Abstract
Brevetoxins are a family of potent lipid-soluble neurotoxins produced by the dinoflagellate Karenia brevis, the organism responsible for Florida red tide. Brevetoxins aerosolized by surf and wind produce irritation of the eyes, nose, and throat in people on or near red tide-affected beaches. The effects of chronic exposures to brevetoxins on healthy and health-compromised individuals are not known. The purpose of this study was to investigate the pulmonary uptake, tissue distribution, and excretion of polyether brevetoxin-3 in mice, a rodent model for investigating the potential systemic adverse health effects associated with repeated brevetoxin inhalation. Male CBA/CaJ mice were administered [3H]brevetoxin-3 by intratracheal instillation. Groups of 3 mice were sacrificed immediately after instillation and at 0.5, 3, 6, 12, 24, 48, and 96 h postinstillation. Four additional mice were placed into metabolism cages for excreta collection up to 168 h postinstillation. Brevetoxin-3 distributed rapidly to all tissues, with the highest initial doses in the liver and gastrointestinal tract. Elimination half-times ranged from approximately 28 h for fat, heart, intestines, kidneys, liver, and muscle to approximately 90 h for brain and testes. The total dose to tissue ranged from 39 ng brevetoxin equivalents-h/g for testes to 406 ng brevetoxin equivalents-h/g for liver. Approximately 90% of excretion had occurred within 96 h, with 11 and 64% of the initial brevetoxin dose excreted in urine and feces, respectively. These results are consistent with earlier reports of rapid absorption and widespread tissue distribution of brevetoxins in rats.
Blooms of the dinoflagellate Karenia brevis (K. brevis) are responsible for what are commonly called Florida red tides. These blooms have spread significantly along the Gulf Coast and the coastal waters of the southeastern United States (Tibbetts, 1998; Van Dolah, 2000; Kirkpatrick et al., 2004; Fleming et al., 2005a). Karenia brevis produces a series of polycyclic ethers, known as brevetoxins (polyether breve toxins, PbTx), with molecular structures consisting of 11 transfused rings (Figure 1). Brevetoxins produce acute neurotoxic shellfish poisoning in individuals who consume K. brevis-contaminated shellfish; however, people can also be exposed to brevetoxins when the toxins become aerosolized in surf and sea spray (Fleming et al., 2005b; Backer et al., 2003, 2005; Kirkpatrick et al., 2004; Pierce et al., 2003). Acute exposures to airborne brevetoxins can produce irritation of the eyes, nose, and throat. Wheezing and altered pulmonary function have been observed among asthmatics exposed to brevetoxin aerosols (Asai et al., 1982; Backer et al., 2003; Kirkpatrick et al., 2004; Fleming et al., 2005b).
FIGURE 1.
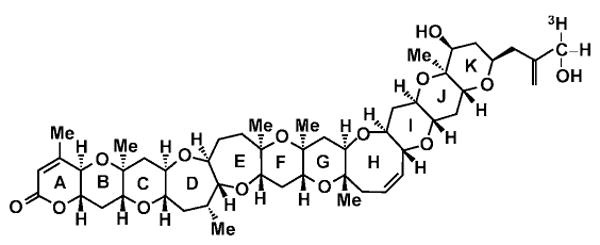
Structure of PbTx-3 showing 3H on C-42.
The fate of PbTx-3 in rats has been determined following administration by several routes (Poli et al., 1990a, 1990b; Cattet & Geraci, 1993; Benson et al., 1999). By all routes, brevetoxin is rapidly distributed primarily to skeletal muscle and liver, and to a lesser extent, to heart, testes, brain, lungs, and spleen. Poli and coworkers have demonstrated hepatic metabolism of PbTx-3 in vivo and in vitro to at least three polar and as yet unidentified metabolites (Poli et al., 1990a, 1990b).
The purpose of this study was to evaluate the pulmonary uptake, tissue distribution, and excretion of PbTx-3 administered intratracheally in mice to aid interpretation of organ-specific toxicity of brevetoxin in this animal model. The results, combined with knowledge of the fate of instilled brevetoxin in rats (Benson et al., 1999), may aid in interpreting any observed differences in the toxic responses of rats and mice to brevetoxins.
Materials and Methods
Chemicals
PbTx-3 radiolabeled with tritium (3H-PbTx-3, 15.5 Ci/mmol) was prepared by the Center for Marine Science at the University of North Carolina at Wilmington, NC (UNCW), using the method described by Poli and coworkers (1986). The tritium label was covalently attached at C-42 (Figure 1) and was nonexchangeable. The radiochemical purity of the 3H-PbTx-3, as determined by high-performance liquid chromatography, exceeded 95%. Unlabeled PbTx-3, purified from the Wilson clone of K. brevis at the UNCW, was added to the 3H-PbTx-3 solution in saline to achieve a final specific activity of 7.86 Ci/mmol (5 μCi/ml; 0.57 μg PbTx-3/ml dosing solution; 0.11 μg/μCi).
Animals
Twenty-seven male, CBA/CaJ mice, 9 wk of age, weighing approximately 20 g at the time of dosing, were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were quarantined for 14 d prior to dosing. Animals were housed in shoebox cages with hardwood chip bedding and filter tops. Mice designated for metabolism collection were conditioned to the polycarbonate metabolism cages located within the animal quarters for 3 d prior to dosing. The animal rooms were maintained at 20 to 22°C with a relative humidity of 20 to 50% and a 12-h light cycle beginning at 0600. Food (Harlan Teklad, Madison, WI) and water were provided ad libitum. All housing was in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The study protocol was reviewed and approved by the Lovelace Respiratory Research Institute's Animal Care and Use Committee.
Dose Justification
The mice were each administered 0.1 ml 3H-PbTx-3 (0.5 μCi/animal; 0.06 μg PbTx-3; 2.6 μg/kg body weight) by intratracheal instillation. This dose was below the median lethal PbTx-3 dose by intratracheal instillation in rats and mice (approximately 10 μg/kg, unpublished observations). The intratracheally administered dose fell within the range expected be deposited in a 120-min exposure of mice by nose only inhalation to 5 or 50 μg PbTx-3/m3 with a mass median aerodynamic diameter of 1 μm, as is the case in our exposure systems (Benson et al., 2004, 2005a, 2005b).
Experimental Design
The mice were anesthetized using 5% halothane in a 60:40 mixture of oxygen and nitrous oxide. A catheter was placed transorally into the trachea for administration of the dose in 0.1 ml saline. Immediately after being dosed, 4 mice were placed into individual metabolism cages for collection of urine and feces over a 168-h period, after which they were sacrificed by intraperitoneal injection of Euthasol. Urine and feces were collected in separate glass containers. Animals sacrificed immediately after dosing (time-zero animals) were frozen, stored at −80°C, and later processed for radiochemical analysis. The remaining dosed mice were sacrificed at 0.5, 3, 6, 12, 24, 48, and 96 h postinstillation. At sacrifice, blood, heart, liver, kidneys, spleen, lung, brain, testes, muscle, perirenal fat, and gastrointestinal (GI) tract with contents were removed and weighed. These samples and the remaining carcass were immediately frozen and stored at −80°C for radiochemical analysis.
Sample Processing and Radiochemical Analysis
Tissues and carcasses of time-zero animals were digested in toto using a 35% solution of tetraethylammonium hydroxide (∼1 ml/g tissue; Sachem, Inc., Austin, TX), and the weight of each sample digest was recorded. Weighed aliquots of digest were neutralized with concentrated hydrochloric acid (VWR International, West Chester, PA), decolorized with 30% hydrogen peroxide (ACS grade; Fisher Scientific, Hampton, NH), and mixed with Ultima Gold XR scintillation cocktail (Packard Instrument Co., Meriden, CT) for quantitation of tritium activity by liquid scintillation counting. Aliquot counts were then used to calculate the total activity for the corresponding sample digest. The carcasses from the three time-zero animals were processed, and the data were used to determine the total initial dose, defined as the initial body burden (IBB).
Fecal samples were homogenized and extracted with 70% methanol in water. The extracts were evaporated to dryness under nitrogen and redissolved in 10 ml fresh solvent. Aliquots of the samples were then mixed with scintillation cocktail for quantitation of tritium activity. The extractions were repeated with fresh solvent until the aliquot counts were at the limit of detection (LOD, up to 5 extractions), and the counts were summed for each sample. Urine samples were mixed with scintillation cocktail and were analyzed for tritium activity without additional processing.
Radioactivity in the samples was quantitated by analysis in a Packard Tri-Carb model 2500TR liquid scintillation analyzer. All samples were counted for 5 min, and quench parameters were determined using the external standard method. The LOD for the instrument and the limit of quantitation (LOQ) for each sample were calculated using the method of Altshuler and Pasternack (1963). The LOD, a function of the background, was 0.51 pg PbTx-3-associated radioactivity (hereafter referred to as PbTx-3 equivalents) for urine and tissues and 0.47 pg PbTx-3 equivalents for feces extracts. The LOQ, a function of background and counting efficiency, ranged from 3.87 to 39.81 pg PbTx-3 equivalents (mean = 9.41) for urine, 2.68 to 18.0 pg PbTx-3 equivalents (mean = 5.4) for feces, and 3.74 to 15.88 pg PbTx-3 equivalents (mean = 4.32) for tissues. The tritium activity in each sample was determined and used to calculate the PbTx-3 equivalents present in the sample. In tissue samples, equivalents of PbTx-3 were normalized to the tissue weight and are expressed in terms of nanograms PbTx-3 equivalents per gram tissue, recognizing that the measured activity represents both parent compound and presumed metabolites. Data for urinary and fecal excretion of 3H-PbTx-3 equivalents were obtained for each mouse and expressed as cumulative nanograms PbTx-3 equivalents excreted as a function of time after instillation.
Statistical Evaluation
Means and standard deviations of tissue concentrations (in terms of ng PbTx-3 equivalents/g tissue and as percent of the IBB) were calculated using Microsoft Excel software. The kinetics of PbTx-3 elimination for each tissue (ng/g tissue) were modeled with single- and two-component exponential decay functions using TableCurve 2D software (SPSS Science, Chicago). Total dose to tissue was calculated by integrating the area under the elimination curve (AUC) from 0.5 to 168 h for each tissue using TableCurve 2D. The total dose to tissue is expressed as ng PbTx-3 equivalents-h/g tissue. Cumulative excretion of brevetoxin equivalents in urine and feces was determined as a function of time after dosing, to 168 h.
Results
The average initial dose or IBB determined for the animals sacrificed immediately after dosing was 0.05 ± 0.004 μg PbTx-3 (2.6 ± 0.22 μg/kg; mean ± SD, n = 3). At 30 min postinstillation, PbTx-3 equivalents were present in all examined tissues. As shown in Figure 2, PbTx-3 equivalents cleared rapidly from the lung and distributed primarily to the liver and gastrointestinal (GI) tract. At 30 min postinstillation, the lungs contained 0.2 to 2% IBB, the liver contained 13 to 32% IBB, the stomach contained 3.5 to 61% IBB, and the intestines contained 10 to 19% IBB. The high variability seen in the stomach may be due to reflux and swallowing after dosing. Significant quantities of PbTx-3 equivalents also were distributed to the kidneys (1 to 3% IBB), fat (3 to 9% IBB), and muscle tissue (14 to 33% IBB). For the remaining tissues, the percent of initial dose at 30 min postinstillation was less than 1%.
FIGURE 2.
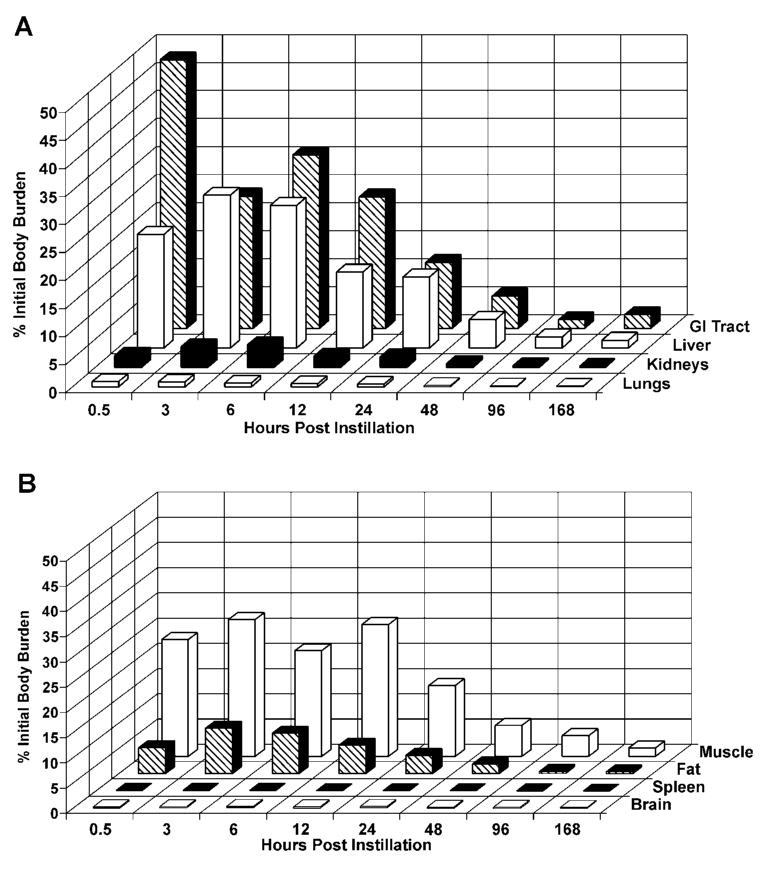
Distribution of PbTx-3 in selected tissues as a function of time after dosing. (A) Percentages of the initial body burden present in GI tract, liver, kidneys, and lungs. (B) Percentages of the initial body burden present in muscle, fat, spleen, and brain. Results are the mean of 3 values except at 12 h, where n = 2.
For all tissues, PbTx-3 elimination was best modeled using a single-component, negative exponential function. Elimination half-times were calculated using the formula t1/2 = (ln 2)/b, where b is the rate constant. Elimination was fastest from the stomach (t1/2 = 10 h; Table 1) and slowest for brain (t1/2 = 89 h) and testes (t1/2 = 90 h). Elimination from fat, heart, intestines, kidneys, liver, and muscle was relatively rapid, with elimination t1/2 values averaging 28 h. Elimination from blood, lungs, and spleen was relatively slow, with elimination t1/2 values averaging 53 h.
TABLE 1.
Brevetoxin Concentrations in Tissue as a Function of Time after Dosing, Estimated Elimination Half-Times, and Calculated Dose to Tissues
| Tissue | ng PbTx-3 equivalents/g-tissuea | t1/2 (h) | Dose to tissue (ng-h/g tissue) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 h (n = 3) |
3 h (n = 3) |
6 h (n = 3) |
12 h (n = 2) |
24 h (n = 3) |
48 h (n = 3) |
96 h (n = 3) |
168 h (n = 4) |
||||
| Blood | Mean | 1.01 | 1.05 | 0.86 | 1.68 | 1.28 | 0.55 | 0.27 | 0.18 | ||
| SD | 0.40 | 0.15 | 0.05 | 0.52 | 0.13 | 0.21 | 0.04 | 0.06 | 65 | 87 | |
| Brain | Mean | 0.37 | 0.55 | 0.45 | 0.49, 0.52 | 0.52 | 0.33 | 0.20 | 0.13 | ||
| SD | 0.16 | 0.05 | 0.05 | 0.02 | 0.03 | 0.02 | 0.02 | 89 | 47 | ||
| Carcass | Mean | 0.95 | 1.15 | 0.91 | 1.68, 0.61 | 0.46 | 0.22 | 0.09 | 0.30 | ||
| SD | 0.50 | 0.22 | 0.17 | 0.02 | 0.02 | 0.02 | 0.01 | 24 | 39 | ||
| Fat | Mean | 0.88 | 1.62 | 1.46 | 1.10, 1.47 | 0.65 | 0.34 | 0.08 | 0.04 | ||
| SD | 0.54 | 0.29 | 0.63 | 0.17 | 0.02 | 0.01 | 0.01 | 28 | 55 | ||
| Heart | Mean | 3.28 | 3.21 | 2.91 | 2.53, 4.12 | 2.10 | 0.86 | 0.49 | 0.25 | ||
| SD | 1.17 | 0.33 | 0.31 | 0.12 | 0.25 | 0.06 | 0.09 | 32 | 153 | ||
| Intestines | Mean | 2.93 | 4.03 | 5.91 | 2.92, 4.93 | 2.14 | 1.02 | 0.25 | 0.37 | ||
| SD | 0.79 | 0.38 | 0.74 | 0.14 | 0.20 | 0.03 | 0.15 | 29 | 183 | ||
| Kidneys | Mean | 2.69 | 4.90 | 5.34 | 2.75, 3.22 | 2.67 | 0.88 | 0.47 | 0.19 | ||
| SD | 1.43 | 0.63 | 0.78 | 0.48 | 0.18 | 0.10 | 0.03 | 30 | 184 | ||
| Liver | Mean | 7.31 | 10.89 | 17.84 | 6.45, 6.87 | 4.94 | 1.88 | 0.74 | 0.38 | ||
| SD | 3.52 | 2.53 | 11.65 | 0.04 | 0.21 | 0.17 | 0.06 | 25 | 348 | ||
| Lungs | Mean | 4.44 | 3.28 | 2.45 | 1.83, 5.28 | 1.84 | 0.88 | 0.63 | 0.25 | ||
| SD | 5.46 | 0.67 | 0.39 | 0.08 | 0.11 | 0.15 | 0.03 | 44 | 163 | ||
| Muscle | Mean | 1.27 | 1.56 | 1.20 | 0.89, 3.33 | 0.80 | 0.35 | 0.24 | 0.07 | ||
| SD | 0.52 | 0.28 | 0.19 | 0.06 | 0.04 | 0.04 | 0.02 | 28 | 56 | ||
| Spleenb | Mean | 0.91 | 1.22 | 1.08 | 0.73 | 0.71 | 0.52 | 0.33 | 0.17 | ||
| SD | 0.44 | 0.22 | 0.16 | 0.08 | 0.11 | 0.06 | 0.03 | 51 | 72 | ||
| Stomach | Mean | 17.54 | 1.85 | 4.64 | 5.64, 23.62 | 1.28 | 0.71 | 0.27 | 0.21 | ||
| SD | 14.21 | 0.53 | 2.75 | 0.46 | 0.20 | 0.09 | 0.05 | 10 | 167 | ||
| Testes | Mean | 0.28 | 0.44 | 0.39 | 0.48, 1.88 | 0.43 | 0.32 | 0.20 | 0.06 | ||
| SD | 0.12 | 0.05 | 0.04 | 0.02 | 0.02 | 0.03 | 0.01 | 90 | 39 | ||
Results are the mean ± SD of 3 values.
At 12 h, n = 2 for all tissues except spleen, where n = 1. Means and individual values are given.
Testes had the lowest total dose of PbTx-3 equivalents, 39 ng PbTx-3 equivalents-h/g, while the liver had the highest total dose, 406 ng-h/g (Table 1). Blood, brain, fat, muscle, and spleen had relatively low doses, averaging 63 ng-h/g. Heart, intestines, kidneys, lungs, and stomach had relatively high total doses, averaging 167 ng-h/g.
The cumulative excretion of PbTx-3 equivalents is illustrated in Figure 3. Little excretion of brevetoxin equivalents in urine and feces occurred before 12 h postdosing. Approximately 90% of excretion occurred within 96 h, with 11% and 64% of the initial brevetoxin dose excreted in urine and feces, respectively (Table 2).
FIGURE 3.
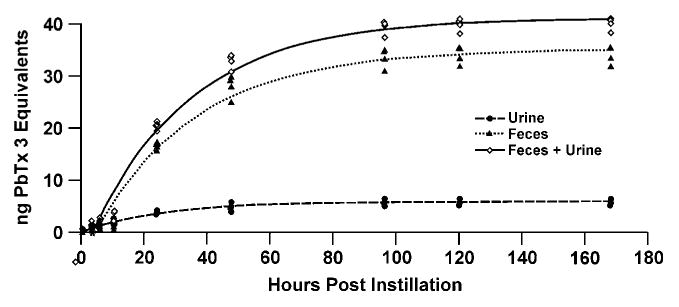
Cumulative excretion of PbTx-3 equivalents.
TABLE 2.
Distribution PbTx-3 Equivalents in Tissues and Excreta 168 h After Dosing
| Sample | ng | Percent of IBB |
|---|---|---|
| Tissues | 3.5 ± 0.5 | 6.6 ± 0.9 |
| Urine | 5.9 ± 0.6 | 11 ± 1.2 |
| Feces | 34 ± 1.7 | 64 ± 3.2 |
| Total recovered | 44 ± 0.9 | 81 ± 1.7 |
Note. Results are the mean ± SD of four values.
Eighty percent of the IBB was recovered in animals held for 168 h postinstillation (Table 2). Slightly over 6.5% of the PbTx-3 activity remained in the tissues at 168 h postinstillation.
Discussion
The purpose of this study was to evaluate the pulmunary absorption, tissue distribution, and kinetics of elimination of PbTx-3 in mice. This discussion focuses on comparing the results obtained in this study in which mice were administered a single dose of 2.6 μg PbTx-3/kg body weight with those of a parallel, earlier study in which rats were administered a single dose of 10.8 μg PbTx-3/kg body weight (Benson et al., 1999). In making these comparisons, dose proportionality between rats and mice over this very low dose range (2.6 to 10.8 μg/kg body weight) is assumed. Use of mice and rats receiving equal doses/kg body weight would provide more direct comparisons of the fate of instilled brevetoxin.
In both mice and rats, intratracheally administered PbTx-3 was rapidly absorbed and distributed to all tissues examined. In both species, the largest proportions of the dose distributed initially to liver and GI tract and carcass (primarily muscle and fat), with excretion primarily in feces. These results are consistent with those reported following administration of 18 μg/kg PbTx-3/kg by oral gavage (Cattet & Geraci, 1993) and 0.7 μg/kg by intravenous injection (Poli et al., 1990). However, mice and rats administered PbTx-3 by intratracheal instillation differed with regard to rates of elimination of PbTx-3 equivalents from tissues, residual dose to tissues, and relative dose to tissues. Comparisons of PbTx-3 tissue elimination rates, tissue concentrations, and calculated dose to tissue are shown in Table 3.
TABLE 3.
Comparisons of PbTx-3 Tissue Concentrations, Elimination Rates, and Calculated Dose to Tissues (AUC) in Mice and Rats
| Tissue | Elimination t1/2 (h)a | Fraction of initial concentration eliminated | Concentration in tissue at 168 h (ng/g)a | Total dose—AUCa | ||||
|---|---|---|---|---|---|---|---|---|
| Mouseb | Ratb | Mousec | Ratd | Mouse | Rat | Mouse, ng-h/g (tissue/carcass) |
Rat, ng-h/g (tissue/carcass) |
|
| Brain | 89 ± 0.001 | 25 ± 0.03 | 0.73 | 0.37 | 0.13 | 1.14 | 47 (1.2) | 203 (0.8) |
| Carcass | 24 ± 0.008 | 5.4 ± 0.05 | 0.99 | 0.86 | 0.3 | 1.00 | 39 (1.0) | 250 (1.0) |
| Kidneys | 30 ± 0.007 | 2.0 ± 0.14 | 0.98 | 0.82 | 0.19 | 6.21 | 184 (4.8) | 1104 (4.4) |
| Liver | 25 ± 0.007 | 13 ± 0.02 | 0.99 | 0.80 | 0.38 | 4.77 | 348 (9.0) | 955 (3.8) |
| Lungs | 44 ± 0.006 | 1.2 ± 0.26 | 0.93 | 0.81 | 0.25 | 38.50 | 163 (4.3) | 6986 (28) |
The average total dose was 2.6 μg PbTx-3/kg body weight for mice and 10.8 μg PbTx-3/kg body weight for rats.
The equation best fitting elimination data for mouse tissue was A = Aoe(−bx) where A is the concentration of PbTx-3 equivalents cleared with a rate constant b. The equation best fitting elimination data for rat tissue was A = Aoe(−bx) + c where A is the concentration of PbTx-3 equivalents eliminated with a rate constant b, and c is the concentration of PbTx-3 equivalents with no calculated rate of elimination. The elimination half-time in hours was calculated from the equation, t1/2 = (ln 2)/b.
The fraction eliminated was estimated by subtracting the concentration present in tissues at 168 h from the initial concentration and dividing by the concentration present in the tissue at time zero.
This number represents the fraction eliminated with short-term rate constant b.
The major differences between mice and rats were in the patterns of elimination of brevetoxin equivalents from tissues. In mice, elimination of PbTx-3 equivalents from tissue was best described by a single-component, negative exponential function. Elimination half-times of brevetoxin equivalents from mouse carcass, kidneys, liver, and lungs ranged from 25 to 44 h (Tables 1 and 3). Elimination from the mouse brain was much slower than from the other tissues, with a half time of 89 h. In rats, elimination of PbTx-3 equivalents from tissue was best described by a single-component, negative exponential function incorporating a constant, indicating that a portion of the material was not being cleared. Therefore, while initial rates of PbTx-3 equivalents from rat tissues were faster than in mice, generally 15 to 20% of the initial dose to tissues remained at 168 h after dosing. Again, the brain was the exception to this pattern, with almost 70% of the initial brevetoxin dose retained at 168 h after dosing. The retained PbTx-3 equivalents in rats resulted in higher tissue concentrations (ng/g) at 168 h after dosing and greater calculated tissue doses for rats compared to mice (Table 3). The differences in tissue concentration and total dose are greater than would be expected based on the fivefold greater PbTx-3 dose to rats compared to mice. The cellular/molecular sites of tissue retention in the rats are not known, although some possibilities include tissue macrophages (Bossart et al., 1998; Benson et al., 2004).
If the total dose of PbTx-3 in tissues (ng-h/g tissue) is normalized for the corresponding total dose to carcass, the relative distributions of PbTx-3 equivalents to the tissues can be compared for rats and mice. If this is done, the relative dose to brain, a target for brevetoxin-induced toxicity, was 50% higher in mice than in rats. For liver, presumably a major site of brevetoxin metabolism, the relative dose of brevetoxin equivalents was two- to fourfold higher for mice than for rats. In contrast, the relative doses of brevetoxin equivalents in lung were sevenfold higher in rats than in mice. Interestingly, no adverse effects were observed in the lungs of rats inhaling PbTx-3 for up to 4 wk (Benson et al., 2005a).
Although total doses of PbTx-3 equivalents to the rat and mouse brain are small, retention in brain is longer than for other tissues. This is important in light of recent findings that short-term inhalation exposure of mice and rats to PbTx-3 produces neuronal damage and loss in several areas of the mouse and rat brain, including the cortex, cerebellum, thalamus, hippocampus, striatum, and median vestibular nucleus (Thomas F. Murray, personal communication). As with other tissues, the cellular and macromolecular sites of brevetoxin retention remain to be determined. The toxicological implications of prolonged brevetoxin retention in brain remain to be determined.
In summary, in mice, as with rats, brevetoxin is rapidly absorbed from the lung and distributes rapidly and widely throughout the body. Tissues with the highest dose, such as GI tract and liver, correlate with organs of metabolism and excretion. For both rats and mice, relative doses of brevetoxin to brain are low compared to other tissues, but retention is longer. Investigation into the cellular and macromolecular distribution of retained brevetoxin equivalents in brain, as well as identification of the molecular forms of the retained material, will be important for interpreting brevetoxin-induced toxicities in that organ.
Acknowledgments
This research was funded under National Institutes of Health contract NIH PO1-ES10594. LRRI animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The authors are grateful for the assistance provided by D. Esparza, A. Dison, and D. Santistevan.
References
- Altshuler B, Pasternack B. Statistical measures of the lower limit of detection of a radioactivity counter. Health Phys. 1963;9:293–298. doi: 10.1097/00004032-196303000-00005. [DOI] [PubMed] [Google Scholar]
- Asai S, Krzanowski JJ, Anderson WH, Martin DF, Polson JB, Lockey RF, Bukantz SC, Szentivanyi A. Effects of toxin of red tide, Ptychodiscus brevis, on canine tracheal smooth muscle: A possible new asthma-triggering mechanism. J Allergy Clin Immunol. 1982;69:418–428. doi: 10.1016/0091-6749(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YC, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Zhou Y, Baden D. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Effects on a healthy worker population. Environ Health Perspect. 2005;113(5):644–649. doi: 10.1289/ehp.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Tischler DL, Baden DG. Uptake, tissue distribution, and excretion of PbTx-3 administered to rats by intratracheal instillation. J Toxicol Environ Health A. 1999;57:345–355. doi: 10.1080/009841099157656. [DOI] [PubMed] [Google Scholar]
- Benson JJ, Hahn FF, Tibbetts BM, Bowen LE, March TH, Sopori MS, Seagrave JC, Gomez A, Bourdelais A, Naar J, Bossart G, Baden D. Inhalation toxicity of PbTx-3 in rats exposed for five days. J Toxicol Environ Health A. 2004;67:1443–1456. doi: 10.1080/15287390490483809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori MJ, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days, Environ. Health Perspect. 2005a;113(5):626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Stagner BB, Martin GK, Friedman M, Durr SE, Gomez A, McDonald J, Fleming LE, Backer LC, Baden DG, Bourdelais A, Naar J, Lonsbury-Martin BL. Cochlear function in mice following inhalation of brevetoxin-3. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005b;191(7):619–626. doi: 10.1007/s00359-005-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing RY, Roberts B, Write SD. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: Gross, histologic, and immunohistochemical features. Toxicol Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Cattet M, Geraci JR. Distribution and elimination of ingested brevetoxin (PbTx-3) in rats. Toxicon. 1993;31:1483–1486. doi: 10.1016/0041-0101(93)90214-4. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida red tide toxins: Exposures and effects. Environ Health Perspect. 2005a;113:618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierce R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van De Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ Health Perspect. 2005b;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R. Literature review of Florida red tide: Implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Lyons J, Cheng YS, Yazzie D, Zhou Y. Brevetoxin concentrations in marine aerosol: human exposure levels during a Karenia brevis harmful algal bloom. Bull Environ Contam Toxicol. 2003;70:161–165. doi: 10.1007/s00128-002-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986;30:129–135. [PubMed] [Google Scholar]
- Poli MA, Templeton CB, Pace JG, Hines HB. Detection, metabolism and pathophysiology of brevetoxins. In: Hall S, Strichartz G, editors. Marine toxins: Origin, structure and molecular pharmacology. Washington, DC: American Chemical Society; 1990a. pp. 176–191. [Google Scholar]
- Poli MA, Templeton CB, Thompson WL, Hewetson JF. Distribution and elimination of brevetoxin PbTx-3 in rats. Toxicon. 1990b;28:903–910. doi: 10.1016/0041-0101(90)90020-8. [DOI] [PubMed] [Google Scholar]
- Tibbetts J. Toxic tides. Environ Health Perspect. 1998;106:A326–A331. doi: 10.1289/ehp.98106a326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dolah FM. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ Health Perspect. 2000;108(1):133–141. doi: 10.1289/ehp.00108s1133. [DOI] [PMC free article] [PubMed] [Google Scholar]


