Abstract
Aims
Sepsis-associated cardiac dysfunction represents an intrinsic impairment of cardiomyocyte function due in part to a decrease in myofilament Ca2+ sensitivity associated with a sustained increase in cardiac troponin I (cTnI) phosphorylation at Ser23/24. Dephosphorylation of cTnI is under regulatory control. Thus, muscarinic and adenosine A1-receptor agonists antagonize β-adrenergic stimulation via activation of protein phosphatase 2A (PP2A). The aim of this study was to determine whether modulation of PP2A and thus cTnI phosphorylation could improve sepsis-induced contractile dysfunction.
Methods and results
Cardiomyocytes were isolated from control or septic mice 16–18 h after an injection of vehicle or lipopolysaccharide (LPS; 9 mg/kg ip) respectively. Protein expression and phosphatase activity were determined in homogenates of control and septic hearts. Our data showed that LPS significantly increased cTnI phosphorylation at Ser23/24 in cardiomyocytes and reduced contraction amplitude without affecting Ca2+-transients. Treatment of cardiomyocytes with the A1 agonist cyclopentyladenosine (CPA) or the protein kinase A inhibitor H89 significantly attenuated the LPS-induced contractile dysfunction without effect on Ca2+-transients. Co-treatment with CPA and H89 completely reversed the contractile dysfunction. Increased cTnI phosphorylation in septic hearts was associated with a significant reduction in the protein expression of both the catalytic and regulatory subunits (B56α) of PP2A and a decrease in PP2A activity. CPA treatment of septic hearts increased PP2A activity. An increase in the protein expression of demethylated PP2A and a decrease in the PP2A-methyltransferase (PPMT; the methyltransferase that catalyses this reaction) were also observed.
Conclusion
These data support the hypothesis that sustained cTnI phosphorylation underlies the contractile dysfunction seen in sepsis.
KEYWORDS: Troponin I, Cardiomyocytes, Myofilaments, Phosphorylation, Protein phosphatase 2A
1. Introduction
Endotoxemia and sepsis involve a major inflammatory response, frequently associated with hypotension, vascular hyporeactivity, cardiac depression, and multiple organ failure. Despite prompt treatment with antibiotics and supportive care, the mortality of septic patients is still high, with the number of deaths from sepsis equalling the number of deaths from myocardial infarction in the USA.1 Myocardial dysfunction is a common complication in patients with severe sepsis and results in a significantly increased mortality.2 Such dysfunction is characterized by depressed ejection fraction and stroke work and increased end-diastolic volumes. Since the reduction in contractility is independent of alterations in cardiac load or heart rate, it largely represents an intrinsic depression of contractile function.3
Previous data from our laboratory have demonstrated that 16–18 h after LPS injection, endotoxemic rat hearts show increased cardiac troponin I (cTnI) phosphorylation at Ser23/24, residues that mediate protein kinase A (PKA)-dependent reduction of myofilament calcium (Ca2+) sensitivity.4 No reduction in the calcium transient was observed, suggesting that a reduction in myofilament Ca2+ sensitivity largely underlies the contractile dysfunction. More recent studies have confirmed the functional importance of increased cTnI phosphorylation in endotoxemia by demonstrating that mice with cardiac-specific replacement of cTnI with slow skeletal TnI (ssTnI), which lacks the PKA phosphorylation sites in the N-terminal, are significantly protected against LPS-induced cardiac dysfunction.5 Moreover, this protection was also observed in Triton-skinned cardiomyocytes. No degradation of cTnI or TnT in LPS-treated animals was observed nor were differences in the phosphorylation of other myofilament proteins between the groups detected.5 Such sustained increase in cTnI phosphorylation could result from either sustained kinase activation or inhibition of dephosphorylation pathways. Although the pathways regulating cTnI phosphorylation at the PKA sites are relatively well understood,6 the mechanism(s) regulating the dephosphorylation pathways remain unclear. A major dephosphorylating enzyme is protein phosphatase 2A (PP2A), a multimeric serine/threonine phosphatase which dephosphorylates both cTnI and phospholamban.7 PP2A predominately exists in vivo as a heterodimer of the catalytic subunit C constitutively bound to the scaffolding subunit A. These can then further complex with one of several regulatory B subunits. The B subunits contain all the targeting information that directs the heterotrimer to distinct intracellular locations.8,9 A number of agonists and compounds have been reported to activate PP2A including the adenosine A1-receptor agonist cyclopentyladenosine (CPA),7,10,11 β2-adrenoceptor agonists,12 cAMP,13 PAK 1,14 and Ca2+.15 CPA has been shown by a number of authors to induce the translocation of the C subunit of PP2A from the cytosol to the membrane7,10 and decrease phosphorylation of a number of protein targets including cTnI.7 Thus, the aim of this study was to investigate whether changes in the regulation of PP2A underlie the sustained phosphorylation of cTnI in myocytes from endotoxemic hearts.
2. Methods
2.1. Animal models
All experiments were performed in accordance with UK Home Office regulations and the investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23 revised 1996). C57/BL6 mice were injected with 7–9 mg/kg ip Escherichia coli bacterial lipopolysaccharide (LPS; serotype 0.11: B4, Sigma Aldrich, UK) or an equivalent volume of saline and sacrificed 16–18 h later.
2.2. Isolated myocytes
Ventricular myocytes were isolated from C57Bl/6 mice following a modified version of the AfCS procedure protocol (# PP00000125) (http://www.afcs.org). The digestion protocol typically yielded 60–70% rod-shaped, viable, Ca2+ tolerant myocytes from both control and septic hearts. The cells were stored in the final buffer at room temperature and used within 5–6 h. Some cells were loaded with the fluorescent Ca2+ indicator Indo-1 AM (2 µmol/L) as described previously.16
Single myocyte contractility and indo-1 AM fluorescence were studied on the stage of an inverted fluorescence microscope (Nikon Diaphot) coupled to a dual emission spectrophotometer (Cairn Research, Faversham, Kent) as described previously.5,17
2.3. Ventricular homogenate preparation
Control and septic hearts were retrogradely perfused with Krebs–Henseleit buffer (KHB) (CaCl2 1.25 mmol/L, glucose 10 mmol/L, and Na–pyruvate 5.0 mmol/L; bubbled with 95%O2:5%CO2 at 37°C) at a constant coronary perfusion pressure of 75 mmHg. Hearts were paced at 588 bpm. In some hearts, once the hearts were stable the perfusate was switched to KHB ± CPA (1 µmol/L) for 10 min, following which ventricles were snap-frozen in liquid nitrogen. All subsequent procedures were carried out at 4°C. Ventricles were thawed in buffer containing 250 mmol/L sucrose, 2 mmol/L EDTA, 2 mmol/L EGTA, 20 mmol/L HEPES, protease inhibitor cocktail, 1 mM sodium ortho-vanadate, and 1 mM NaF, pH 7.4, and homogenized (10% w/v) by a hand-held ground glass grinder and then sonicated three times in 10 s bursts. Samples were centrifuged (100 000 g for 1 h) to separate cytosolic and particulate fractions.
2.4. Gel electrophoresis and immunoblot analysis
Samples were re-suspended in Laemlli buffer with reducing agents and the protein levels were determined by RC-DC Bio-Rad protein assay with BSA as standard. For western analysis, 30 µg of soluble extract protein was used per lane. Samples were subjected to electrophoresis (10% gel) and transferred to nitrocellulose. After blocking (3%BSA/TBST 1 h, RT), the membranes were probed using a range of primary antibodies against PP2A B56α subunit (BD Transduction Laboratories), PP2A-C subunit, PP2A-A subunit, PP2A demethylated, P-TnI Ser23/24, total cTnI (Cell Signalling Technologies), anti-PP2A-methyltransferase/PPMT1, clone 4A4, anti-Rac-1 clone 23A8 (Upstate Cell Signalling Solutions), and HRP-conjugated secondary antibodies. The bound antibodies were visualized by chemiluminescence detection and protein levels were quantified by scanning densitometry using TotalLab© software.
2.5. Real-time reverse transcriptase–polymerase chain reaction
Total RNA was purified from heart homogenate using an SV RNA extraction kit (Promega, UK) and reverse transcribed using MLV-RT (Promega). Expression of the B56α subunit of PP2A was analysed by real-time RT–PCR using fluorescent SYBR Green technology on a Prism 7000 HT system (Applied Biosystems, USA), and with β-actin mRNA used for normalization. To obtain quantitative results, standard curves were constructed from cDNA standards for the B56α gene. Results were then normalized by expression as a molar/molar ratio to β-actin. The following primers were used (5′–3′): β-actin forward CGTGAAAAGATGACCCAGATCA, reverse TGGTACGACCAGAGGCATACAG; B56α forward TACAGTTGGTGTATGAATTCTTCTTGAG, reverse GAGCTGTTGGACAAACTTCTGATC.
2.6. Protein phosphatase 2A activity assay
Protein phosphatase 2A activity was measured in whole heart homogenate with a Malachite Green Phosphatase assay kit (Millipore). Samples were homogenized with a Dounce homogenizer in ice cold buffer containing 250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgSO4, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 0.1 mg/mL PMSF, 45 µg/µL aprotinin, and 1 mM sodium vanadate. Samples were then sonicated three times in 10 s bursts. The baseline phosphatase activity was measured and subtracted from all samples. Detergent was not included in the assay buffer as it was found that detergent reduced PP2A activity by ∼40% (data not shown). Samples were diluted to 25 µg of protein with phosphate-free buffer containing 0.1 M NaOH, 60 mM β-mercaptoethanol, 1 mM MgCl2, 1 mM EGTA, 0.1 mM MnCl2 1 mM DTT, 10% glycerol, and 0.1 mg/mL BSA, pH 7.4, and added to the wells of a 96-well plate. After addition of 5 µL of the phosphopeptide, samples were incubated for 30 min at room temperature. The reaction was terminated by addition of Malachite green solution. The colour was allowed to develop for 15 min at room temperature and the free phosphate levels were determined by measuring absorbance at 612 nm. The proportion of signal that could be inhibited by okadaic acid (10 nM) was considered as the PP2A activity, which was expressed as a percentage of total phosphatase activity.
2.7. Statistical analysis
All data are shown as mean ± SEM of at least three different experiments. Significance was determined either with an unpaired two-way Student's t-test or with one-way ANOVA with Student–Newman–Keul's post hoc analysis (Prism 4.0, GraphPad, USA). P < 0.05 was considered significant.
3. Results
3.1. Depression of cardiac function in septic myocytes is reversed by the combination of an A1 agonist and a protein kinase A inhibitor
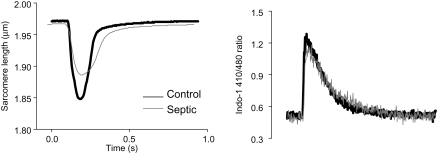
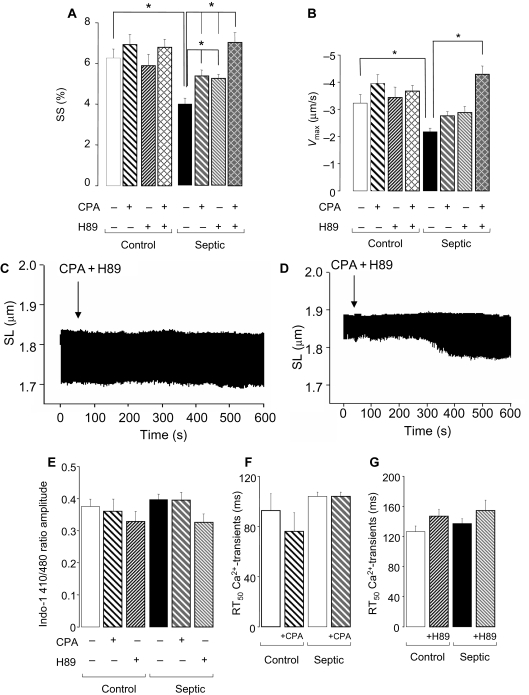
In line with our previous studies,5 unloaded sarcomere shortening (SS) and the maximum shortening velocity (Vmax) were significantly reduced in septic myocytes (P < 0.05; Figures 1 and 2A and B) 16–18 h following LPS administration. This reduction in contractility was not associated with any changes in the Ca2+-transients (Figures 1B and 2E–G), suggesting that a decrease in myofilament Ca2+ sensitivity underlies the contractile dysfunction induced by LPS. Treatment of control cells with CPA (1 µmol/L) or H89 (1 µmol/L) (individually or in combination) had no effect on SS or Vmax (Figure 2A and B). In contrast, in septic cells, SS was significantly improved with either CPA or H89 (from 3.9 ± 0.3% to 5.4 ± 0.3% and 5.2 ± 0.2%, respectively; P < 0.05; Figure 2A) although the reduction in Vmax was not significantly altered (Figure 2B). Furthermore, when septic cells were treated with both CPA and H89 together there was a further improvement in SS (over and above either treatment alone) to 7.0 ± 0.5% (P < 0.05 vs. control septic or septic + CPA or H89; Figure 2A, C, and D). Thus, the combination restored contraction amplitude such that it was not significantly different from that of control myocytes. None of these treatments affected the Ca2+ transient (Figure 2E–G). The combination of CPA and H89 significantly improved Vmax to control levels (from −2.2 ± 0.1 to −4.3 ± 0.3 µm/s; P < 0.01; Figure 2B).
Figure 1.
(A) Representative trace of sarcomere shortening from representative myocytes from saline (black line)- or lipopolysaccharide (LPS; grey line)-treated hearts. (B) Associated intracellular Ca2+-transients (Indo-1 410/480 ratio) of myocytes from saline (black trace)- or LPS (grey trace)-treated hearts.
Figure 2.
(A) Averaged contraction data from saline- or lipopolysaccharide-treated mouse myocytes in the presence of cyclopentyladenosine (CPA) (1 µmol/L), H89 (1 µmol/L), or co-treatment with both CPA and H89 (n = 80–100 cells from three hearts; *P < 0.01) and (B) maximum shortening velocity (Vmax, µm/s). (C and D) Representative traces for single cells showing the effect of co-treatment with both CPA and H89 for 10 min on a control (C) and septic cell (D). (E) Amplitude of Ca2+-transients (Indo-1 410/480 ratio) and RT50 (ms) of Ca2+-transients in the presence of (F) CPA or (G) H89. Bars represent mean ± SE of the mean.
3.2. Cyclopentyladenosine reverses increased cardiac troponin I phosphorylation associated with sepsis
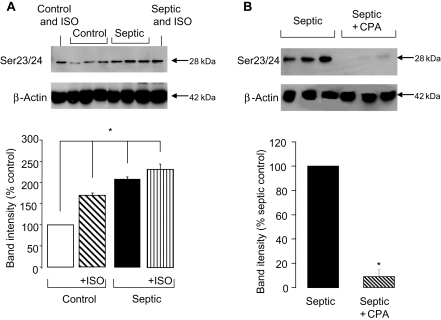
In line with previous studies,4 the depression in contractile function in septic myocytes was associated with a significant (∼2-fold) increase in cTnI phosphorylation at Ser23/24 (P < 0.05; Figure 3A). The increase in phosphorylation in septic myocytes was equivalent to that achieved in control myocytes with isoprenaline treatment. However, in septic myocytes, isoprenaline was unable to elicit any additional phosphorylation at Ser23/24 suggesting that these sites may be already maximally phosphorylated.
Figure 3.
(A) Representative blot of cardiac troponin I (cTnI) phosphorylation at Ser23/24 with β-actin loading control and quantification of Ser23/24 phosphorylation in control and septic myocytes in the presence and absence of 10 nmol/L isoprenaline (ISO; n = 3 hearts/gp, *P < 0.05). (B) Representative blot and quantification of cTnI phosphorylation at Ser23/24 in septic hearts in the presence and absence of 1 µmol/L cyclopentyladenosine (n = 3 hearts/gp, *P < 0.001). Band intensities are normalized to β-actin as a loading control.
In line with the hypothesis that increased cTnI phosphorylation is a fundamental mechanism underlying the contractile defect in septic myocytes, CPA treatment completely reversed the LPS-induced increase in Ser23/24 phosphorylation (Figure 3B).
3.3. Protein phosphatase 2A-C expression is significantly decreased in septic hearts
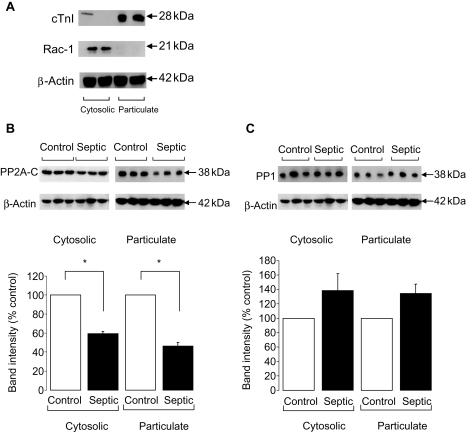
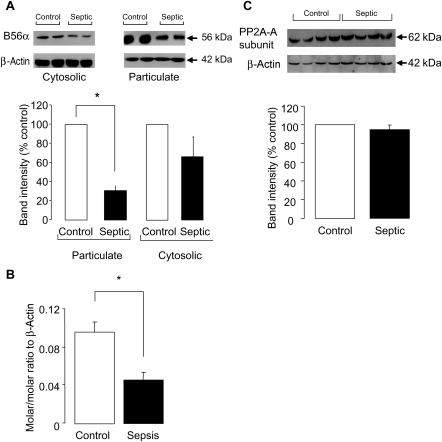
The increase in cTnI phosphorylation could result from either a sustained kinase activation or decreased phosphatase (particularly PP2A) activity. Hence, we undertook a series of experiments to investigate PP2A expression and activity in control and septic hearts. Since the vast majority of the C subunit resides in the cytosol and a relatively small percentage of this is translocated to the particulate fraction upon activation7,10 for most experiments, western blot analysis was performed on cytosolic and particulate fractions that were probed for cTnI and Rac-1, respectively, to ascertain fractionation (Figure 4A). In both cytosolic and particulate fractions, PP2A-C expression was significantly decreased in septic ventricles (P < 0.05; Figure 4B). In contrast, PP1 expression was not significantly altered in septic ventricles in either the cytosolic or particulate fractions (Figure 4C).
Figure 4.
(A) Representative blots showing fractionation of heart homogenate into cytosolic and particulate fractions using cardiac troponin I and Rac-1, respectively. (B) Representative blot and quantification of the amount of total protein phosphatase 2A (PP2A)-C in control and septic ventricular homogenates (n = 3 hearts/gp, *P < 0.05). (C) Representative blot and quantification of the amount of total PP1 in control and septic ventricular homogenates (n = 3 hearts/gp, P = ns). Band intensities are normalized to β-actin as a loading control.
3.4. Protein phosphatase 2A-B56α expression is significantly decreased in septic hearts
The B subunit of PP2A is thought to contain the targeting information that directs the heterotrimer to specific cellular compartments. Hence, loss of the B56α subunit may prevent translocation of the catalytic unit to the required location. Western blot analysis demonstrated a dramatic reduction in the expression of the B56α subunit by 69 ± 5% in septic myocytes in the particulate fraction (Figure 5A). A trend to a reduction in the cytosolic fraction was also observed, although this did not reach statistical significance (Figure 5A). To determine whether this reduction in protein levels was a result of a reduction at the mRNA level, we analysed mRNA expression in whole heart homogenate. As shown in Figure 5B, mRNA levels of B56α subunit showed a >50% reduction in septic hearts (LPS 0.046 ± 0.008 vs. control 0.096 ± 0.01 molar/molar ratio to β-actin). No change in protein expression of PP2A-A subunit was observed (Figure 5C).
Figure 5.
(A) Representative blot and quantification of the amount of the B56α subunit of protein phosphatase 2A (PP2A) in control and septic hearts separated into particulate and cytosolic fractions (n = 3 hearts/gp, *P < 0.001). Band intensities are normalized to β-actin as a loading control. (B) Amount of mRNA of the B56α subunit present in control and septic hearts determined by RT–PCR (n = 3 hearts/gp, *P < 0.01). (C) Representative blot and quantification of the amount of PP2A-A subunit in septic and control hearts (n = 4 hearts/gp). Band intensities are normalized to β-actin as a loading control.
3.5. Methylation of the C subunit is decreased in septic hearts
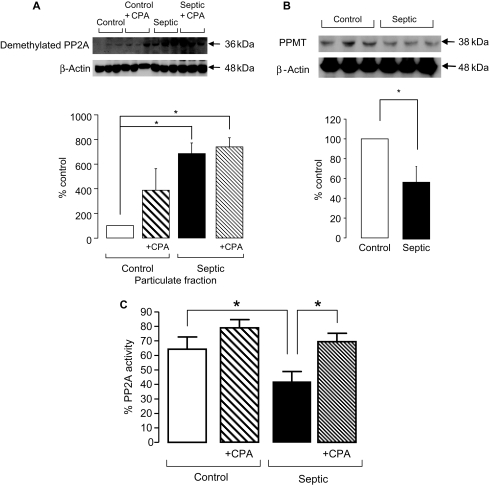
Post-translational methylation at Leu309 of the C subunit is thought to be an important mechanism for regulating the activity of PP2A. Methylation is thought to activate the enzyme and allow binding of the B subunit. We used an antibody specific for the demethylated (i.e. inactive) form of PP2A-C since there is no antibody available which recognizes the methylated form. Figure 6A demonstrates that there was a significant increase in the level of the demethylated form of PP2A-C in the particulate fraction of septic hearts. No changes were observed with CPA treatment, suggesting that CPA would not increase PP2A activity by increasing the methylation status of the catalytic subunit in septic hearts. No changes were observed in the cytosolic fractions (data not shown). Furthermore, the expression of the specific methyltransferase (PPMT) required to methylate PP2A-C18 was also shown to be significantly decreased in septic hearts (by ∼60% and 50%, respectively; Figure 6B).
Figure 6.
(A) Representative blot and quantification of the amount of demethylated protein phosphatase 2A (PP2A)-C in the particulate fractions of control and septic hearts (n = 3 hearts/gp, *P < 0.01). (B) Representative blot and quantification of the amount of PPMT in control and septic hearts (n = 3 hearts/gp, *P < 0.01). Band intensities are normalized to β-actin as a loading control. (C) Averaged data from three separate experiments of PP2A activity assay in septic and control hearts in the presence and absence of cyclopentyladenosine as determined by malachite green assay with absorbance measured at 612 nm (n = 4–7 hearts/gp, *P < 0.05).
3.6. Cyclopentyladenosine treatment increases protein phosphatase 2A activity in septic hearts
In line with the significant reduction in protein expression of PP2A-B and -C subunits, a significant (P < 0.05) reduction in PP2A activity was also observed in septic heart homogenate compared with controls (Figure 6C). Treatment with CPA significantly increased PP2A activity in septic hearts (P < 0.05; Figure 6C). Taken in conjunction with the other data presented here, this finding strongly supports the hypothesis that a decrease in PP2A activity in sepsis results in increased cTnI phosphorylation and thus the pronounced contractile dysfunction observed in sepsis.
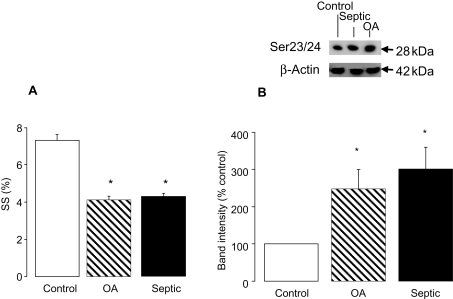
3.7. Okadaic acid mimics the contractile dysfunction seen in septic hearts
Okadaic acid is a protein phosphatase inhibitor that demonstrates selectivity for PP2A at concentrations <1 µmol/L.19,20 We reasoned that if the effects of sepsis are due in part to decreased PP2A activity, treatment of control myocytes with okadaic acid at doses specific for PP2A should mimic the contractile effects of sepsis. Figure 7 demonstrates that pre-treatment of control cardiomyocytes with okadaic acid (1 nmol/L) for 10 min reduced SS to almost exactly the same level as LPS treatment (Figure 7A). A similar effect was observed on Vmax (data not shown). In line with this, cTnI phosphorylation on Ser23/24 was increased by 248 ± 52% following okadaic acid treatment, a level similar to the increase found in septic myocytes (301 ± 59%) (Figure 7B).
Figure 7.
(A) Averaged contraction data for control, control + okadaic acid (OA) (1 nmol/L), and septic myocytes (n = 60–80 cells from three hearts; *P < 0.05); and (B) representative blot and quantification of Ser23/24 phosphorylation for control, control + OA (1 nmol/L), and septic myocytes. Band intensities are normalized to β-actin as a loading control.
4. Discussion
Previous data from our laboratory have demonstrated that endotoxemia is associated with a sustained phosphorylation of cTnI at the PKA sites, serines 23/24, that results in a reduction in myofilament calcium sensitivity.4,5 Such an sustained increase in cTnI phosphorylation could result from either prolonged kinase activation or phosphatase inhibition. Pathways regulating the phosphorylation of cTnI at the PKA sites are relatively well understood;6 however, potential regulatory mechanisms controlling the dephosphorylation of cTnI remain unclear.
It was first suggested that PP2A was responsible for dephosphorylating cTnI by Mumby et al.21 Neuman et al.22 then described how inhibition of PP2A resulted in increased phosphorylation of contractile proteins such as cTnI and phospholamban without reducing cAMP levels. PP2A is thought to be the phosphatase mainly responsible for the dephosphorylation of both cTnI7 and α-tropomyosin23 in the sarcomere and phospholamban in the sarcoplasmic reticulum.7 PP2A has an important role in maintaining normal cardiac function, highlighted by the following observations: (i) inhibition of PP2A resulted in increased phosphorylation of cTnI and phospholamban without affecting cAMP levels22 and (ii) transgenic mice that over-expressed the catalytic PP2A Cα in the heart developed cardiac hypertrophy, cardiac dilatation, and reduced contractility.24 The latter study also reported significant reduction in the phosphorylation of phospholamban, cTnI, and eukaryotic elongation factor 2.
The major finding of the present study is that PP2A expression, regulation, and activity are significantly impaired in septic hearts. Also there is a significant reduction in the protein expression of PPMT which methylates PP2A and a concomitant increase in the level of demethylated (inactive) PP2A present in the septic hearts. This impairment in PP2A is associated with an increase in cTnI phosphorylation at Ser23/24 and a marked impairment of cardiac myocyte contractile function. The A1 adenosine agonist CPA significantly increased PP2A activity, reduced cTnI phosphorylation, and improved contractile function in septic myocytes.
Protein phosphatase 2A is a serine/threonine phosphatase in which the catalytic (C) subunit is constitutively bound to the scaffolding (A) subunit. This heterodimer can then further complex with one of several regulatory B subunits, the identity of which is believed to determine the enzymatic activity, substrate specificity, and subcellular localization of the holoenzyme.8,9,25 Although PP2A is thought to exist predominantly as a trimer, there are several studies which suggest that the AC dimer and/or C alone may play significant roles in vivo.26–28
Regulation of PP2A activity is complex and mediated by several posttranslational modifications of both the B and C subunit. The C subunit can undergo posttranslational modifications such as carboxyl methylation of Leu309 at the C-terminus by a methyltransferase, and phosphorylation at Thr307 whereby methylation activates the enzyme and allows binding to the regulatory B subunit29,30 and phosphorylation inhibits activity.31 Methylation of the C unit is a highly conserved mechanism and therefore is thought to play a crucial role in PP2A regulation. Further regulation of PP2A activity is provided by two non-competitive inhibitor proteins termed I1PP2A and I2PP2A,32. I1PP2A is thought to be largely nuclear,33 whereas I2PP2A is diffuse within the cytosol.34 Both inhibitor proteins have acidic tails through which they may mediate their inhibitory effect on the phosphatase.35
Deregulation of PP2A is known to occur in Alzheimer's disease where an accumulation of phosphorylated tau proteins is a central pathological feature.18,36,37 Sontag et al.36 have demonstrated decreased expression levels of all the subunits of PP2A in the brains of Alzheimer's patients, in addition to changes in the carboxymethylation status of PP2A-C.18
Under basal conditions, the majority of PP2A-C resides in the cytoplasm and activation of the A1 receptor induces translocation to the particulate fraction.7,10 In the current study, we have demonstrated a significantly decreased expression of the catalytic subunit of PP2A in both the cytosolic and particulate fractions of septic hearts when compared with controls (P < 0.05) (Figure 4B). As such, activation of PP2A by CPA (Figure 6A) was associated with a marked decrease in cTnI phosphorylation and an improvement in contractile function (Figures 2 and 3). This is in line with the hypothesis that impaired PP2A activity and localization underlies the contractile dysfunction.
As discussed above, the B subunits are thought to regulate the site of action of PP2A. Hence, the decrease in expression of the B56α subunit in the septic hearts, which is markedly clear in the particulate fraction, may provide one mechanism for the increased cTnI phosphorylation observed in these hearts. This decrease in protein expression was paralleled by a decrease in mRNA expression by ∼50% and again mirrors the results in Alzheimers disease.36 Similarly, Glaser et al.,30 in an established model of sustained JNK activation, reported a 70% decrease in expression of B56α in both neonatal and adult cardiomyocytes.30 A variety of other stress-related stimuli, such as p38 MAPK activation, phorbol esters, and LPS, also decreased B56α expression in this isolated myocyte preparation.30
Although several authors7,10,37 have demonstrated that CPA can induce translocation of PP2A-C to the membrane fraction, the mechanisms that regulate this process are far from clear. As discussed above, α-carboxyl methyl esterification of the C subunit has been reported to regulate PP2A by modulating holoenzyme assembly.29 Therefore, we hypothesized that decreased methylation would correlate with a decreased association with the B subunit, decreased activity, and possibly changes in localization. Using an antibody that specifically recognizes the demethylated form of PP2A-C, we demonstrated a significant increase (∼35-fold) in the demethylated form of PP2A-C in the particulate fraction of septic hearts (Figure 6A). Given that the overall expression of PP2A-C is significantly decreased, this represents a large decrease in the amount of the methylated form. However, we could not demonstrate a reduction in demethylated PP2A-C following CPA treatment in either control or septic hearts. This is in contrast to the results of Liu and Hofmann7 who demonstrated a decrease in the level of demethylated PP2A-C following a 5 min of incubation with CPA. Methylation of PP2A-C is catalysed by a specific Leu carboxyl methyltransferase PPMT.18,29 Hence, we examined the protein expression of PPMT in the cytosolic and particulate fractions of control and septic hearts. In line with the increase in demethylated PP2A-C (reflecting a decreased methylation), the amount of PPMT was significantly decreased in septic hearts (Figure 6B). Thus, the increased cTnI phosphorylation observed in septic hearts could reflect a decreased PP2A-C methylation as a result of a significant decrease in the methyltransferase that methylates this subunit. The consequence of this would be a decreased association of the B subunit with the A/C heterodimer, an association required for targeting and activation of the holoenzyme. Furthermore, the decreased expression of B56α at both protein and mRNA level may reflect a failure to associate with the catalytic subunit since only the heterotrimeric forms of PP2A appear to be stable in intact cells.9 Additional support for a role for PP2A in the modulation of contractile function via dephosphorylation of cTnI is provided by the demonstration that septic hearts have significantly lower PP2A activity compared with control cells. Furthermore, treatment with CPA not only improved contractile function (Figures 1 and 2) and reduced cTnI phosphorylation (Figure 3B) but also increased PP2A activity (Figure 6C). Also, incubation of control cells with okadaic acid, at a concentration that specifically inhibits PP2A, mimicked the effects of sepsis in terms of both decreased contractile function and increased cTnI phosphorylation (Figure 7).
Treatment of septic cardiomyocytes with the PKA inhibitor H89 improved contractile function to a similar extent to CPA treatment (Figure 2), while the combination of CPA and H89 further improved contractile function to baseline levels. Both of these treatments were independent of changes in the Ca2+ transient, suggesting that the improvement in contractile function reflected a restoration in myofilament Ca2+ sensitivity. The mechanism underlying the beneficial effect of H89 requires further investigation. While it is likely that H89 is acting by directly inhibiting PKA-mediated phosphorylation of cTnI, it is also possible that the mechanism involves dephosphorylation and uncoupling of the β2-adrenoceptor from Gi. Hence, Daaka et al.38 demonstrated that stimulation of MAPK via Gi requires phosphorylation of the β2-receptor by PKA and that H89 inhibited isoprenaline-mediated activation of MAPK. Similarly, Faucher et al.39 recently demonstrated that PKA activation was a prerequisite for the negative inotropic effect induced by the β2-agonist salbutamol in guinea-pig papillary muscles. As such, H89 significantly reduced the negative inotropic effect induced by salbutamol. Furthermore, okadaic acid (at a dose that would inhibit both PP1 and PP2A) significantly increased the sensitivity of the β2-adrenoceptor to salbutamol suggesting phosphatases modulate the response. Lastly, in adult myocytes isolated from rats injected with LPS, the decrease in cell shortening and Ca2+-transients could be reversed by the Gi inhibitor, pertussis toxin,40 again suggesting that Gi protein signalling plays a role in the negative inotropic effect of LPS. In the light of these studies, the results of the current study suggest that in septic cardiomyocytes, the β2-adrenoceptor is phosphorylated thereby switching the signalling to Gi. Importantly, use of a specific cGMP-dependent protein kinase inhibitor (Rp-8-Br-cGMPs; 10 µM) had no effect on either SS or the Ca2+ transient in control or septic cardiomyocytes (data not shown). This excludes a non-specific effect of H89 on PKG. However, such a mechanism would require that Gi signalling from the A1-receptor activates different signalling pathways to β2-mediated Gi signalling if the beneficial effects of CPA and H89 are to be reconciled. Further studies will be required to explore this protective effect.
In conclusion, the studies presented here strongly support the hypothesis that the increased cTnI phosphorylation and reduced contractility apparent in cardiomyocytes from LPS-treated mice results, at least in part, from an inhibition of PP2A activity. This reduction in PP2A activity may reflect a decrease in PPMT expression resulting in decreased PP2A-C methylation, an inability to assemble the holoenzyme and thus increased cTnI phosphorylation. The inability to form the holoenzyme may underlie the reduction in expression of the B56α and C subunits in septic hearts.
Conflict of interest: none declared.
Funding
This study was supported by a British Heart Foundation (BHF) Project Grant (PG/03/107) and BHF award (CH/99001). Funding to pay the Open Access charge was provided by the BHF.
References
- 1.Maeder M, Fehr T, Rickli H, Ammann P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 2.Parrillo JE. Pathogenic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 3.Grocott-Mason RM, Shah AM. Cardiac dysfunction in sepsis: new theories and clinical implications. Intensive Care Med. 1998;24:286–295. doi: 10.1007/s001340050570. [DOI] [PubMed] [Google Scholar]
- 4.Tavernier B, Li J-M, El-Omar MM, Lanone L, Yang Z-K, Trayer IP, et al. Cardiac contractile impairment associated with increased phosphorylation of troponin I in endotoxemic rats. FASEB J. 2001;15:294–296. doi: 10.1096/fj.00-0433fje. [DOI] [PubMed] [Google Scholar]
- 5.Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19:1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- 6.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Hofmann PA. Antiadrenergic effects of adenosine A1 receptor-mediated protein phosphatase 2a activation in the heart. Am J Physiol. 2002;283:H1314–H1321. doi: 10.1152/ajpheart.00343.2002. [DOI] [PubMed] [Google Scholar]
- 8.Turowski P, Myles T, Hemmings BA, Fernandez A, Lamb NJ. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol Biol Cell. 1999;10:1997–2015. doi: 10.1091/mbc.10.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snabaitis AK, D'Mello R, Dashnyam S, Avkiran M. A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem. 2006;29:20252–20262. doi: 10.1074/jbc.M600268200. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Hofmann PA. Modulation of protein phosphatase 2A by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol. 2003;285:H97–H103. doi: 10.1152/ajpheart.00956.2002. [DOI] [PubMed] [Google Scholar]
- 12.Pullar CE, Chen J, Isseroff RR. PP2A activation by β2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555–22562. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- 13.Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ. A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther. 2002;302:111–118. doi: 10.1124/jpet.302.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 15.Yasuoka C, Ihara Y, Ikeda S, Miyahara Y, Kondo T, Kohno S. Antapoptotic activity of AKT is down-regulated by Ca2+ in myocardial H9c2 cells. J Biol Chem. 2004;279:51182–51192. doi: 10.1074/jbc.M407225200. [DOI] [PubMed] [Google Scholar]
- 16.Layland J, Li J-M, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layland J, Grieve DJ, Cave AC, Sparks E, Solaro RJ, Shah AM. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J Physiol. 2004;556:835–847. doi: 10.1113/jphysiol.2004.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sontag E, Hladik C, Montgomery L, Luangpiprom A, Mudrak I, Ogris E, et al. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63:1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- 19.Douglas P, Moorhead GBG, Ye R, Lees-Miller SP. Protein phosphatases regulate DNA-dependent protein kinase activity. J Biol Chem. 2001;276:18992–18998. doi: 10.1074/jbc.M011703200. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, Mumby MC. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2A. J Biol Chem. 1999;274:31917–31924. doi: 10.1074/jbc.274.45.31917. [DOI] [PubMed] [Google Scholar]
- 21.Mumby MC, Russell KL, Garrard LJ, Green DD. Cardiac contractile protein phosphatases. J Biol Chem. 1987;262:6257–6265. [PubMed] [Google Scholar]
- 22.Neumann J, Botnik P, Bodor GS, Jones LR, Schmitz W. Effects of adenosine receptor and muscarinic cholinergic receptor agonists on cardiac protein phosphorylation. Influence of pertussis toxin. J Pharmacol Exp Ther. 1994;269:1310–1318. [PubMed] [Google Scholar]
- 23.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, et al. P38-MAPK induced dephosphorylation of α-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- 24.Gergs U, Botnik P, Buchwalow I, Fabritz L, Matus M, Justus I, et al. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem. 2004;279:40827–40834. doi: 10.1074/jbc.M405770200. [DOI] [PubMed] [Google Scholar]
- 25.Ikehara T, Ikehara S, Imamura S, Shinjo F, Yasumoto T. Methylation of the C-terminal leucine residue of the PP2A catalytic subunit is unnecessary for the catalytic activity and the binding of regulatory subunit (PR55/B) Biochem Biophys Res Commun. 2007;354:1052–1057. doi: 10.1016/j.bbrc.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 26.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: Abundant expression of both forms in cells. Mol Cell Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata K, Wu J, Brautigan DL. B cell receptor-associated protein α4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung H, Nairn AC, Murata K, Brautigan DL. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favours association with the α4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry. 1999;38:10371–10376. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- 29.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaser ND, Lukyanenko YO, Wang Y, Wilson GM, Rodgers TB. JNK activation decreases PP2A regulatory subunit B56α expression and mRNA stability and increases AUF1 expression in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291:H1183–H1192. doi: 10.1152/ajpheart.01162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Parsons S, Brautigan DL. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem. 1994;269:7957–7962. [PubMed] [Google Scholar]
- 32.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 33.Fornerod M, Boer J, van Baal S, Jaegle M, von Lindern M, Murti KG, et al. Relocation of the carboxyterminal part of CAN from the nuclear envelope to the nucleus as a result of leukemia-specific chromosome rearrangements. Oncogene. 1995;10:1739–1748. [PubMed] [Google Scholar]
- 34.Vaesen M, Barnikol-Watanabe S, Götz H, Awni LA, Cole T, Zimmermann B, et al. Purification and characterization of two putative HLA class II associated proteins: PHAPI and PHAPII. Biol Chem Hoppe Seyler. 1994;375:113–126. doi: 10.1515/bchm3.1994.375.2.113. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Makkinje A, Damuni Z. Molecular identification of I, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry. 1996;35:6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- 36.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, et al. Altered expression levels of the protein phosphatase 2A ABαC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 37.Brust TB, Cayabyab FS, Zhou N, MacVicar BA. p38 mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat hippocampus. J Neurosci. 2006;26:12427–12438. doi: 10.1523/JNEUROSCI.4052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the [beta]2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 39.Faucher FA, Gannier FE, Lignon JM, Cosnay P, Malécot CO. Roles of PKA, PI3K and cPLA2 in the NO-mediated negative inotropic effect of {beta}2-adrenoceptor agonists in guinea pig right papillary muscles. Am J Physiol Cell Physiol. 2008;294:C106–C117. doi: 10.1152/ajpcell.00231.2007. [DOI] [PubMed] [Google Scholar]
- 40.Mittra S, Bourreau JP. Gs and Gi coupling of adrenomedullin in adult rat ventricular myocytes. Am J Physiol. 2006;290:H1842–H1847. doi: 10.1152/ajpheart.00388.2005. [DOI] [PubMed] [Google Scholar]