Abstract
Brevetoxin-3 was shown previously to adversely affect central auditory function in goldfish. The present study evaluated the effects of exposure to this agent on cochlear function in mice using the 2f1-f2 distortion-product otoacoustic emission (DPOAE). Towards this end, inbred CBA/CaJ mice were exposed to a relatively high concentration of brevetoxin-3 (∼400 μg/m3) by nose-only inhalation for a 2-h period. Further, a subset of these mice received a second exposure a day later that lasted for an additional 4 h. Mice exposed only once for 2 h did not exhibit any notable cochlear effects. Similarly, mice exposed two times, for a cumulative dose of 6 h, exhibited essentially no change in DPOAE levels.
Keywords: Brevetoxin, CBA/CaJ mice, Distortion-product otoacoustic emissions, Cochlear function
Introduction
Brevetoxins are potent neurotoxins produced by the marine alga, Karenia brevis. K. breve blooms cause the red tides that frequently occur in the Gulf of Mexico and along the southeastern Atlantic coast. Although the biochemical and neurophysiologic actions of the brevetoxins in regard to neurotoxic shellfish poisoning (NSP) are well documented (Baden 1989), little is known of the human health effects from environmental exposures that occur when brevetoxins are aerosolized by the wind and surf, as occurs during onshore winds. Concentrations of marine aerosols of brevetoxins-2 and 3 on affected beaches have been reported in the range of 10–100 ng/m3 (Backer et al. 2003; Pierce et al. 2003). Recently, Backer et al. (2003) documented respiratory-tract symptoms associated with recreational exposures to brevetoxins during a typical red tide. Specifically, significant increases in eye and throat irritations, coughing, and chest tightness were reported by individuals exposed to <10–36 ng brevetoxin/m3. In addition, significant increases in nasal congestion and wheezing were described by individuals exposed to 20–93 ng brevetoxin/m3.
To provide evidence that such health effects are attributable to the aerosolized brevetoxin, a sensitive, rapid, non-invasive biomarker of brevetoxin exposure and its after-effects is needed in humans. Methods such as ELISA (Naar et al. 2002) and immunohistochemistry of nasal epithelial cells (Bossart et al. 1998) have been used effectively in experimental models, but, to date, these methods have not been successful in humans. In part, this may reflect the significantly lower concentrations of brevetoxins in humans exposed under natural conditions, than in experimental exposures in laboratory animals (Fleming et al. 2002).
Recently, brevetoxin-3 was reported to transiently reduce auditory functioning in goldfish administered acute, sublethal doses by intraperitoneal injection (Lu and Tomchik 2002). These findings in the fish model using auditory brainstem response (ABR) measures suggest that brevetoxin-3 may potentially affect human hearing. It has long been known that the outer hair cell (OHC) class of auditory receptor located in the mammalian cochlea is particularly sensitive to the effects of ototoxic compounds including aminoglycoside antibiotics and platinum-based anti-tumor agents, which preferentially destroy these sensory cells (e.g., Stavroulaki et al. 2001; Hilton et al. 2002). Histopathologically, the initial loss of OHCs occurs in the basal region of the cochlea, which functionally corresponds to a high-frequency hearing loss.
The harmful effects of ototoxins on cochlear function can be measured rapidly and non-invasively in humans and laboratory animals by using distortion-product otoacoustic emissions (DPOAEs), which reflect the normal electromotile microvibrations of intact OHCs (Brownell 1990). Since the brevetoxins are rapidly distributed throughout the body following administration by a variety of routes (Poli et al. 1990; Cattet and Gerachi 1993; Benson et al. 1999), we hypothesized that brevetoxin-3, which was ototoxic in goldfish, would reduce or eliminate the OHC activity of a mammalian species like the mouse. The selection of mice as experimental subjects was based on the knowledge that the mouse is rapidly becoming the biomedical experimental model of choice, because of the great availability of genetic information and the ease of using powerful molecular methodologies on mice. Thus, the present study used DPOAEs to assess whether aerosolized brevetoxin-3 adversely affected OHC activity in a mouse model of normal cochlear function.
Materials and methods
Animals
Ten female CBA/CaJ mice, approximately 9 to 11-weeks old, were obtained commercially (Jackson Laboratories, Bar Harbor, ME, USA). CBA/CaJ mice were selected, because they are commonly used in hearing research as an experimental model of normal cochlear function (e.g., Jimenez et al. 1999). Additionally, female mice were used, because they are much less aggressive in contrast to their male counterparts. Mice were housed under standard vivarium conditions in shoebox cages with hardwood-chip bedding. Food (Teklad 8604) and water were available ad libitum, except during the brevetoxin-exposure intervals. Animal rooms were maintained at ∼22°C, with a 12-h light/dark cycle. Prior to exposure, the mice were conditioned to nose-only restraint tubes for 0.5, 1, and 2 h, over a 3-day period, respectively. The study protocol was approved by the Lovelace Respiratory Research Institute (LRRI) Institutional Animal Care and Use Committee.
Aerosol generation and characterization of brevetoxin-3
Brevetoxins-2 and 3 were isolated and purified from K. breve cultures at the Center for Marine Sciences at the University of North Carolina in Wilmington. Stock solutions of brevetoxin-3 were prepared in 100% ethanol (0.5 mg/ml). Generator solutions were prepared daily by diluting stock solutions with 0.9% saline to achieve a final concentration of 0.15 mg brevetoxin-3/ml. Aerosols of this solution were generated by nebulization (Hospitak Inc, Farmingdale, NY, USA), with the resulting aerosol being diluted appropriately with air, dried, and delivered to a 36-port nose-only inhalation chamber (InTox Products, Edgewood, NM, USA). Flow rate through the chamber was 10 l/min, and O2 concentration and temperature within the chamber were monitored continuously.
Total aerosol concentration was determined gravimetrically. Specifically, throughout the exposure period, aerosol was collected every 30 min from the animal's breathing zone onto 25-mm filters (Zeflour, SKC Gulf Coast, Houston, TX, USA) at a flow rate of 2 l/min. The brevetoxin-3 concentration was estimated from the gravimetric results based on its fraction of the total mass (0.0164) in the generator solution. The stability of the aerosol concentration during the exposure was monitored using a real-time aerosol monitor (DustTrak, TSI Industries, Shoreview, MN, USA). The particle size distribution of the brevetoxin-3 aerosol was determined by a cascade impactor.
Brevetoxin-3 concentration in the exposure atmosphere was verified by a liquid chromatography/mass spectrometry analysis performed on a subset of filter samples. Sample filters and filters used to prepare the brevetoxin-3 standard curve were spiked with 500 ng brevetoxin-2 as an internal standard and extracted with 5 ml acetone. Extracts were concentrated to 500 μl under a stream of N2 and then diluted to a final volume of 1 ml with a methanol:water (50:50) solution. Samples were injected onto a high-performance liquid chromatograph (10ADVP HPLC, Shimadzu Company, Kyoto, Japan) equipped with a 75×2 mm analytic column (Aqua 3 μm C18, Phenomenex USA, Torrence, CA, USA). Compounds were eluted using a methanol–water mobile phase containing 1 mM ammonium acetate. The eluant was directed into an electrospray mass spectrometer (API 365, Applied Biosystems, Foster City, CA, USA), which was monitored for ion pairs consisting of 895.559/877.550 (brevetoxin-2) and 897.590/725.404 (brevetoxin-3).
To determine the concentration of ethanol vapor in the exposure atmosphere, a sample of the control-chamber exposure atmosphere was collected into a Tedlar bag obtained using a rigid air-sample box (Vac-U Chamber, SKC Inc, Eighty Four PA, USA). A glass fiber filter was placed in line to remove particles from the sample before it entered the sampling bag. The ethanol concentration in the sample was measured by gas chromatography with flame ionization (GC-17A/FID, Shimadzu Scientific Instruments, Columbia, MD, USA) using a 30×0.32 mm (5-μm film width) analytic column (Rtx-1, Restek Corporation, Bellefonte, PA, USA). The chromatograph oven was operated isothermally at 50°C, while the injector and detector temperatures were 200°C. A five-point ethanol vapor standard curve was used over a concentration range of 1.16 to 9.4 g/m3.
DPOAE Measurements
The 2f1–f2 DPOAE measurements were performed as previously described for mice (Jimenez et al. 1999; Vazquez et al. 2001, 2004). Briefly, DPOAEs were produced by the simultaneous presentation of two pure tones at the f1 and f2 primary frequencies (f2/f1 ratio =1.25) and levels (L1,L2) specified below. The primary tones were generated by a dual-channel synthesizer (3326A, Hewlett-Packard, Palo Alto, CA, USA) and attenuated under the control of a personal computer system using customized software. The two primary tones were presented over separate ear speakers (Realistic Dual Radial Horn Tweeters, Tandy Corp, Dallas, TX, USA) and delivered through a commercially available acoustic probe incorporating a calibrated microphone assembly (ER-10B+, Etymotic Research, Elk Grove, IL, USA) that was inserted snugly into the outer ear canal. The f1 and f2 primary tones were allowed to mix acoustically in the ear canal to avoid artifactual distortion.
The primary measure was in the form of a DP-gram, i.e., DPOAE level as a function of test frequency, which was obtained for geometric-mean [GM=(f1× f2)0.5] frequencies ranging from 5.6 kHz to 48.5 kHz (f2=6.3–54.2 kHz), in 0.1-octave intervals, at three primary-tone levels (L1=L2=55, 65, 75 dB SPL). To collect a DP-gram, ear-canal sound pressure was sampled and synchronously averaged for a series of ascending test frequencies using two approaches. For the lower GM frequencies ranging from 5.6 kHz to 19.7 kHz (f2=6.3–22.5 kHz), such sampling and averaging were performed by a digital signal processor (DSP) on board the microcomputer. DPOAE levels were measured automatically over the 92-ms duration of the primary tones from the amplitude spectrum resulting from a 4,096-point (bandwidth=10.8 Hz) fast Fourier transform (FFT) of eight averaged samples. To determine the corresponding noise floors (NFs), the levels for the ear-canal sound pressure for five FFT frequency bins above and below the DPOAE-frequency bin (i.e., ±54 Hz) were averaged.
For the higher GM frequencies ranging from 20.1 kHz–48.5 kHz (f2=21.5–54.2 kHz), a computer-controlled dynamic-signal analyzer (3561A, Hewlett Packard, Palo Alto, CA, USA) was used. Over this range of GM-test frequencies, related NFs were estimated by averaging the levels of the ear-canal sound pressure for the two FFT frequency bins below the DPOAE frequency (i.e., for 3.75 Hz below the DPOAE). Using either the DSP- or signal-analyzer systems, no artifactual DPOAEs were ever measured in a hard-walled cavity that approximated the size of the mouse outer ear canal.
Experimental protocol
For the brevetoxin-3 exposures, each of the ten mice served as its own control. Specifically, each mouse was anesthetized using halothane (5% in O2 for induction; 2% for maintenance). Baseline DPOAE measurements were then performed on both ears. After each mouse had fully recovered from the anesthetic, which typically took a few minutes, it was exposed for 2 h to a target concentration of 500 μg brevetoxin-3/m3 using the nose-cone restraint system. Such a highly concentrated dose of brevetoxin was selected to approximate possible effects associated with repeated exposure to lower, more environmentally relevant concentrations of air-borne brevetoxin-3. Upon completion of the brevetoxin-3 exposure, the mice were anesthetized as described above, and the DPOAE measurements were repeated on both ears. The time between completion of the aerosol exposure and initiation of the post-exposure DPOAE measurements was generally less than 30 min, depending on how quickly the subjects were safely washed and transported from the exposure to test areas.
Because no noteworthy effects were observed immediately after the 2-h exposures, on the following day, the ten mice were subdivided into two groups consisting of five mice each. The DPOAE measurements were repeated on one of these groups of five mice in the absence of further exposure to brevetoxin-3 to determine if there were any delayed effects on cochlear function from the 2-h exposures of the previous day. The second group of five mice underwent a second brevetoxin-3 exposure for an additional 4 h and thus received a cumulative exposure of 6 h. Immediately following the second exposure, these animals were anesthetized and DPOAE measurements were performed on both ears. For both groups of mice, i.e., the 2- and 6-h exposed animals, comparisons were made to the individual pre-exposure baseline values obtained on the previous day, i.e., before any exposure to brevetoxin-3 occurred.
Because the DPOAE-measurement system was set up at a remote test site at the Lovelace Respiratory Research Institute and because the mice exhibited essentially no significant brevetoxin-3 induced changes in DPOAE levels, a control procedure was conducted to ensure that the acquired functional measures were valid. Toward this end, one subject (#L530), belonging to the 2-h exposure group, was further exposed at the remote test site to a standard sound over-stimulation episode that was known to produce a frequency-specific reduction in mouse DPOAE levels (Jimenez et al. 2001; Candreia et al. 2004; Vazquez et al. 2004). Specifically, following baseline DP-gram measures for equilevel (L1=L2) primary tones at 55, 65, and 75 dB SPL, mouse #L530 was exposed monaurally (left ear), under closed sound-field conditions, to a 10-kHz pure tone at 105-dB SPL for 5 min. Immediately following this brief overexposure episode, post-exposure DP-grams were obtained to document the frequency effects of the acoustic overstimulation. Based on past experience, the expectation was that the sound-induced reductions in DPOAEs would be maximal for test frequencies at about one-half to an octave above the 10-kHz exposure frequency and less effective at both lower (<10kHz) and higher (>20 kHz) frequencies.
Verification of brevetoxin-3 absorption
In order to confirm that the mice absorbed brevetoxin-3 from the exposure atmosphere, the five mice exposed for a cumulative duration of 6 h were transferred to metabolism cages for excreta collection over an interval lasting ∼18 h. Because previous studies have shown that brevetoxins are excreted primarily in feces, generally within 48 h of exposure (Poli et al. 1990; Benson et al. 1999), and that volumes of urine collected were small, feces samples were analyzed for brevetoxin. Toward this end, feces were homogenized in 10 ml methanol and then sonicated for at least 20 min. Samples were then centrifuged and the methanol supernatant from each sample was removed and concentrated under a stream of N2. The pellets were next re-extracted with 10 ml methanol and the process repeated. Feces from unexposed mice served as control (blank) samples. Extracts were then analyzed by enzyme-linked immunosorbent assay (ELISA) for brevetoxin as described by Naar et al. (2002).
Data processing and statistical analysis
Data were routinely extracted as difference DP-grams, i.e., plots of the difference between post-exposure and pre-exposure DPOAE levels as a function of the GM-test frequency. Difference DP-grams were assembled for each level of primary-tone stimulation, i.e., for each of the equilevel primary-tone paradigms at 55, 65, and 75 dB SPL, for each mouse, and then averaged across the population of mice. Although DPOAEs were measured in both ears of each mouse, the data points represented in the mean difference DP-grams of Figs. 1 and 2 were computed based on an average DPOAE level value contributed by the right and left ears of each mouse subject. That is, at each test frequency and for each primary-tone level condition, DPOAE magnitudes were averaged between the right and left ears so that a mean value represented a particular data point for any one mouse. For the sound-exposure experiment, the difference DP-gram was assembled for an n=1.
Fig. 1.
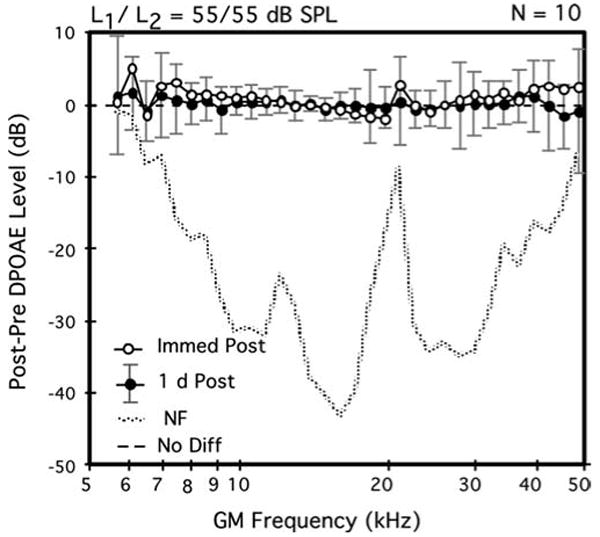
Average difference DP-grams elicited by equilevel (L1=L2=55 dB SPL) primary tones showing the immediate (open circles) versus 1-day (solid circles) post-exposure aftereffects on DPOAE levels of a 2-h inhalation exposure to brevetoxin-3. Although data points were slightly elevated for both the very low and very high test frequencies immediately post-exposure, by 1 day post-exposure, note that DPOAE levels were back to baseline levels as evidenced by the distribution of data points round the ‘0’ line. For both this average difference DP-gram plot and that in Fig 2, the dotted line in the lower portion of the plot represents the post-2-h exposure minus the pre-exposure NF levels. Error bars indicate the ±1 SD ranges for the 1-day post-exposure DPOAE levels. A total of n=10 mice (each mouse contributed one DPOAE-level data point per condition that represented the average of its right and left ears) contributed to the plot
Fig. 2.
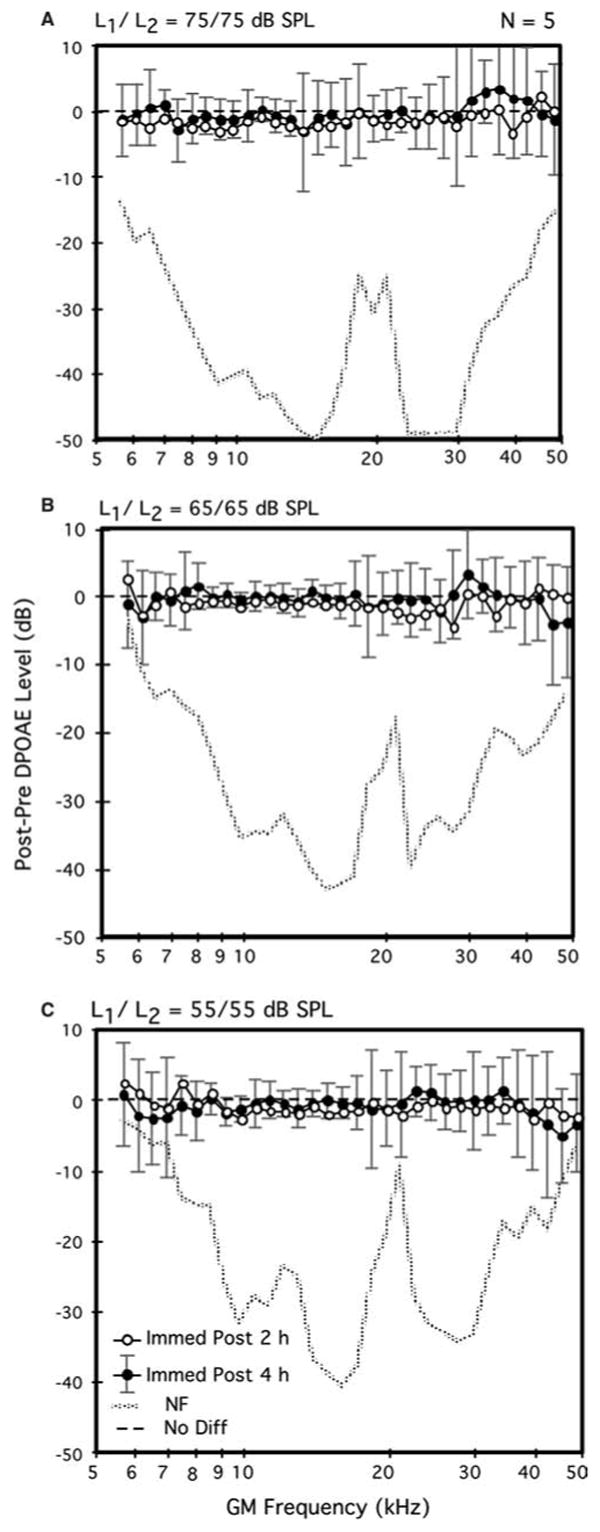
Average difference DP-grams elicited by 75- (A), 65- (B), and 55-dB SPL (C) primary tones (L1=L2) comparing the immediate post-exposure aftereffects of 2- (open circles) versus 4-h (solid circles) exposures to brevetoxin-3 in the same mice. Note that this subset of mice exhibited DPOAE levels for the four highest test frequencies immediately following the 2-h exposure that were slightly, but non-significantly, lower than their baseline counterparts. A total of n=5 mice (as for Fig. 1, each mouse contributed one DPOAE-level data point per condition that represented the average of its right and left ears) contributed to each plot
For both the brevetoxin-3 and noise-exposure experiments, paired t-tests were used to compare pre- to post-exposure DPOAE measures. A commercially available software package (StatView, v.4.5, Abacus Concepts, Berkeley, CA, USA) was used to perform these quantitative comparisons. The adopted level of statistical significance at 0.01 was conservative given the use of multiple t-test comparisons across the set of frequencies that made up the DP-gram.
Results
All mice including the ones that were re-exposed on the second day of the study for a period of time that was twice as long as the exposure conducted on day 1 survived the experiments. There were no visible signs that these mice had any toxin-induced health problems.
Aerosol characteristics and estimates of brevetoxin-3 deposition in mice
On the first day of the study, ten mice were exposed for 2 h to an average concentration of 295 μg brevetoxin-3/m3. A slightly lower average concentration of 255 μg brevetoxin-3/m3 was measured on the second exposure day for the five mice that were re-exposed for an additional 4 h. The mass median aerodynamic diameter (MMAD) and geometric standard deviation of the aerosol were 1.3 and 2 μm, respectively. The concentration of ethanol vapor in the exposure atmosphere was 5.9 g/m3.
Deposition of inhaled particles was calculated based on the formula: μg deposited=aerosol concentration (μg/l) × minute volume (l/min) × exposure duration (min) × deposition fraction (dependent on species and aerosol size). Mice have a minute respiratory volume of ∼0.04 l/min (Schlesinger 1989), and the total respiratory tract deposition for a 1-μm aerosol is estimated at 80% [based on deposition of a 1-μm particle in rat (Schlesinger 1985)]. Therefore, total respiratory-tract deposition of brevetoxin-3 in mice exposed for 2 h to 295-μg brevetoxin-3/m3 was 1.13 μg (57 μg/kg) and for 4 h to 255-μg brevetoxin-3/m3 was 1.96 μg (98 μg/kg).
DPOAE measurements: 2-h brevetoxin-3 exposures
The 2-h exposures to brevetoxin-3 on day 1 produced no clear deficits in cochlear function as measured with DPOAEs at any of the three test levels. In fact, as illustrated in the average difference DP-gram of Fig. 1 for the most sensitive 55-dB SPL primaries, immediately after the exposure period (open circles), on average, DPOAE levels for the GM-test frequencies ≤ 10 kHz and ≥40 kHz were, in general, slightly elevated. However, these apparent post-brevetoxin-3 enhancements in DPOAE levels were not statistically significant. Although not shown here, the average difference DP-grams elicited by the more intense primary-tone levels of 65 and 75 dB SPL immediately post-exposure showed no changes from their corresponding baseline functions.
The average difference DP-gram of Fig. 1 also shows that, by 1 day post-exposure (solid circles), DPOAE magnitudes were essentially at their pre-exposure baseline levels as indicated by the distribution of data points around the 0-difference line. Thus, any minor changes associated with the 2-h exposure to brevetoxin-3 were only transitory. Note that the rather large ± 1 standard-deviation (SD) error bars, which are associated with the 1-day post-exposure data infer that there was great variability in recovery-period effects, particularly for DPOAEs either <8 or >18 kHz.
DPOAE measurements: 4-h brevetoxin-3 exposures
Figure 2 displays mean difference DP-grams elicited at all three primary-tone levels (55, 65, 75 dB SPL) for the subset of five mice that were re-exposed to brevetoxin-3 on day 2 of the study for an additional 4 h. These mice thus received a cumulative dose of brevetoxin-3 of ∼0.77 μg during the 6 h of exposure that was distributed over a 2-day period. For this subset of mice, the difference DP-grams for the immediate post-exposure intervals are provided for both their 2- (open circles) and 4-h (solid circles) exposures. In contrast to the minor enhancements evidenced by the overall group of ten mice exposed on day 1 for 2 h (see Fig. 1), the subset of animals that were re-exposed on day 2 for an additional 4 h showed, on average, a slight, but non-significant, brevetoxin-3 related reduction in DPOAEs (open circles) that was observed at all levels of stimulation. Further, following the 4-h exposure (solid circles), for the lower test levels of 55 (Fig. 2C) and 65 (Fig. 2B) dB SPL, a small brevetoxin-3-induced reduction in DPOAE levels was again observed, particularly for test frequencies >38–40 kHz. However, such changes in response to the 4-h brevetoxin-3 exposure were not statistically significant for any of these higher test frequencies.
Control sound over-exposure
Finally, as a test of the validity of mouse DPOAE measures in a new experimental setting, as explained above, a brief sound-exposure experiment was conducted on the left ear of one mouse (L530-L). The data in Fig. 3 illustrate the outcome of this tonal over-stimulation episode. In this case, the post-exposure difference DP-gram elicited by 55-dB SPL primaries indicates a notable reduction in post-exposure DPOAEs, particularly, between 10 kHz and 20 kHz, thus encompassing a frequency region that extends approximately one octave above the frequency of the 10-kHz exposure tone. In addition, DPOAEs elicited by the 65- and 75-dB SPL primaries exhibited similar, but less drastic aftereffects. Based on the laboratory's considerable experience with this type of acoustic over-exposure in mice (e.g., Jimenez et al. 2001; Vazquez et al. 2001, 2004; Candreia et al. 2004), the sound-induced post-exposure aftereffect established the validity of DPOAE measures in brevetoxin-exposed mice conducted at the remote laboratory site.
Fig. 3.
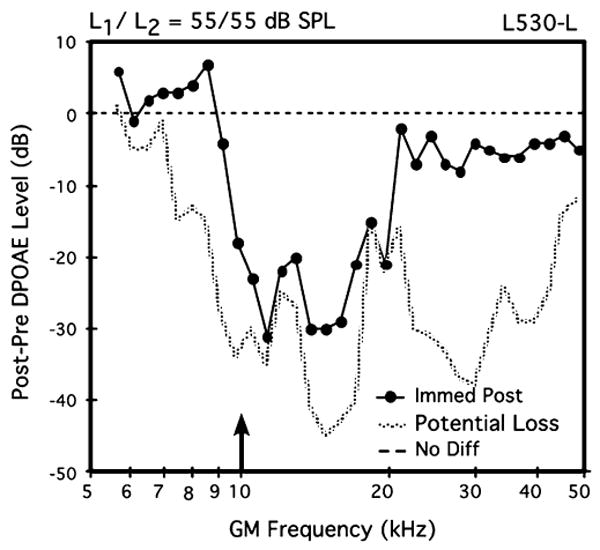
Difference DP-gram in response to equilevel (L1=L2) 55-dB SPL primary tones showing the effects of a 5-min, 105-dB SPL pure-tone exposure at 10 kHz (arrow) for the left ear of mouse #L530. Note the drastically reduced DPOAEs from about 10–20 kHz, which were essentially at NF levels. These findings showing a sound-induced reduction in DPOAE activity verified that the DPOAE-monitoring equipment was functioning correctly at the remote laboratory site. Potential loss=post-exposure NF minus pre-exposure DPOAE
Verification of brevetoxin-3 absorption
Feces samples from five mice exposed to brevetoxin-3 for 6 h contained 0.56±0.12 μg (mean ±1 SD) of the toxin. Moreover, brevetoxin-like compounds as determined by ELISA confirmed absorption of the inhaled brevetoxin-3. Further, no brevetoxin was detected in the feces from non-exposed control mice.
Discussion
The present findings established that brevetoxin-3, a powerful neurotoxin, is not acutely hazardous to the sensitive hearing processes supported by the OHC system of the peripheral ear, at least, for mice. Specifically, non-significant changes in DPOAE levels were observed in mice exposed to brevetoxin-3 concentrations that were orders of magnitude greater than those typically encountered on a Florida beach affected by red tide (Backer et al. 2003). In fact, after 2 h of exposure to brevetoxin-3, DPOAE levels were slightly, but not significantly enhanced, especially for the lowest and the highest test frequencies. Similarly, a subset of mice exposed for 2 h on day 1 of the study, and then re-exposed for an additional 4 h to comparable levels of brevetoxin-3 on day 2, showed only minor post-exposure decreases in DPOAE levels for, particularly, test frequencies >38 kHz.
On the whole, then, brevetoxin-3 did not appreciably affect the processing of sound at the cochlear level in the mouse model. However, it is of note that even though the toxic aftereffects of brevetoxin-3 were negligible, after 6 h of cumulative exposure, there was a trend for a subtle reduction that targeted the high-frequency range of cochlear function, specifically for the DPOAEs that were elicited by the less-intense primary-tone protocols. Intriguingly, this is the frequency region most susceptible to the majority of known ototoxic chemical compounds. The use of a larger population of experimental subjects in a study similar to the present one would likely result in more significant effects.
According to the prior experimental findings of Lu and Tomchik (2002), brevetoxin-3 harmfully affected hearing in goldfish as evaluated with ABR measures. This particular physiological index of auditory function is most sensitive to the neural activity generated by central auditory nervous system structures that are distributed within the initial brainstem pathways that support hearing. Thus, it is not completely unexpected that exposure to brevetoxin-3, a potent neurotoxin, would reduce ABR amplitudes representing central auditory function rather than affect the processes of the peripheral auditory nervous system.
It has long been known that mice are vulnerable to an aminoglycoside-induced hearing loss only during their pre-adult stage of development (Henry et al. 1981). Recently, Schacht and his coworkers (Wu et al. 2001, 2002) attempted to determine the appropriate dosing variables for gentamicin ototoxicity in mature mice, using a series of different drug concentrations. However, mice succumbed to the systemic toxicity of the drug before a measurable hearing loss could be obtained. Further, these investigators also showed that doses of kanamycin that typically produced appreciable threshold shifts in guinea pigs were ineffective in mice. It is important to note, however, that extreme dosing with kanamycin using twice daily injections of a compound that was more toxic than that typically used in rats or guinea pigs over a period of several weeks, as was achieved in the Wu et al. (2001) study, eventually produced deficits in auditory function as measured with ABRs. Thus, these findings in combination show that high doses of familiar aminoglycosides that commonly cause ototoxicity in typical laboratory animals are well tolerated by mice.
Such observations suggest that mice may not make the most sensitive animal model with respect to documenting the potential ototoxic effects of brevetoxin-3 on cochlear function in mammalian species. However, at least in Wu et al.'s (2001) experiments, extremely high doses that did not produce systemic toxicity eventually did result in ototoxicity. Thus, the lack of a systemic toxicity along with the knowledge that the mice in the present study, which were exposed to brevetoxin-3 levels that were many times more toxic than those observed under natural seaside conditions, showed no significant signs of ototoxicity support the likelihood that this toxin does not affect the most sensitive sensory-cell system of the peripheral ear.
Clearly, a natural follow-up experiment would be to assess whether brevetoxin-3 acts more like a neurotoxin than an ototoxin in mice as it does in goldfish. Interestingly, DPOAEs can also be used to provide information about central auditory system function using specialized acoustical-stimulation protocols that permit a measure of the intactness of the descending auditory efferent system in mice (Sun and Kim, 1999). Thus, using this approach, in future studies we can more fully investigate the potential neural effects of brevetoxin-3 on auditory pathways that are more central to the cochlea than the OHC system.
Acknowledgments
This research was funded in part by grants from the National Institutes of Health (ES10594, DC00613, DC03114). The authors thank Amber Dison, Carolyn Elliott, Dolores Esparza, Dean Kracko, Colleen Santistevan, and Thomas Argubright for technical assistance. The experiment was conducted in compliance with federal laws of the United States of America and guidance concerning the use of animals in scientific research
Abbreviations
- ABR
Auditory brainstem response
- DP-gram
Distortion-product otoacoustic emission gram
- DPOAE
Distortion-product otoacoustic emission
- DSP
Digital signal processor
- FFT
Fast Fourier transform
- GM
Geometric mean
- MMAD
Mass median aerodynamic diameter
- NF
Noise floor
- OHC
Outer hair cell
- SD
Standard deviation
Contributor Information
Janet M. Benson, Lovelace Respiratory Research Institute, Albuquerque, NM, USA
Barden B. Stagner, Department of Otolaryngology, University of Colorado Health Sciences Center, Denver, CO, USA Research Service (151), Jerry Pettis Memorial, Veterans Medical Center, 11201 Benton Street, Loma Linda, CA, 92357 USA.
Glen K. Martin, Department of Otolaryngology, University of Colorado Health Sciences Center, Denver, CO, USA.
Melissa Friedman, NIEHS Marine and Freshwater Biomedical Sciences Center, University of Miami, Miami, FL, USA.
Sarah E. Durr, Lovelace Respiratory Research Institute, Albuquerque, NM, USA
Andrea Gomez, Lovelace Respiratory Research Institute, Albuquerque, NM, USA.
Jacob McDonald, Lovelace Respiratory Research Institute, Albuquerque, NM, USA.
Lora E. Fleming, NIEHS Marine and Freshwater Biomedical Sciences Center, University of Miami, Miami, FL, USA
Lorraine C. Backer, National Center for Environmental Health, Center for Disease Control and Prevention, Atlanta, GA, USA
Daniel G. Baden, Center for Marine Science Research, University of North Carolina at Wilmington, Wilmington, NC, USA
Andrea Bourdelais, Center for Marine Science Research, University of North Carolina at Wilmington, Wilmington, NC, USA.
Jerome Naar, Center for Marine Science Research, University of North Carolina at Wilmington, Wilmington, NC, USA.
Brenda L. Lonsbury-Martin, Department of Otolaryngology, University of Colorado Health Sciences Center, Denver, CO, USA
References
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Baden DG. Brevetoxins: unique polyether dinoflagellate toxins. FASEB J. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- Benson JM, Tischler DL, Baden DG. Uptake, tissue distribution, and excretion of Brevetoxin 3 administered to rats by intratracheal instillation. J Toxicol Environ Health A. 1999;57:345–355. doi: 10.1080/009841099157656. [DOI] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: gross, histologic, and immunohistochemical features. Toxicol Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candreia C, Martin GK, Stagner BB, Lonsbury-Martin BL. distortion-product otoacoustic emissions show exceptional resistance to noise exposure in MOLF/Ei mice. Hear Res. 2004;194:109–117. doi: 10.1016/j.heares.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Cattet M, Geraci JR. Distribution and elimination of ingested Brevetoxin (PbTx3) in rats. Toxicon. 1993;31:1483–1486. doi: 10.1016/0041-0101(93)90214-4. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Backer L, Rowan A. The epidemiology of human illnesses associated with harmful algal blooms. In: Baden D, Adams D, editors. Neurotoxicology Handbook. Vol. 1. Humana Press Inc; Totowa: 2002. pp. 363–381. [Google Scholar]
- Henry KR, Chole RA, McGinn MD, Frush DP. Increased ototoxicity in both young and old mice. Arch Otolaryngol. 1981;107:92–95. doi: 10.1001/archotol.1981.00790380022006. [DOI] [PubMed] [Google Scholar]
- Hilton M, Chen J, Kakigi A, Hirakawa H, Mount RJ, Harrison RV. Middle ear instillation of gentamicin and streptomycin in chinchillas: electrophysiological appraisal of selective ototoxicity. Clin Otolaryngol. 2002;27:529–535. doi: 10.1046/j.1365-2273.2002.00614.x. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion-product otoacoustic emissions in four mouse strains. Hear Res. 1999;138:91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Susceptibility of DPOAEs to sound over-exposure in inbred mice with AHL. J Assoc Res Otolaryngol. 2001;2:233–245. doi: 10.1007/s101620010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Tomchik SM. Effects of a red-tide toxin on fish hearing. J Comp Physiol A. 2002;188:807–813. doi: 10.1007/s00359-002-0369-8. [DOI] [PubMed] [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DB. A competitive ELISA to detect PbTxs from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Lyons J, Cheng YS, Yazzie D, Zhou Y. PbTx concentrations in marine aerosol: Human exposure levels during a Karenia brevis harmful algal bloom. Bull Environ Contam Toxicol. 2003;70:161–165. doi: 10.1007/s00128-002-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli MA, Templeton CB, Thompson WL, Hewetson JF. Distribution and elimination of Brevetoxin PbTx-3 in rats. Toxicon. 1990;28:903–910. doi: 10.1016/0041-0101(90)90020-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans; a review. J Toxicol Environ Health. 1985;15:197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB. Deposition and clearance of inhaled particles. In: McClelland RO, Henderson RF, editors. Concepts in Inhalation Toxicology. Hemisphere Publishing Co; New York: 1989. pp. 163–192. [Google Scholar]
- Stavroulaki P, Apostolopoulos N, Segas J, Tsakanikos M, Adamopoulos G. Evoked otoacoustic emissions–an approach for monitoring cisplatin induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 2001;59:47–57. doi: 10.1016/s0165-5876(01)00455-4. [DOI] [PubMed] [Google Scholar]
- Sun XM, Kim DO. Adaptation of 2f1-f2 distortion-product otoacoustic emission in young-adult and old CBA and C57 mice. J Acoust Soc Am. 1999;105:3399–3409. doi: 10.1121/1.424668. [DOI] [PubMed] [Google Scholar]
- Vazquez AE, Lonsbury-Martin BL, Martin GK, Luebke AE. Temporary and permanent noise-induced changes in distortion-product otoacoustic emissions in CBA/CAJ mouse. Hear Res. 2001;156:31–43. doi: 10.1016/s0378-5955(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Vazquez AE, Jimenez AM, Martin GK, Luebke AE, Lonsbury-Martin BL. Evaluating cochlear function and the effects of noise exposure in the B6.CAST+ahl mouse with distortion-product otoacoustic emissions. Hear Res. 2004;194:87–96. doi: 10.1016/j.heares.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurootol. 2002;7:171–174. doi: 10.1159/000058305. [DOI] [PubMed] [Google Scholar]


