibodies directed against F. tularensis DnaK (kindly provided by Dr A. G. Savitt, SUNY, Stony Brook, NY). Antibody responses against GroEL and Bfr were further confirmed by probing purified recombinant GroEL and Bfr proteins (kindly provided by Dr Daniel Clemens, University of California, Los Angeles, CA) with day 14 post-challenge serum from the LVS or sodBFt vaccinated mice. The blots were developed and the results were recorded as described above.
Statistical analysis
All results were expressed as mean ± SEM and comparisons between the groups were made using one-way ANOVA followed by Bonferroni’s correction, nonparametric Mann-Whitney test, or Student’s t-test. The survival data were analyzed using Log-rank test and P values were determined. Differences between the experimental groups were considered significant at a P< 0.05 level.
Results
SodBFt vaccination offers protection against the highly virulent SchuS4 strain of F. tularensis
To test the efficacy of sodBFt in protecting mice against virulent F. tularensis SchuS4 challenge, C57BL/6 mice were immunized i.n. with ~5×103 CFU of sodBFt or LVS. On day 21 after primary immunization, all the vaccinated mice were challenged with 14 CFU of F. tularensis SchuS4. It was observed that sodBFt vaccinated C57BL/6 mice not only had a significantly extended MTD as compared to LVS vaccinated or unvaccinated mice, but, 40% (4/10) of the sodBFt vaccinated mice survived the challenge. All naïve C57BL/6 mice challenged with a similar dose of SchuS4 strain succumbed to infection within six to eight days post-challenge (Table 2).
Table 2.
Survival of mice vaccinated with sodBFt and LVS following SchuS4 challenge.
| Vaccinationa | Dose (CFU) | SchuS4 Challenge Dose (CFU) c | Median Time to Death (Days) | No. of Mice Survived/No. of mice Challenged (% Survival)d | |
|---|---|---|---|---|---|
| Primary | Boosterb | ||||
| Unvaccinated | None | None | 14 | 7 | 0/10 (0) |
| LVS | 5.14 × 103 | None | 14 | 12e | 0/10 (0) |
| sodBFt | 5.27 × 103 | None | 14 | UDf | 4/10 (40) |
| Unvaccinated | None | None | 103 | 6 | 0/12 (0) |
| LVS | 5.23 × 102 | 1.37 × 103 | 103 | 15g | 0/12 (0) |
| sodBFt | 5.1 6 × 102 | 1.21 × 103 | 103 | UDh | 5/12 (42) |
Mice were vaccinated i.n.
21 days after primary immunization.
42 days after primary immunization.
Mice were monitored for morbidity and mortality for a period of 21–30 days post-challenge.
Significantly different from unvaccinated mice (P<0.01).
Significantly different from unvaccinated (P<0.001) and LVS vaccinated mice (P<0.01).
Significantly different from unvaccinated mice (P<0.01).
Significantly different from unvaccinated (P<0.001) and LVS vaccinated mice (P<0.001).
Data were analyzed using Log-rank test and are representative of two experiments conducted.
UD =Undefined, as 40–42% of the mice survived the infection.
It was next tested whether a low dose immunization and inclusion of a low dose boost would protect mice against a higher challenge dose of F. tularensis SchuS4. C57BL/6 mice were immunized and boosted with LVS or sodBFt mutant 21 days after primary immunization. The mice were then challenged with 103 CFU of SchuS4 on day 42 after the primary immunization. All the unvaccinated mice died within days 6–7 post-challenge. LVS vaccinated C57BL/6 mice showed an extended MTD (15 days) as compared to the unvaccinated mice (6 days); whereas a significant proportion, 42% (5/12) of the sodBFt vaccinated C57BL/6 mice survived until day 30 post-challenge (Table 2). These results demonstrate that the sodBFt vaccinated mice are better protected against SchuS4 challenge than the LVS vaccinated mice and 289 inclusion of a boost enhances resistance to a higher challenge dose. All the mice that survived 14 and 103CFU challenge dose of SchuS4 were sacrificed to isolate bacteria which were found to persist in the lung, liver and spleen of the surviving mice at 21–30 days post challenge however were cleared completely by day 45 post-challenge.
We next examined the duration of immunity induced by sodBFt vaccination in C57BL/6 mice. C57BL/6 mice were vaccinated with LVS or sodBFt followed by a booster dose of the respective strains after 21 days. All the LVS vaccinated mice challenged with 104 CFU of SchuS4 at day 134 post-primary immunization succumbed to infection, whereas 16% of sodBFt vaccinated mice survived the challenge until the end of the experiment. However, no statistically significant differences in the MTD were observed between LVS and sodBFt vaccinated groups (Table 3). In another experiment when the vaccinated mice were challenged with 1.29 × 106 300 CFU (~100LD100) of LVS 210 days after primary immunization, 86% of the sodBFt vaccinated mice survived. On the contrary, 100% of the LVS vaccinated mice succumbed to infection in a manner similar to naïve mice (Table 3). These results suggest that although sodBFt vaccination provides protection against a lethal LVS challenge; immunity against the highly virulent SchuS4 strain is not long lasting and gradually wanes off over a period of time.
Table 3.
Survival of mice vaccinated with sodBFt and LVS following a delayed SchuS4 and LVS challenge.
| Vaccinationa | Dose (CFU) | SchuS4 Challenge Dose (CFU) c | Median Time to Death (Days) | No. of Mice survived/No. of mice challenged (%Survival)e | |
|---|---|---|---|---|---|
| Primary | Boosterb | ||||
| Unvaccinated | None | None | 104 | 6 | 0/6 (0) |
| LVS | 5.3 × 102 | 1.1 × 103 | 104 | 9f | 0/9 (0) |
| sodBFt | 5.19 × 102 | 1.2 × 103 | 104 | 11g | 1/6 (16.6) |
| LVS Challenge Dose (CFU)d | |||||
| Unvaccinated | None | None | 1.29 × 106 | 9 | 0/7 (0) |
| LVS | 5.3 × 102 | 1.1 × 103 | 1.29 × 106 | 9.5 | 0/7 (0) |
| sodBFt | 5.19 × 102 | 1.2 × 103 | 1.29 × 106 | UDh | 6/7 (86) |
Mice were vaccinated i.n.
21 days after primary immunization.
132 days after primary immunization.
210 Days after the primary immunization.
Mice were monitored for morbidity and mortality for a period of 21–30 days postchallenge.
Significantly different from unvaccinated mice (P<0.05).
Significantly different from unvaccinated mice (P<0.01).
Significantly different from unvaccinated (P<0.001) and LVS vaccinated mice (P<0.01).
Data were analyzed using Log-rank test and are representative of two experiments conducted.
UD =Undefined.
SodBFt vaccinated mice control F. tularensis SchuS4 replication more efficiently than the LVS vaccinated mice
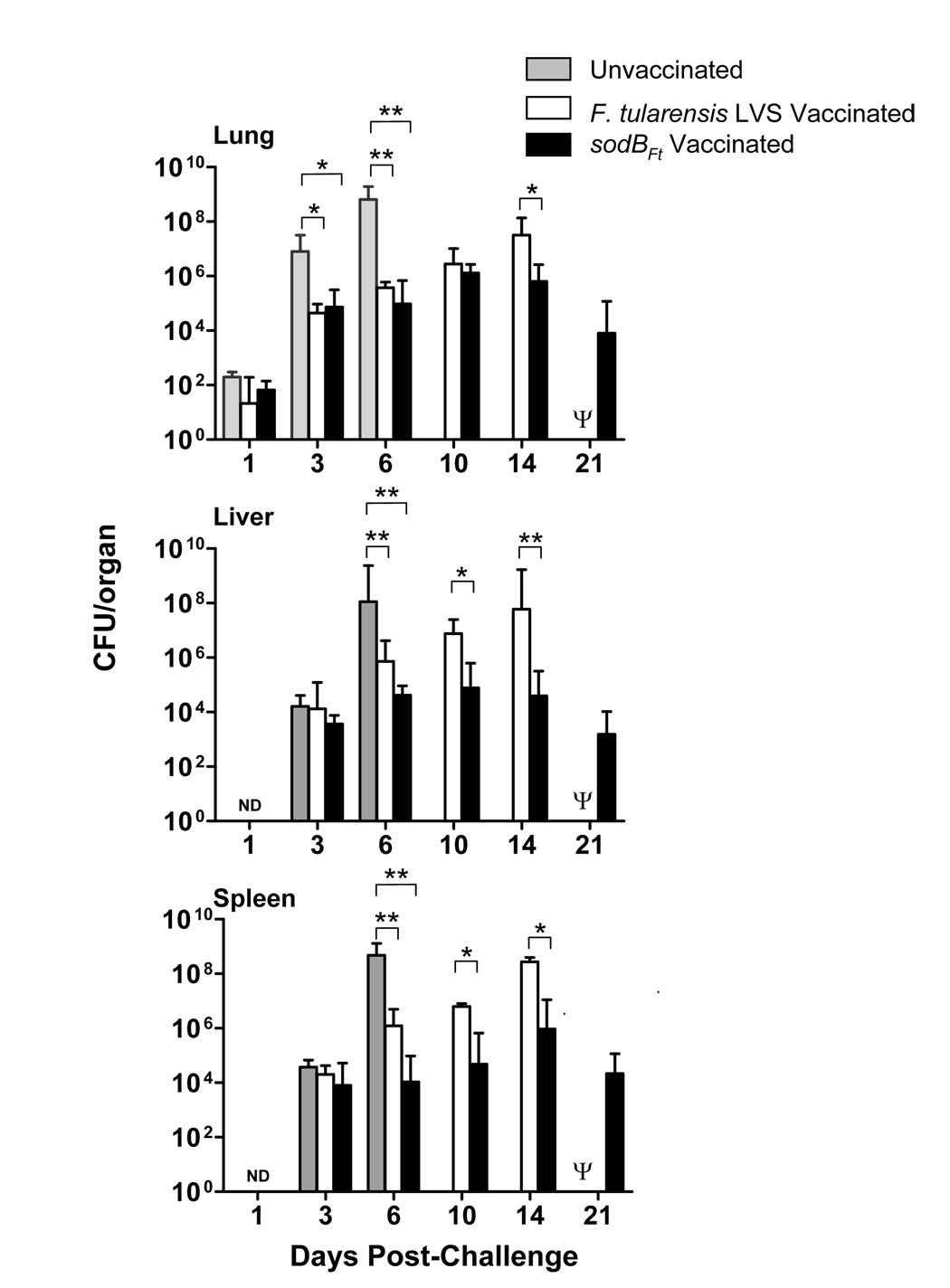
To determine why sodBFt vaccinated mice were better protected than the LVS vaccinated mice, a time course experiment was conducted. C57BL/6 mice were vaccinated with sodBFt or LVS and then challenged at day 21 with 16 CFU of SchuS4. Mice were sacrificed at the indicated times and bacterial burdens were quantitated in the lung, liver and spleen. At days three and six post-challenge, bacterial numbers were significantly higher in the lungs of the unvaccinated C57BL/6 mice compared to the sodBFt or LVS vaccinated mice. The majority of the unvaccinated mice succumbed to infection shortly thereafter (Fig. 1). Both the sodBFt and LVS vaccinated C57BL/6 mice showed a steady increase in bacterial numbers between days three and six post-challenge in all the tested organs. The LVS vaccinated mice revealed significantly higher bacterial burden at day 14 in the lungs as compared to sodBFt vaccinated mice (Fig. 1). In the liver and spleen, LVS vaccinated mice harbored significantly higher numbers of bacteria at day 10 and 14 post-challenge compared to the sodBFt vaccinated mice (Fig. 1). All the sodBFt vaccinated mice that survived the SchuS4 challenge until day 21, were still found to carry bacteria in their lungs, liver and spleen however, the bacteria were cleared completely in the surviving mice by day 45 post-challenge (not shown). These results demonstrate that vaccination of C57BL/6 mice with either LVS or sodBFt results in the control of SchuS4 replication, but vaccination with sodBFt in particular exhibit a better control of bacterial replication in the lung, liver and spleen than LVS vaccinated mice.
Figure 1. SodBFt vaccinated mice exhibit enhanced bacterial clearance following SchuS4 challenge.
(A) C57BL/6 mice were vaccinated i.n. with F. tularensis LVS or sodBFt and challenged with F. tularensis SchuS4 on day 21 post-primary vaccination. At the times indicated, mice were sacrificed and homogenates of the lung, liver, and spleen were plated for determination of bacterial burden. Results shown are the mean ± SEM and are cumulative of two independent experiments conducted (n = 6–8 mice per group). * p < 0.05, ** p < 0.001 using the one-way ANOVA. Ψ All the LVS vaccinated mice died by day 15–17 post-challenge and hence were unavailable for comparisons.
SodBFt vaccinated mice exhibit less severe histopathology than the LVS vaccinated mice after SchuS4 challenge
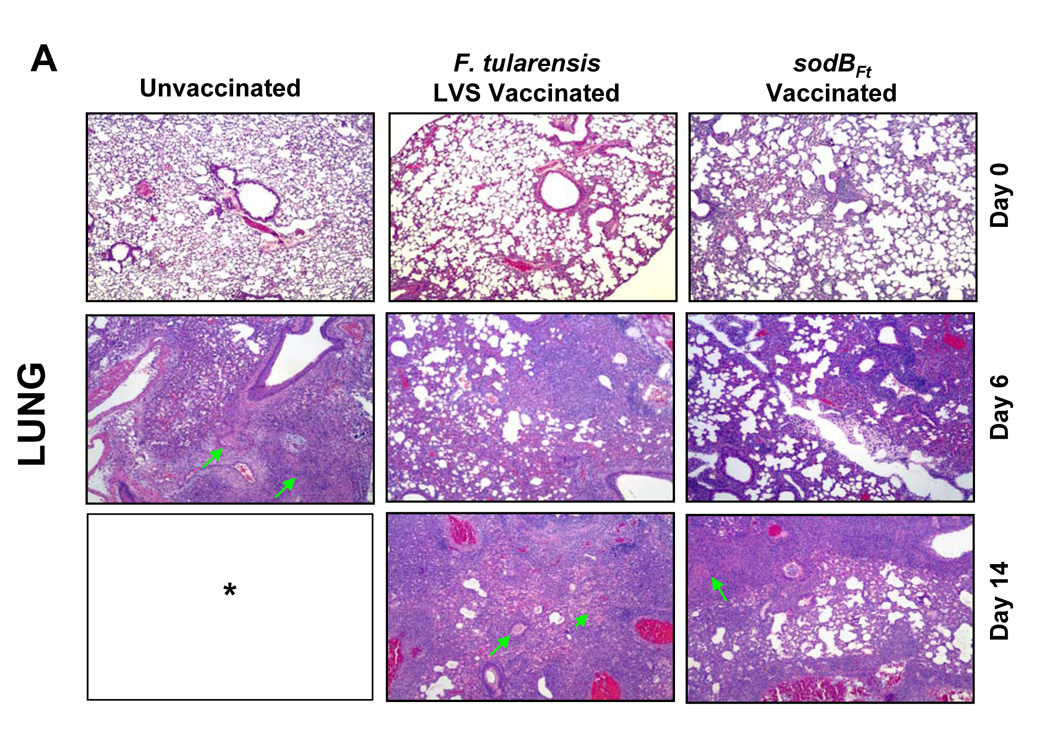
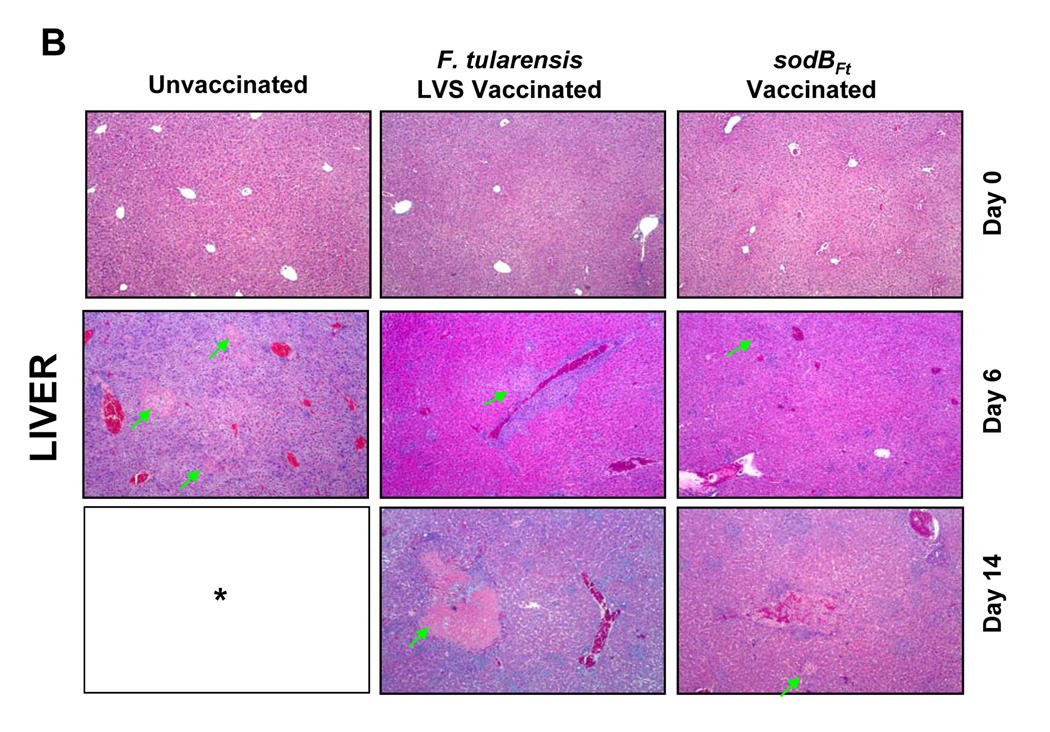
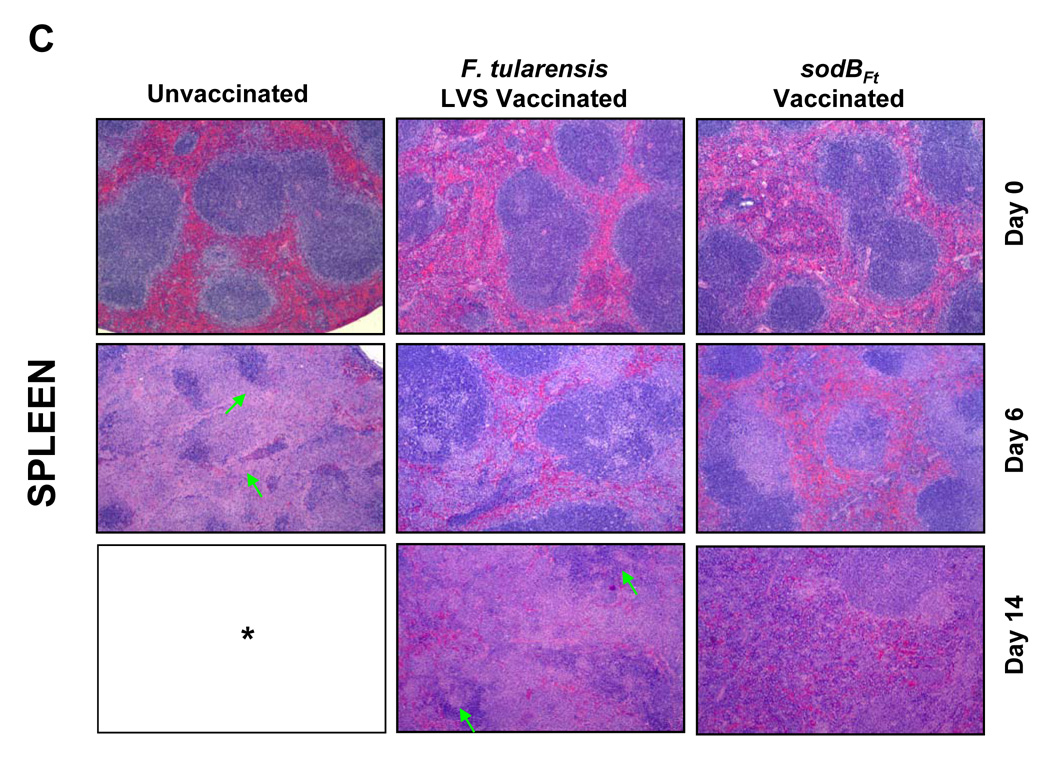
Since sodBFt vaccinated C57BL/6 mice revealed significantly less bacterial numbers in the lung, liver and spleen following SchuS4 challenge, it was of interest to examine if these differences were also reflected in the histopathological lesions in these organs. Histological analysis was performed prior to, and after the SchuS4 challenge at days three, six, 10, 14 and 21. LVS or sodBFt vaccinated mice prior to challenge revealed mild inflammatory foci in the lungs and small granulomatous lesions in the liver and spleen (Fig. 2A, B and C, top panels). The unvaccinated mice revealed severe tissue damage and extensive necrotic lesions in the lung, liver and spleen at day six post-challenge. However, lesions in the lungs of sodBFt and LVS vaccinated mice at day six post-challenge consisted mostly of mild to severe peribronchial and perivascular inflammation and focal patches of pulmonary pneumonia which developed into necrotizing pneumonia in LVS vaccinated mice by day 14 post-challenge (Fig. 2A). The livers of sodBFt and LVS vaccinated mice revealed nondiscrete, granulomatous lesions with no indication of necrosis at day six and 10 post-challenge. The lesions became more pronounced at day 14 post-challenge in the LVS vaccinated mice exhibiting large areas of severe necrotic and pyogranulomatous lesions as compared to the sodBFt vaccinated mice (Fig. 2B). Lesions in the spleen consisted of multifocal to coalescing areas of neutrophilic to pyogranulomatous necrosis that involved the splenic red pulp. Spleens from sodBFt and LVS vaccinated mice showed inflammation with greater numbers of infiltrating cells, however complete disruption of the splenic architecture with necrotic splenitis was more prominently observed in the latter group at day 14 post-challenge (Fig. 2C). The sodBFt vaccinated mice that survived the challenge showed resolution of inflammatory lesions in the lung, liver and spleen by day 21 post-challenge (not shown).
Figure 2. SodBFt vaccinated mice exhibit less severe histopathological changes than LVS vaccinated mice following SchuS4 challenge.
H and E stained sections from A. Lung; B. Liver and C. Spleen sections from unvaccinated and LVS or sodBFt vaccinated mice prior to challenge (day 0) and at days six and 14 post-SchuS4 challenge. Arrows in the lung panel indicate necrotizing pneumonia, in the liver indicate necrotic granulomas and those in the spleen indicate an area of intense lymphoproliferation and necrotizing splenitis. * All the unvaccinated mice died shortly after day 6 post-challenge. (Magnification × 40X).
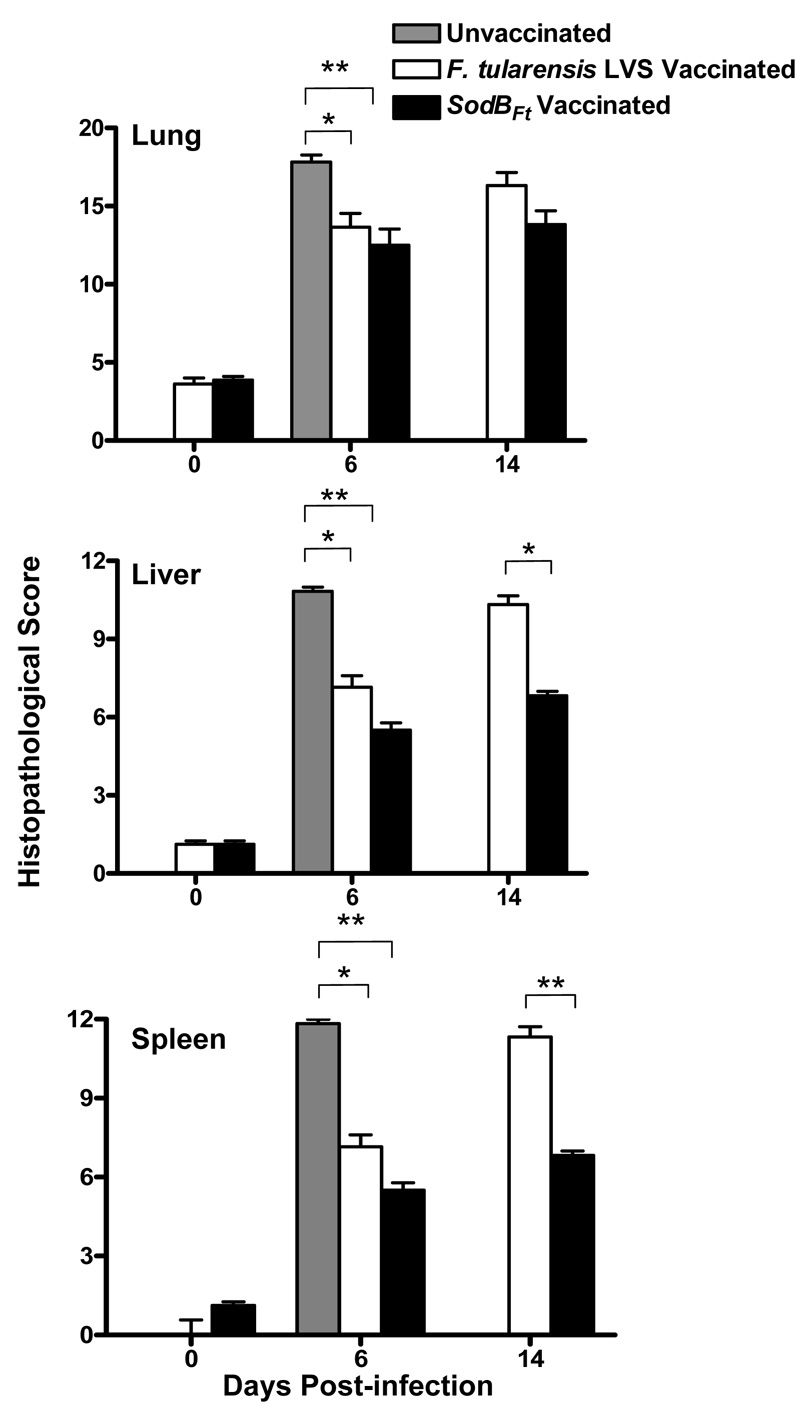
The histopathological lesions in the lung, liver and spleen of the vaccinated and unvaccinated mice challenged with SchuS4 were quantitated by a HSP scoring scoring. At day six post-challenge, unvaccinated mice revealed significantly higher HSP scores for lung, liver and spleen as compared to LVS and sodBFt vaccinated mice. However significant differences were not observed between the vaccinated groups at this time point. Higher HSP scores observed at day 14 post-challenge in the lungs of LVS vaccinated compared to sodBFt vaccinated mice reflected severe inflammation but these differences were not statistically significant (Fig. 3). In contrast, significantly higher HSP scores were observed for liver and spleen in the LVS vaccinated mice as compared to sodBFt vaccinated counterparts at day 14 post-SchuS4 challenge, indicating greater tissue damage (Fig. 3). Collectively, reduced histopathology and tissue destruction associated with lower bacterial burdens and mortality in the sodBFt vaccinated mice suggests that sodBFt vaccination induces a strong clearance mechanism following SchuS4 challenge.
Figure 3. Quantitation of histopathological lesions in the lung, liver and spleen from unvaccinated or vaccinated mice challenged with SchuS4.
H and E stained tissue sections from unvaccinated and LVS or sodBFt vaccinated mice were evaluated for histopathological lesions prior to challenge (day 0) and at days six and 14 post SchuS4 challenge. The values represent cumulative histopathological scores (n=6 mice per group) based on the criteria described in Table 1. The results are expressed as mean ± SE and P values were determined using the Student’s t- test (*p < 0.05, **p < 0.01).
Mice vaccinated with sodBFt exhibit regulated production of pro-inflammatory cytokines following SchuS4 challenge
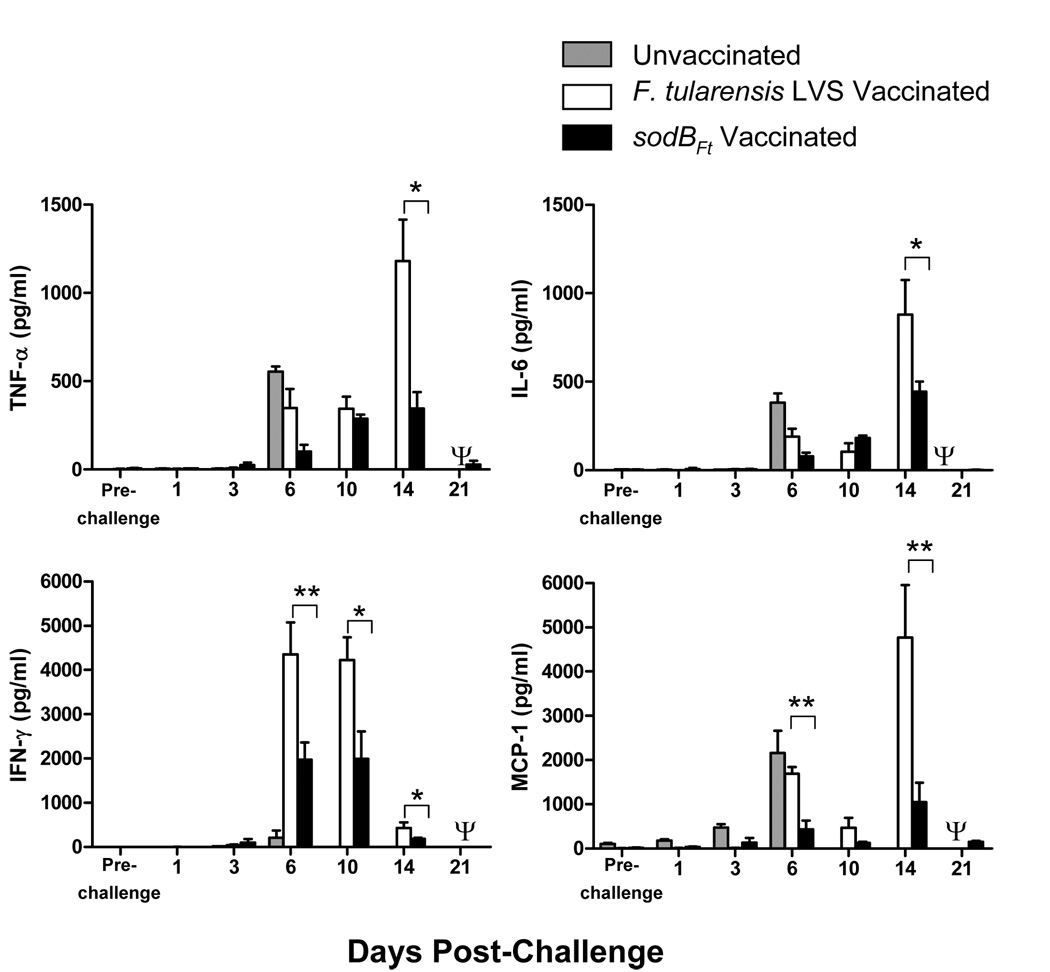
Dysregulated production of cytokines in response to F. tularensis infection is associated with more severe histopathological lesions observed in the lung, liver and spleen of infected mice [16;19;20]. The levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interferon-gamma (IFN-γ), and monocyte chemoattractant protein-1 (MCP-1) were determined in the lung homogenates of LVS or sodBFt vaccinated C57BL/6 mice following SchuS4 challenge. Elevated MCP-1 and IL-6 levels are indicators of critical illness and sepsis [21], whereas increased levels of TNF-α and IFN-γ indicate a greater degree of tissue inflammation and destruction [16;19]. IFN-γ levels at days six and 10, and MCP-1 levels at day six post-challenge were significantly elevated in the LVS compared to sodBFt vaccinated mice. The significantly elevated levels of TNF-α, IL-6, IFN-γ and MCP-1 were also observed in LVS vaccinated mice at day 14 post-challenge, the time after which majority of mice succumb to infection (Fig. 4). In contrast, the cytokine levels in sodBFt vaccinated mice rose steadily and peaked by days 10–14 post-challenge and subsequently returned to their baseline values by day 21 post-challenge (Fig. 4) indicating that cytokine production is more tempered in sodBFt vaccinated mice following a lethal SchuS4 challenge compared to LVS vaccinated mice. This observation is consistent with our earlier studies showing that susceptible population of mice produce higher levels of pro-inflammatory cytokines prior to death [16;19]. No Detectable levels of Th2 cytokines IL-4 and IL-5 were observed in the lung homogenates of LVS or sodBFt vaccinated mice at days 6, 10 and 14 post- SchuS4 challenge (not shown).
Figure 4. SodBFt vaccinated mice produce regulated levels of proinflammatory cytokines following SchuS4 challenge.
Unvaccinated and SodBFt or LVS vaccinated and SchuS4 challenged C57BL/6 mice were sacrificed at the times indicated and cytokine levels were measured in homogenates of the lungs. The results shown are the mean ± SEM and are cumulative of two independent experiments conducted (n = 6–8 mice per time point). *p < 0.05, **p < 0.01 using the one-way ANOVA with Bonferroni’s multiple comparison test. Ψ All the LVS vaccinated mice died by day 15–17 post-challenge and hence were unavailable for comparisons.
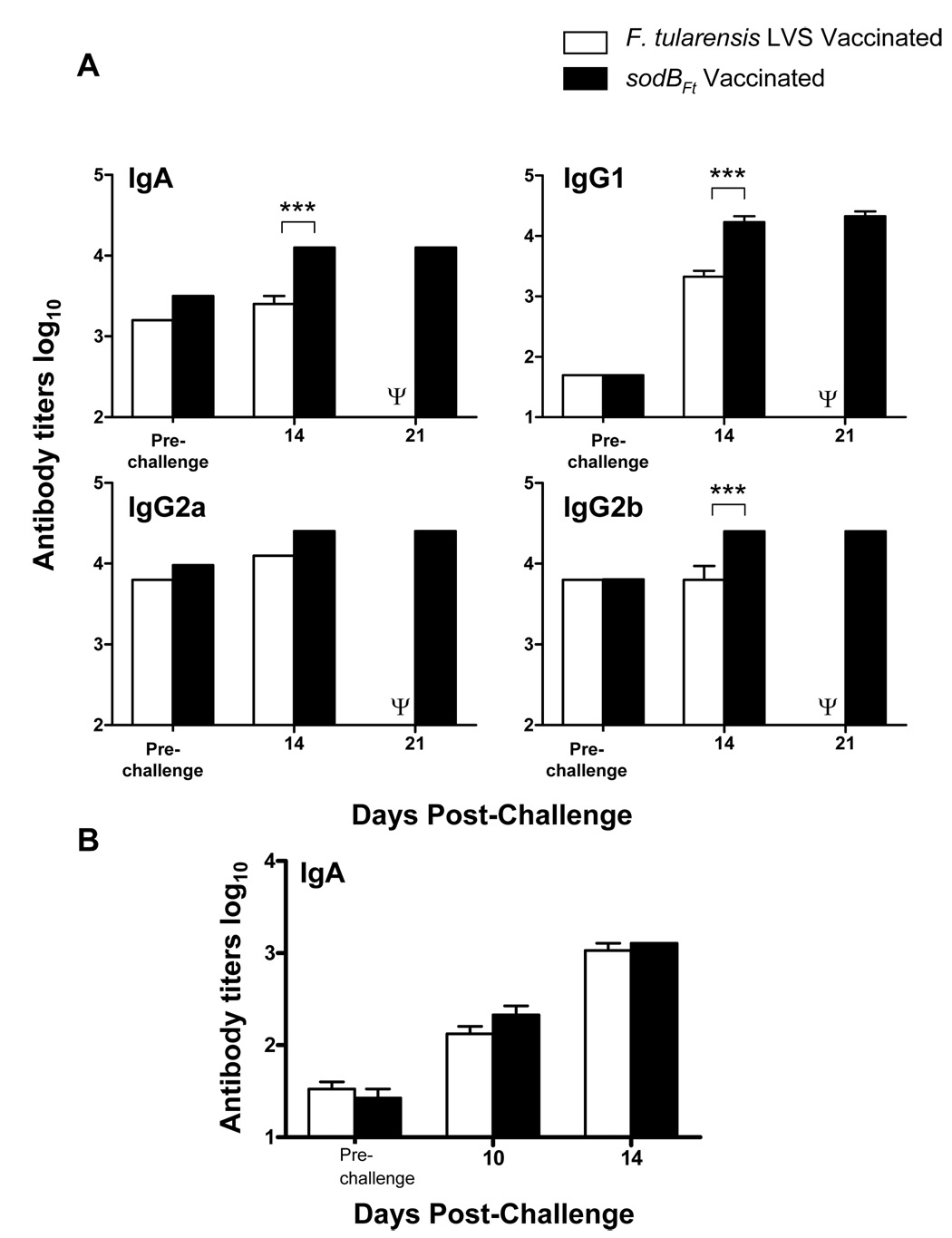
SodBFt vaccinated C57BL/6 mice exhibit elevated antibody levels compared to the LVS vaccinated mice following SchuS4 challenge
Given that sodBFt vaccinated mice are better protected against a lethal SchuS4 challenge than the LVS vaccinated mice, it was next investigated whether this improved protection was due to differences in the antibody responses of these animals. It was observed that both sodBFt and LVS vaccinated mice had similar levels of IgM, IgA, IgG1, IgG2a, and IgG2b antibody levels prior to challenge with SchuS4. However, sodBFt vaccinated mice exhibited significantly elevated levels of IgA, IgG2b and IgG1 levels at day 14 post-challenge compared to the LVS vaccinated mice (Fig. 5A). The results demonstrate that vaccination with sodBFt induces a potent humoral immune response in the vaccinated C57BL/6 mice following SchuS4 challenge. The results also indicate that in addition to a potent Th1 humoral immune response, significant proportion of Th2 type antibodies are also produced in the sodBFt vaccinated mice following SchuS4 challenge. Additionally, the IgA levels in the lung homogenates of both the LVS and sodBFt vaccinated mice rose steadily at days 10 and 14 following SchuS4 challenge, however no significant differences were observed between the two vaccinated groups (Fig. 5B).
Figure 5. SodBFt vaccinated C57BL/6 mice exhibit elevated levels of anti- F. tularensis specific antibodies following SchuS4 challenge.
(A) Anti-F. tularensis antibody isotypes were determined by ELISA in sera, and (B) IgA levels in the lung homogenates from LVS or sodBFt vaccinated and SchuS4 challenged mice at the indicated times. The data represent an average of 3–4 mice per group. P values were determined using ANOVA. ** p < 0.01; *** p < 0.001. Ψ All the LVS vaccinated mice died by day 15–17 post-challenge and hence were unavailable for comparisons.
SodBFt vaccination mediated protection requires both CD4 and CD8 T cells
In addition to the differences observed in antibody responses between LVS and sodBFt vaccinated mice, it was examined next if cellular responses also were critical for providing protection against SchuS4 challenge in the sodBFt vaccinated mice. CD4−/− and CD8−/− mice vaccinated with LVS or sodBFt were challenged with 111 CFU of SchuS4. No increased susceptibility was observed in the unvaccinated CD4−/− or CD8−/− mice compared to the wild type mice. However, all the mice vaccinated with sodBFt succumbed to infection similar to LVS with a small extended MTD which was not statistically significant (Table 4). Thus, the results demonstrate that in the absence of CD4 and CD8 T cells, partial protection against SchuS4 is lost suggesting that both CD4 and CD8 T cells are required to mediate the protection in sodBFt vaccinated mice.
Table 4.
Survival of C57BL/6CD4−/− and CD8−/− mice vaccinated with sodBFt and LVS following SchuS4 challenge.
| Mouse Strain | Vaccinationa | Dose (CFU) | SchuS4 Challenge Dose (CFU)c | Median Time to Death (Days) | No. of Mice Survived/ No. of mice Challenged (% Survival)d | |
|---|---|---|---|---|---|---|
| Primary | Boosterb | |||||
| CD4−/− | Unvaccinated | None | None | 111 | 7 | 0/6 (0) |
| C57BL/6 | Unvaccinated | None | None | 111 | 6 | 0/6 (0) |
| CD4−/− | LVS | 5.33×102 | 1.17×103 | 111 | 14.5e | 0/6 (0) |
| CD4−/− | sodBFt | 5.29×102 | 1.44×103 | 111 | 16.5f | 0/6 (0) |
| CD8−/− | Unvaccinated | None | None | 111 | 6 | 0/6 (0) |
| C57BL/6 | Unvaccinated | None | None | 111 | 6 | 0/6 (0) |
| CD8−/− | LVS | 5.33×102 | 1.17×103 | 111 | 12g | 0/6 (0) |
| CD8−/− | sodBFt | 5.29×102 | 1.44×103 | 111 | 14h | 0/6 (0) |
Mice were vaccinated i.n.
21 days after primary immunization.
42 days after primary immunization.
Mice were monitored for morbidity and mortality for a period of 21–30 days post-challenge.
Significantly different from unvaccinated mice (P<0.05).
Significantly different from unvaccinated mice (P<0.01).
Significantly different from unvaccinated mice (P<0.01).
Significantly different from unvaccinated mice (P<0.01).
Data were analyzed using Log-rank test.
SodBFt exhibit upregulation of stress related proteins
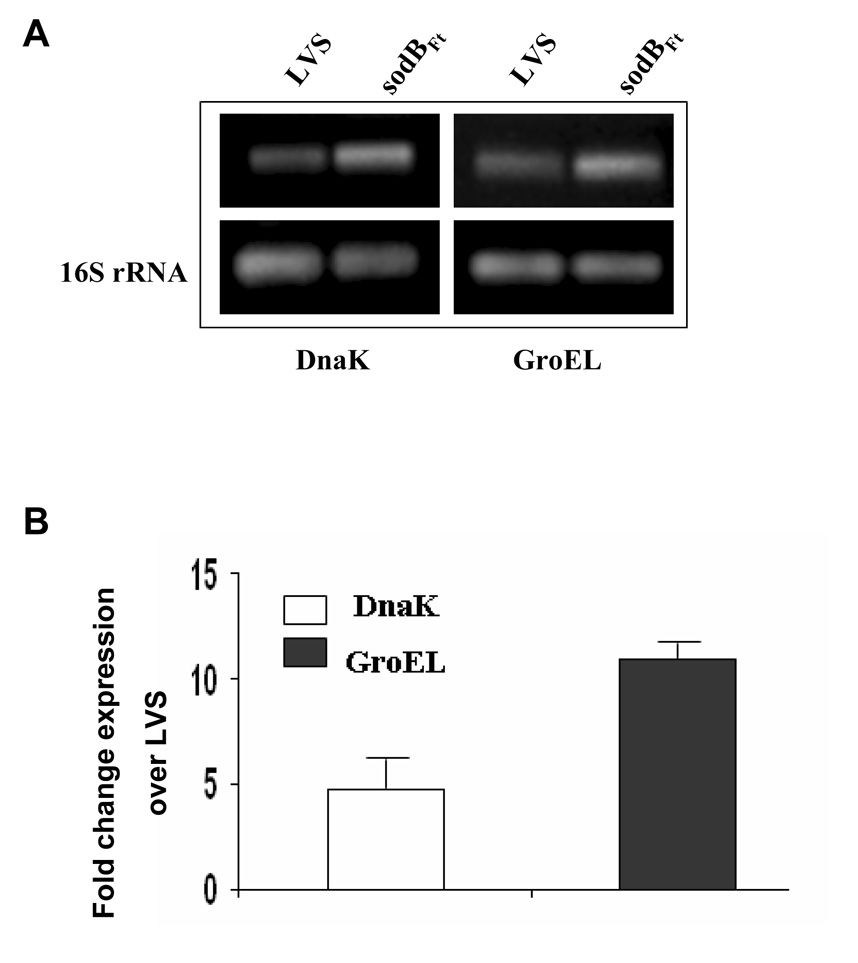
It has previously been reported that exposure of bacteria to oxidative stress leads to the induction of several proteins including highly immunogenic heat shock proteins [22;23]. We hypothesized that oxidative stress due to lowered expression of FeSOD may lead to upregulation of several immunogenic proteins in sodBFt and thus make it a better vaccine than LVS. Microarray analysis revealed increased transcription of several genes out of which DnaK, GroEL and Bfr were prominently upregulated in sodBFt as compared to LVS (Table 5). RT-PCR and qRT-PCR analysis further confirmed upregulation DnaK and GroEL in sodBFt as compared to LVS (Fig. 6A and B). The increased transcript levels also correlated well with the differences in the expression of both the DnaK and GroEL proteins in sodBFt mutant when analyzed by 2-dimensional gel electrophoresis (not shown).
Table 5.
Microarray analysis for differential expression of stress related genes in sodBFt mutant over LVS.
| Locus | Gene | Protein | Fold Change over LVSa |
|---|---|---|---|
| FTL_1191 | DnaK | Chaperone protein (Heat shock protein family 70 protein) | 2.98 (P<0.001)b |
| FTL_1714 | GroEL | Chaperone protein | 2.40 (P<0.001) |
| FTL_1715 | GroES | Chaperone protein | 2.04 (P<0.001) |
| FTL_0617 | Bfr | Bacteroferritin | 2.447 (P<0.001) |
| FTL_0359 | PulG | Type IV pili fiber building block protein | 2.15 (P<0.001) |
| FTL_1303 | rpmE | 50S Ribosomal protein L31 | 1.647 (P<0.01) |
Results represent cumulative values from four replicate RNA samples prepared under identical conditions and are representative of two experiments conducted with identical results.
The P values were adjusted for multiple testing using the Benjamini and Hochberg method within Limma. P values of <0.05 were considered differentially regulated.
Figure 6. SodBFt exhibits increased levels of DnaK and GroEL as compared to F. tularensis LVS.
(A) RT-PCR analysis to determine the transcript levels of DnaK and GroEL (B). qRT-PCR for the quantitation of DnaK and GroEL transcripts. The results are representative of three experiments conducted.
Elevated levels of antibodies are generated against stress proteins in the sodBFt vaccinated mice challenged with SchuS4
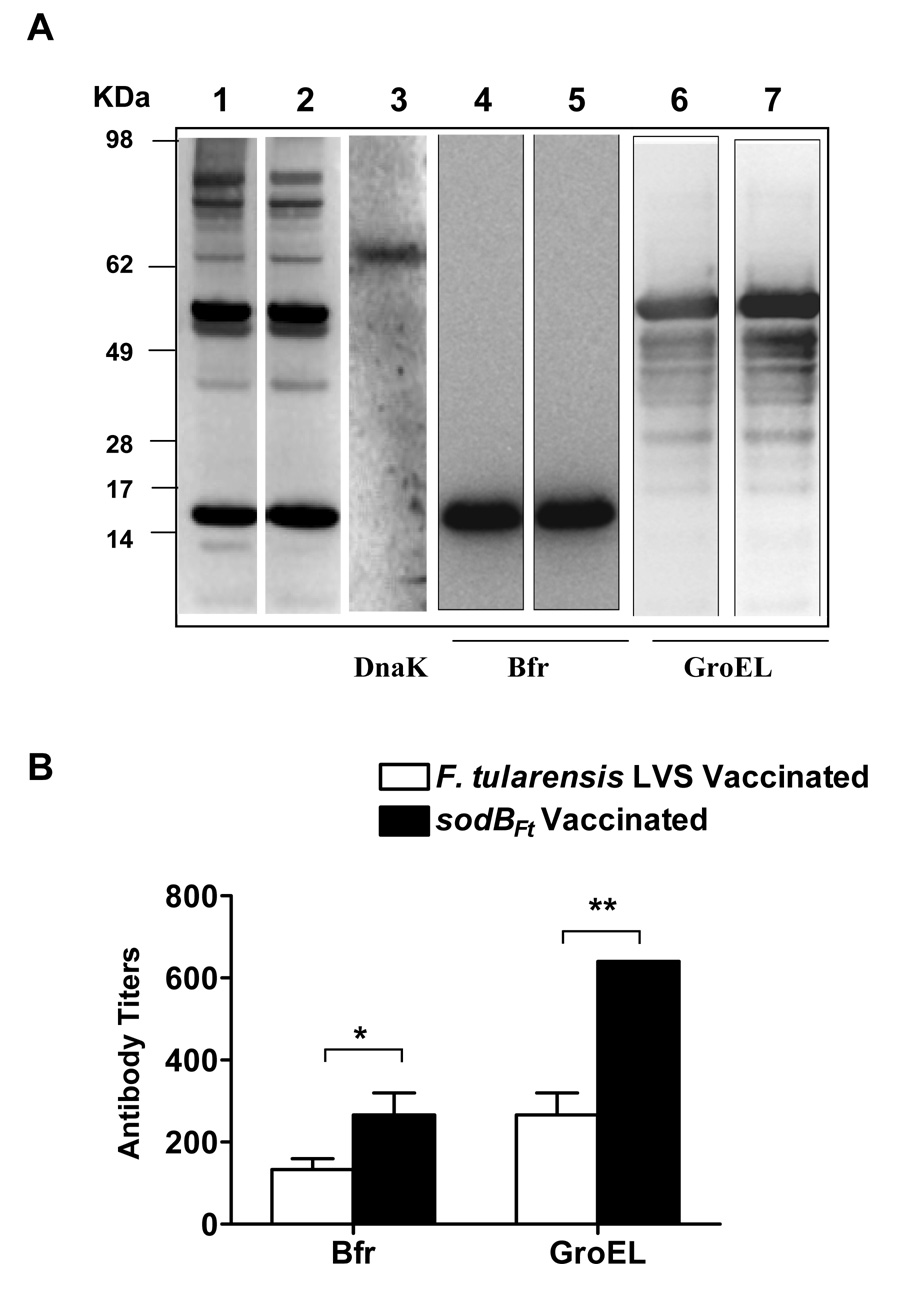
We next assessed production of antibodies against the immunogenic stress proteins GroEL, Bfr and DnaK in the LVS or sodBFt vaccinated mice following SchuS4 challenge. Probing of SchuS4 lysates with the pooled serum collected at day 14 post-SchuS4 challenge revealed generation of antibodies against several immunogenic proteins in both the groups of vaccinated mice (Fig. 7A; lanes 1 and 2). Strong antibody responses were generated against ~17 and ~59 kDa proteins. These proteins were identified as Bfr and GroEL respectively, by probing purified recombinant Bfr and GroEL proteins with the pooled serum from challenged mice (Figure 7A; lanes 4–7). In contrast, a weak antibody response observed against a protein of ~70 kDa in both the LVS and sodBFt vaccinated and SchuS4 challenged mice was identified as DnaK by probing the SchuS4 lysate with anti-F. tularensis DnaK monoclonal antibodies (Fig. 7A; lane 3). The results show that antibodies are generated against the immunogenic stress proteins GroEL and Bfr in the vaccinated mice, however similar to earlier report [24], our results also show that C57BL/6 mice generate a weak antibody response against DnaK.
Figure 7. Determination of antibody responses against stress proteins GroEL, DnaK and Bfr in LVS or sodBFt vaccinated mice at day- 14 post-SchuS4 challenge.
(A). The western blot analysis was performed as described in the materials and methods. The SchuS4 lysates were probed with pooled sera (n=3 mice) from LVS vaccinated mice (Lane 1), or sodBFt vaccinated mice (Lane 2) collected at day- 14 post-schuS4 challenge. The blots were stripped and reprobed with anti-DnaK antibodies to identify DnaK protein (Lane 3). For determination of antibody responses against Bfr and GroEL, purified recombinant proteins were probed with day- 14 post- SchuS4 challenge serum from LVS vaccinated (Lanes 4 and 6) and sodBFt vaccinated mice (Lanes 5 and 7), respectively. The protein bands observed for Bfr (Lanes 4–5), GroEL (Lane 6–7) and DnaK (Lane 3) corresponds to ~17 kDa, ~59 kDa and ~70 kDa bands in the SchuS4 lysates (Lane 1 and 2). (B). Quantitation of anti- Bfr and GroEL antibodies in day- 14 post- SchuS4 challenge serum from LVS and sodBFt vaccinated mice by ELISA. The data are expressed as mean ± S.D. (n=3 mice per group). P values were determined using Student’s t- test. * p ≤ 0.05; ** p < 0.01.
The strong antibody responses observed against Bfr and GroEL in the vaccinated mice at day 14 post-SchuS4 challenge by western blot analysis were further quantitated by ELISA. It was observed that levels of anti-Bfr and GroEL antibodies were significantly higher in the sodBFt vaccinated mice at day 14 post-SchuS4 challenge than their LVS vaccinated counterparts (Fig. 7B). These results demonstrate that sodBFt vaccinated mice generate a potent antibody response against the immunogenic stress proteins.
Discussion
A mutant of F. tularensis with attenuated virulence and enhanced protective efficacy should be a safer and more effective vaccine candidate than the original LVS. Several mutants of F. tularensis exhibit an attenuated virulence in mice, but have been inefficient in inducing a protective immune response against the virulent SchuS4 strain. Deletion mutants of both the SchuS4 and LVS in the Francisella pathogenicity island (FPI) protein IglC are avirulent but fail to confer protection against challenge with the virulent strains [25]. Although, several other attenuated mutants of Francisella lacking FPI proteins MglA and PdpB [26], an orphan response regulator gene [27], the O-antigen polysaccharide [28;29], and the auxotrophic mutants [30;31] are effective in providing protection against the parental strains, they are either incapable or their ability to provide protection against challenge with SchuS4 has not been assessed. The only mutant that has been shown to induce protection against a systemic or aerosol challenge with the virulent type A strain to-date is the FTT0918 deletion mutant of SchuS4 [25]. The present study describes an improved live vaccine candidate.
Mice serve as a useful model to screen F. tularensis vaccine candidates [32]. Earlier studies have shown that susceptibility to F. tularensis infection in mice varies from strain to strain [8;33]. C57BL/6 mice are moderately susceptible to LVS but exhibit high susceptibility to the SchuS4 and other virulent type A strains of F. tularensis [33;34]. It has been reported that BALB/c but not the C57BL/6 mice immunized with LVS are protected against challenge with the virulent type A strains of F. tularensis [7;15;33]. In agreement with these observations, our initial studies showed that sodBFt vaccination in BALB/c mice provides 100% protection against a lethal 100LD100 challenge of SchuS4 suggesting that despite being attenuated for virulence, sodBFt retains its antigenic properties (not shown). In the present study C57BL/6 mice were used for testing the protective properties of sodBFt mutant and its potential as a vaccine candidate against experimental SchuS4 infection. Based on the ability of i.n. route of vaccination to confer optimal protection against respiratory tularemia and the possibility of aerosols of F. tularensis being used in a bioterrorist attack, i.n. vaccination and challenge route was preferred over other routes of inoculation [20].
Our data demonstrates that a single or a low dose sodBFt vaccination followed by a booster were sufficient to induce a partial protective immune response in the vaccinated mice. Boosting mice with sodBFt but not LVS significantly increased their resistance to a ~100LD100 dose of SchuS4. In addition, vaccination with sodBFt induced a long lasting immunity against a ~100LD100 challenge dose of LVS. In contrast, the protective response in sodBFt immunized C57BL/6 mice was lost against the highly virulent SchuS4 strain after a long-term challenge, suggesting a need for repeated immunizations for the maintenance of immunity against SchuS4.
The extremely high virulence of SchuS4 may be attributed to its rapid rate of replication and systemic dissemination that leads to extensive damage to the liver and spleen. It has been proposed that systemic dissemination and replication of bacteria rather than the initial pulmonary infection is the major cause of death in the infected mice [15]. Even though dissemination of SchuS4 occurred at similar rates, the sodBFt vaccinated mice exhibited a better control of bacterial replication in the liver and spleen than the LVS vaccinated counterparts. The controlled bacterial replication in sodBFt vaccinated mice also was reflected in the extent of tissue damage observed in the liver and spleen as the severity of the lesions did not reach to the levels that observed in unvaccinated or LVS vaccinated mice.
Exaggerated production of pro-inflammatory cytokines especially MCP-1 and IL-6 cause sepsis and organ failure [21;35]. There is evidence that the pattern of cytokines produced in the lungs during Francisella infection changes over time and correlates with the type and magnitude of tissue injury [19]. In addition, levels of TNF-α and IFN-γ together with IL-6 and MCP-1 serves as a “cytokine code” that determines the outcome of the F. tularensis infection in mice [16;20]. In this study, it was noted that the SchuS4 challenge in the LVS vaccinated mice produced progressive disease associated with higher levels of pro-inflammatory cytokines. In contrast, sodBFt vaccinated mice induced a lower but regulated increase in the levels of these cytokines. It appears that a delayed exaggerated cytokine response observed in the LVS vaccinated mice might have been the result of damage to the host tissues. Similar to our findings, Chiavolini et al [21] also have shown significantly elevated levels of pro-inflammatory cytokines in moribund as compared to those mice that survive the Francisella infection. Our data also indicates that immune response in the vaccinated mice following SchuS4 challenge does not get skewed towards Th2 type, as no detectable levels of IL-4 or IL-5 were observed.
It has been demonstrated for several intracellular pathogens that greater protection is achieved only when a vaccination strategy that can evoke both cell-mediated and humoral immunity is employed [36]. IgA, IgG2a and IgG2b subtypes have been shown to be required for protection against F. tularensis [20;37;38]. Our data demonstrates that sodBFt vaccination induced a potent humoral immune response following SchuS4 challenge. The sodBFt vaccinated mice had significantly higher levels of F. tularensis specific total antibodies at day 14 post-challenge than the LVS vaccinated mice (not shown). The higher levels IgA, IgG2a and IgG2b antibody subtypes in sodBFt vaccinated mice following SchuS4 challenge correlated well with the enhanced bacterial clearance and protective immunity observed in this group of mice. In addition, IgG1 levels were also found to be elevated in the sodBFt vaccinated group of mice following SchuS4 challenge. The role of IgG1 in providing protection against SchuS4 is not known and need further investigations.
Although antibodies alone are shown to be effective in the control of infection with LVS [39–43], protection against SchuS4 require both humoral and cellular immune responses [15;44]. We observed that protection following sodBFt vaccination specifically required both CD4 and CD8 T cells, a finding consistent with previous reports that depletion of these cell types in the LVS vaccinated BALB/c mice results in loss of protection against type A Francisella strains [5;7]. Based on these observations, it is tempting to speculate that sodBFt vaccination results in the expansion of CD4 and CD8 T cells that recognize antigens expressed in abundance on sodBFt in addition to those shared by LVS and are required for protection against SchuS4 challenge. Collectively, data suggests that vaccination with sodBFt generates a better cell-mediated immune response which in conjunction with antibody mediated immune response provides an effective bacterial clearance mechanism in the sodBFt vaccinated mice.
Bacterial proteins expressed in response to heat shock and oxidative stress have been demonstrated to play an important role in the induction of a protective humoral and cell mediated immune response [23;45;46]. Evidences suggest that loss of a key antioxidant gene might in turn elevate the expression of other stress response genes through redox-sensitive transcription machinery [47]. We observed that oxidative stress in the sodBFt mutant leads to increased expression of several immunogenic stress proteins and a potent antibody response was generated against Bfr and GroEL proteins in the sodBFt vaccinated mice. Francisella produce these conserved prokaryotic proteins in abundance on exposure to heat and hydrogen peroxide [23;48]. These proteins are highly immunogenic in mammals and have strong T cell stimulatory properties [23;49;50]. It has been shown that following infection with F. tularensis, GroEL is released into the cytosol, processed and presented by the macrophages resulting in the proliferation of CD4 and CD8 T-cell [51]. However, it has also been demonstrated that seroconversion of these T cell antigens is not a correlate of protection, as these proteins are also recognized by antibodies from unprotected vaccinated mice [24;52;53]. These findings are in 549 concurrence with our observations in the LVS vaccinated mice. Although, detailed studies are currently underway to understand the mechanism of protection in the sodBFt vaccinated mice, upregulation of immunodominant proteins in the sodBFt mutant may offer an explanation for improved protection observed in mice vaccinated with sodBFt.
To our knowledge this is the first report that demonstrates i.n. vaccination with an attenuated mutant of LVS reproducibly protects C57BL/6 mice against i.n. SchuS4 challenge. Although only a partial protection was observed in the present study, the level of protection may further be improved by the use of adjuvants in combination with sodBFt vaccination. Additionally, levels of protection may further be improved by using an attenuated mutant generated on a SchuS4 background and sodB gene appears to be a most suitable target for achieving such a goal.
Acknowledgements
Excellent technical support was provided by Michelle Wyland-O’Brien, Sally Catlett, Bradley Fairchild, Seth D Caldon and Yili Lin of Center for Immunology and Microbial Disease, Albany Medical College, and Huaixin Zheng from SUNY Stony Brook, NY. We also thank Dr Daniel Clemens from University of California, Los Angeles, CA for providing recombinant GroEL and 566 Bfr proteins and Dr A. G. Savitt from SUNY Stony Brook, NY for providing anti-DnaK monoclonal antibodies. This work was supported by National Institutes of Health grant P01 AI056320.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004 Dec;2(12):967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 2.Saslaw S, Carlisle HN. Studies with tularemia vaccines in volunteers. IV. Brucella aggiutinins in vaccinated and nonvaccinated volunteers challenged with Pasteurella tularensis. Am J Med Sci. 1961 Aug;242:166–172. doi: 10.1097/00000441-196108000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Microbiol Mol Biol Rev. 1966 Sep 1;30(3):532–538. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eigelsbach HT, Hunter DH, Janssen WA, Dangerfield HG, Rabinowitz SG. Murine model for study of cell-mediated immunity: protection against death from fully virulent Francisella tularensis infection. Infect Immun. 1975 Nov;12(5):999–1005. doi: 10.1128/iai.12.5.999-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan JW. Vaccines against Francisella tularensis--past, present and future. Expert Rev Vaccines. 2004 Jun;3(3):307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- 7.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005 May;73(5):2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Chen W, Conlan JW. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine. 2004 Jun 2;22(17–18):2116–2121. doi: 10.1016/j.vaccine.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.KuoLee R, Harris G, Conlan JW, Chen W. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis. Vaccine. 2007 May 10;25(19):3781–3791. doi: 10.1016/j.vaccine.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sansone A, Watson PR, Wallis TS, Langford PR, Kroll JS. The role of two periplasmic copper- and zinc-cofactored superoxide dismutases in the virulence of Salmonella choleraesuis. Microbiology. 2002 Mar 1;148(3):719–726. doi: 10.1099/00221287-148-3-719. [DOI] [PubMed] [Google Scholar]
- 11.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003 Oct 1;149(10):2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 12.Larsson P, Oyston PC, Chain P, et al. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005 Feb;37(2):153–159. doi: 10.1038/ng1499. [DOI] [PubMed] [Google Scholar]
- 13.Bakshi CS, Malik M, Regan K, et al. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol. 2006 Sep;188(17):6443–6448. doi: 10.1128/JB.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil H, Platz GJ, Forestal CA, et al. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proc Natl Acad Sci U S A. 2006 Aug 22;103(34):12897–12902. doi: 10.1073/pnas.0602582103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wayne CJ, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005 Mar 31;23(19):2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006 Jun;74(6):3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentleman R, Carey V, Bates D, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth GK. Linear models and empirical Bytes methods for assessing differential expression in microarray experiments. Statistical Application in Genetics and Molecular Biology. 2008;3(1) doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 19.Malik M, Bakshi CS, McCabe K, et al. Matrix Metalloproteinase 9 Activity Enhances Host Susceptibility to Pulmonary Infection with Type A and B Strains of Francisella tularensis. J Immunol. 2007 Jan 15;178(2):1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 20.Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis Live Vaccine Strain Protects against Respiratory Tularemia by Intranasal Vaccination in an Immunoglobulin A-Dependent Fashion. Infect Immun. 2007 May 1;75(5):2152–2162. doi: 10.1128/IAI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiavolini D, Alroy J, King CA, et al. Identification of Immunologic and Pathologic Parameters of Death versus Survival in Respiratory Tularemia. Infect Immun. 2008 Feb 1;76(2):486–496. doi: 10.1128/IAI.00862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen Peroxide-Inducible Proteins in Salmonella typhimurium Overlap with Heat Shock and Other Stress Proteins. PNAS. 1986 Nov 1;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ericsson M, Tarnvik A, Kuoppa K, Sandstrom G, Sjostedt A. Increased synthesis of DnaK,GroEL, and GroES homologs by Francisella tularensis LVS in response to heat and hydrogen peroxide. Infect Immun. 1994 Jan;62(1):178–183. doi: 10.1128/iai.62.1.178-183.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twine SM, Petit MD, Shen H, Mykytczuk NC, Kelly JF, Conlan JW. Immunoproteomic analysis of the murine antibody response to successful and failed immunization with live anti-Francisella vaccines. Biochem Biophys Res Commun. 2006 Aug 4;346(3):999–1008. doi: 10.1016/j.bbrc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Twine S, Bystrom M, Chen W, et al. A mutant of Francisella tularensis strain SCHU S4lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005 Dec;73(12):8345–8352. doi: 10.1128/IAI.73.12.8345-8352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tempel R, Lai XH, Crosa L, Kozlowicz B, Heffron F. Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect Immun. 2006 Sep;74(9):5095–5105. doi: 10.1128/IAI.00598-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohapatra NP, Soni S, Bell BL, et al. Identification of an orphan response regulator required for Francisella virulence and transcription of pathogenicity island genes. Infect Immun. 2007 Apr 23; doi: 10.1128/IAI.00351-07. IAI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastian S, Dillon ST, Lynch JG, et al. A Defined O-Antigen Polysaccharide Mutant of Francisella tularensis Live Vaccine Strain Has Attenuated Virulence while Retaining Its Protective Capacity. Infect Immun. 2007 May 1;75(5):2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raynaud C, Meibom KL, Lety MA, et al. Role of the wbt Locus of Francisella tularensis in Lipopolysaccharide O-Antigen Biogenesis and Pathogenicity. Infect Immun. 2007 Jan 1;75(1):536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quarry JE, Isherwood KE, Michell SL, Diaper H, Titball RW, Oyston PCF. A Francisella tularensis subspecies novicida purF mutant, but not a purA mutant, induces protective immunity to tularemia in mice. Vaccine. 2007 Mar 1;25(11):2011–2018. doi: 10.1016/j.vaccine.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Pechous R, Celli J, Penoske R, Hayes SF, Frank DW, Zahrt TC. Construction and characterization of an attenuated purine auxotroph in a Francisella tularensis live vaccine strain. Infect Immun. 2006 Aug;74(8):4452–4461. doi: 10.1128/IAI.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons R, Wu T. Animal Models of Francisella tularensis Infection. Ann NY Acad Sci. 2007 Mar;29 doi: 10.1196/annals.1409.003. annals. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Shen H, Webb A, Kuolee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003 Sep 8;21(25–26):3690–3700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005 May;73(5):2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulloa L, Tracey KJ. The [`]cytokine profile': a code for sepsis. Trends in Molecular Medicine. 2005 Feb;11(2):56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Healey GD, Elvin SJ, Morton M, Williamson ED. Humoral and Cell-Mediated Adaptive Immune Responses Are Required for Protection against Burkholderia pseudomallei Challenge and Bacterial Clearance Postinfection. Infect Immun. 2005 Sep 1;73(9):5945–5951. doi: 10.1128/IAI.73.9.5945-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyles JE, Unal B, et al. Immunodiminant Francisella tularensis antigens identified using proteome microarray. Proteomics. 2007;7 doi: 10.1002/pmic.200600985. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Rawool DB, Bitsaktsis C, Li Y, et al. Utilization of Fc Receptors as a Mucosal Vaccine Strategy against an Intracellular Bacterium, Francisella tularensis. J Immunol. 2008 Apr 15;180(8):5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenmark S, Lindgren H, Tarnvik A, Sjostedt A. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003 Aug;35(2):73–80. doi: 10.1016/s0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 40.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994 Aug;308(2):83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001 Aug 14;19(31):4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 42.Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006 Apr;74(4):2063–2071. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007 Jul 1;179(1):532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 44.Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002 Oct 4;20(29–30):3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 45.Twine SM, Mykytczuk NC, Petit MD, et al. In vivo proteomic analysis of the intracellular bacterial pathogen, Francisella tularensis, isolated from mouse spleen. Biochem Biophys Res Commun. 2006 Jul 14;345(4):1621–1633. doi: 10.1016/j.bbrc.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz MA, Lee BE, Dillon BJ, Harth G. Protective Immunity Against Tuberculosis Induced by Vaccination with Major Extracellular Proteins of Mycobacterium tuberculosis. PNAS. 1995 Feb 28;92(5):1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christman MF, Storz G, Ames BN. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A. 1989;86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenco J, Pavkova I, Hubalek M, Stulik J. Insights into the oxidative stress response in Francisella tularensis LVS and its mutant [Delta]iglC1 + 2 by proteomics analysis. FEMS Microbiology Letters. 2005 May 1;246(1):47–54. doi: 10.1016/j.femsle.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 49.Ericsson M, Kroca M, Johansson T, Sjostedt A, Tarnvik A. Long-lasting recall response of CD4+ and CD8+ alphabeta T cells, but not gammadelta T cells, to heat shock proteins of francisella tularensis. Scand J Infect Dis. 2001;33(2):145–152. doi: 10.1080/003655401750065562. [DOI] [PubMed] [Google Scholar]
- 50.Golovliov I, Ericsson M, Sandstrom G, Tarnvik A, Sjostedt A. Identification of proteins of Francisella tularensis induced during growth in macrophages and cloning of the gene encoding a prominently induced 23-kilodalton protein. Infect Immun. 1997 Jun;65(6):2183–2189. doi: 10.1128/iai.65.6.2183-2189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BY, Horwitz MA, Clemens DL. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect Immun. 2006 Jul;74(7):4002–4013. doi: 10.1128/IAI.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Havlasova J, Hernychova L, Brychta M, et al. Proteomic analysis of anti-Francisella tularensis LVS antibody response in murine model of tularemia. Proteomics. 2005 May;5(8):2090–2103. doi: 10.1002/pmic.200401123. [DOI] [PubMed] [Google Scholar]
- 53.Havlasova J, Hernychova L, Halada P, et al. Mapping of immunoreactive antigens of Francisella tularensis live vaccine strain. Proteomics. 2002 Jul;2(7):857–867. doi: 10.1002/1615-9861(200207)2:7<857::AID-PROT857>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]