Abstract
Brevetoxins and ciguatoxins are closely related potent marine neurotoxins. Although ciguatoxins accumulate in fish to levels that are dangerous for human consumption, live fish have not been considered as potential sources of brevetoxin exposure in humans. Here we show that, analogous to ciguatoxins, brevetoxins can accumulate in live fish by dietary transfer. We experimentally identify two pathways leading to brevetoxin-contaminated omnivorous and planktivorous fish. Fish fed with toxic shellfish and Karenia brevis cultures remained healthy and accumulated high brevetoxin levels in their tissues (up to 2675 ng g−1 in viscera and 1540 ng g−1 in muscle).
Repeated collections of fish from St. Joseph Bay in the Florida panhandle reveal that accumulation of brevetoxins in healthy fish occurs in the wild. We observed that levels of brevetoxins in the muscle of fish at all trophic levels rise significantly, but not to dangerous levels, during a K. brevis bloom. Concentrations were highest in fish liver and stomach contents, and increased during and immediately following the bloom. The persistence of brevetoxins in the fish food web was followed for 1 year after the K. brevis bloom.
Keywords: Brevetoxins, Ciguatoxins, Karenia brevis, Gambierdiscus toxicus, Neurotoxic Shellfish Poisoning (NSP), Ciguatera Fish Poisoning (CFP), Food web transfer, Vector, Ichthyotoxins, Human health
1. Introduction
Brevetoxins and ciguatoxins are potent marine neurotoxins. The source for the former is the planktonic red tide dinoflagellate Karenia brevis (McFarren et al., 1965; Baden et al., 1979) and for the latter is the epibenthic dinoflagellate Gambierdiscus toxicus (Yasumoto et al., 1977; Lewis et al., 2000). Both groups of toxins have similar chemical natures and similar biological activities. They are lipid-soluble polycyclic polyether compounds and are the only molecules known to activate voltage-sensitive sodium channels in mammals through a specific interaction with site 5 of the alpha subunit of the sodium channel (Poli et al., 1986; Lombet et al., 1987; Dechraoui et al., 1999). Both are toxic to mammals, and in humans the ingestion of brevetoxin-contaminated shellfish (Halstead, 1978; Steidinger, 1993) and of ciguatoxin-contaminated fish (Bagnis et al., 1979) results in severe forms of food poisoning—Neurotoxic Shellfish Poisoning (NSP) and Ciguatera Fish Poisoning (CFP), respectively. Because both toxins possess similar modes of action, the clinical manifestations following ingestion, although more severe, varied and longer-lasting for genuine ciguatera, are quite similar, with gastrointestinal, neurological and cardiovascular components (Bagnis et al., 1979; Baden et al., 1995; Poli et al., 2000; Kirkpatrick et al., 2004). The reversal of temperature discrimination, also known as paradoxical dysthesia, is probably the most characteristic symptom associated with ciguatera poisoning (Bagnis et al., 1979), but is also documented in severe cases of brevetoxin poisoning (Baden et al., 1995).
Despite these similarities, these toxins present obvious differences in their impacts on fish. Ciguatoxins are well known to accumulate in fish by trophic transfer in tropical fish food webs (Legrand, 1991), and have not been documented in association with fish mortalities. In contrast, K. brevis blooms (Florida red tides) characteristically result in massive fish kills (Steidinger et al., 1973; Landsberg, 2002). Because of the fish kills routinely observed along the west coast of Florida during red tides, it has been assumed that the ichthyotoxicity of brevetoxins precluded their vectoring or accumulation via live fish.
In 2004, a mass mortality of bottlenose dolphins (Tursiops truncatus) in the Florida panhandle clearly indicated that fish are not always killed by K. brevis red tides and that they have the potential to vector brevetoxins to higher trophic levels (Flewelling et al., 2005). Besides a coincident mortality of large redfish (Sciaenops ocellatus), no obvious indicators of a red tide were reported (i.e. there was no obvious bloom or other animal mortalities). However, high levels of brevetoxins were measured in multiple tissues of all bottlenose dolphins examined (n = 36). Tissues from six undigested menhaden (Brevoortia sp.) recovered from the stomach contents of some dolphins contained excessive levels of brevetoxins, with concentrations reaching 33,200 ng g−1 in viscera and 1500 ng g−1 in muscle. At least eight other species of fish collected live from the area while the mortality was ongoing also contained elevated, but much lower, concentrations of brevetoxins in their tissues (Flewelling et al., 2005). During this event, we confirmed that bottlenose dolphins are susceptible to brevetoxicosis and that planktivorous fish can vector lethal concentrations of brevetoxins to higher trophic levels.
The goals of the present study were to experimentally identify pathways by which brevetoxins may accumulate in the tissues of live fish, and to determine whether the brevetoxin accumulation that we previously observed in live fish (Flewelling et al., 2005) was an exception or alternatively, an undocumented common occurrence.
2. Materials and methods
2.1. Experimental exposure of omnivorous fish to contaminated shellfish
Hard clams (Mercenaria sp.) naturally contaminated with brevetoxins were collected from Charlotte Harbor, Florida during a red tide in March of 2003 and stored at −20 °C for 2 months prior to the experiments. Locally harvested nontoxic hard clams (M. mercenaria) were purchased in North Carolina immediately before the experiments began.
Adult pinfish (Lagodon rhomboides; 12–17 cm total length) and Atlantic croakers (Micropogonias undulatus; 15–22 cm total length) were collected using hook-and-line gear from the Intracoastal Waterway in Wilmington, NC, USA. Fish (36 per species) were maintained in separate 24-l aquaria (3 fish per aquarium, 24 aquaria) in natural filtered seawater under constant flow and aeration. Salinity of the seawater source (Intracoastal Waterway) varied between 20 and 32 ppt. Fish were acclimated to aquaria conditions for 1 week, during which they were fed shucked nontoxic clams (7–10 g each, one clam per fish) twice per day. After acclimation, fish were fed shucked toxic hard clams (7–10 g each, one clam per fish) twice daily for 14 days, and feeding was confirmed by direct observation. Fish were then fed nontoxic shucked hard clams (7–10 g each, one clam per fish) from North Carolina twice daily for another 14 days. Three pinfish and three croakers each were sampled at days 0, 7, 14, 21 and 28. Control fish were similarly fed nontoxic hard clams throughout the experiment and were sampled in duplicate at days 0, 7, 14, 21 and 28.
2.2. Experimental exposure of planktivorous fish to K. brevis culture
Juvenile striped mullet (Mugil cephalus; 3–4 cm total length) were collected using a dip net from the Intracoastal Waterway in Wilmington, NC, and acclimated to synthetic seawater media (NH15) under aeration in 4-l aquaria for 48 h. Then 10 fish each were exposed to four densities of K. brevis cells (Wilson clone). K. brevis cultures were grown in NH15 media at 30 ppt. Only K. brevis cultures in exponential growth phase were used. Cell density of the culture was determined by enumeration of Lugol's iodine-preserved aliquots at 100× using a Nikon light microscope. Aeration in the aquaria was stopped before addition of the culture to prevent lysis of the K. brevis cells. After addition of the culture, final cell densities were 500, 1000, 2000 and 4000 cells ml−1, all within the range of densities observed in the Gulf of Mexico during red tides. Cell integrity was confirmed by microscopic examination. Five fish were sampled from each aquarium after 6 h and again after 24 h. Dissolved brevetoxin concentrations and total brevetoxin concentrations in the water were measured after 6 and 24 h. Control fish (juvenile striped mullet held in NH15 media and not exposed to K. brevis) were sampled on the same schedule.
To demonstrate brevetoxin ichthyotoxicity, identical experiments were performed, but K. brevis cells were lysed by sonication (15 min) before adding the culture to the aquaria. After adding the lysed K. brevis culture, fish were continually monitored and the time to death was recorded to the nearest minute. Juvenile striped mullet held in NH15 media and not exposed to K. brevis served as control fish.
2.3. Field collections
Collections of live fish were made from St. Joseph Bay in northwest Florida in February, June, September and November of 2005, and in May, August and November of 2006. Fish were collected using a 3.6-m cast net (19-mm mesh) as well as hook-and-line gear. Water samples were collected using a horizontal PVC beta bottle (3.2 l; Wild-co™) from 0.5 m below the surface and from 1 m above the bottom at fish collection sites and multiple other sites (10–20) throughout St. Joseph Bay. Aliquots (500 ml) for brevetoxin analysis were stored on ice in polyethylene bottles while in the field and frozen at −20 °C immediately upon return until processed.
K. brevis cells were enumerated from Lugol's iodine-preserved aliquots. Samples were mixed by inverting (> 10×) and 3 ml placed in a Lab-Tek chamber (Nalge Nunc # 155380). Cells were allowed to settle for at least 1 h prior to enumerating. Samples were identified and enumerated at 100× and 400× using a Zeiss Axiovert 25 or Olympus IX71 inverted microscope. Identifications were based on Haywood et al. (2004) and descriptions published in Steidinger et al. (in press). In addition to the water samples we obtained during each fish collection, K. brevis cell counts were also obtained for five fixed sites in St. Joseph Bay that were sampled every month by the Florida Department of Environmental Protection's St. Joseph Bay Aquatic Preserve staff from January 2005 through December 2006.
2.4. Sample extractions
Fish from exposure studies were euthanized by exposure to a lethal concentration of MS-222 anesthetic (tricaine methanesulfonate, 50 mg l−1). Death was confirmed by absence of ventilation and absence of reaction after stimulus. From pinfish and croaker, muscle, liver and remaining viscera were extracted separately. Due to the small size of the juvenile mullet, muscle was separated from viscera and each was extracted separately. Field-caught fish were weighed and their total length was measured. Muscle, liver and stomach contents were sampled, weighed and extracted separately. Brevetoxins in fish tissues and stomach contents were extracted by homogenization in 100% acetone (4 ml g−1 tissue). Homogenates were centrifuged (10 min at 3200g), the supernatants were retained and the pellets were extracted a second time in the same manner. The supernatants were pooled and evaporated to dryness. The extracts were then re-dissolved in 80% aqueous methanol and partitioned twice with 100% hexane (1:1 v:v). The methanol fraction was evaporated to dryness, and re-dissolved in 100% methanol. For LC-MS analyses, a 1 g equivalent in a subsample of extracts was diluted to 25% methanol and applied to a pre-conditioned C18 SPE cartridge (Supelco, 500 mg, 3 ml). The column was washed with 25% methanol and toxins were eluted with 100% methanol. The methanol extract was then evaporated to dryness and re-dissolved in 1 ml of 100% methanol.
Water samples collected from fish exposure aquaria were either gravity filtered by passing through a cotton-packed glass Pasteur pipette (dissolved brevetoxins) or sonicated for 15 min (total brevetoxins) and immediately analyzed. Total brevetoxins were extracted from field-collected sea-water samples by passing 500 ml through a pre-conditioned C18 SPE disk (3 M Empore™). The disk was then rinsed twice with 10 ml of deionized water, and brevetoxins were eluted with 20 ml of 100% methanol. The methanol extracts were evaporated to dryness and re-dissolved in 2 ml of 100% methanol. Fish and water extracts were stored at −20 °C until analyzed.
2.5. Brevetoxin analyses
Brevetoxin concentrations were measured in fish tissues, stomach contents, shellfish and water extracts by competitive ELISA according to Naar et al. (2002). This assay has been shown to be an effective tool to assess brevetoxin and brevetoxin metabolite contamination in a wide range of biological and environmental matrices including seawater and seaspray (Cheng et al., 2005; Pierce et al., 2005), shellfish (Naar et al., 2002, 2004; Dickey et al., 2004; Plakas et al., 2004; Pierce et al., 2006), dolphin and manatee tissues and body fluids (Flewelling et al., 2005), and seagrass (Flewelling et al., 2005). The antibodies used in this assay were obtained following goat immunization with PbTx-3-KLH conjugates (Trainer and Baden, 1991). Because standards for brevetoxin metabolites are unavailable, the exact affinity of these polyclonal antibodies for the different brevetoxin metabolites is not known, but the good correlation observed between results from ELISA and HPLC-MS analyses of shellfish tissues (Dickey et al., 2004; Plakas et al., 2004; Pierce et al., 2006) indicates a similar affinity of our antibodies for the brevetoxins and their derivatives. Results are expressed as PbTx-3 equivalents and reflect the overall concentration of brevetoxins and brevetoxin-like compounds present in the sample. As performed, the limit of detection by ELISA was 5 ng g−1 in tissues and 0.1 μg l−1 in extracted seawater.
Toxicity of clams used in exposure studies was determined by mouse bioassay conducted according to the official method (APHA, 1970).
Brevetoxin confirmation and composition was determined in a subset of samples by LC-MS performed on a ThermoFinnigan AqA HPLC/MS. The AqA single quad system scanned from 204 to 1216 AMU with AqA Max 40 V, and a scan rate of 1.1 scans per second. All analyses were conducted using electrospray ionization with the probe at 3 kV and 250 °C. The column was a Phenomenex Security Guard C-18 guard column with a Phenomenex Luna C-18 5Fm 250×2 mm2 analytical column. The solvent gradient was composed of acidified (0.3% acetic acid) ACN/H2O with initial 50:50 ACN/H2O to 95:5 ACN/H2O over 40 min. Parent brevetoxins (PbTx-1:867, PbTx-2:895, PbTx-3:897, PbTx-6:911, PbTx-7:869, PbTx-9:899, PbTx-10:871, Brevenal:657) and brevetoxin metabolites (Cyst-PbTx-2:1018, Ox-Cyst-PbTx-2:1034, Cyst-PbTx-1:990, Ox-Cyst-PbTx-1:1006) were monitored at indicated masses. The instrument was calibrated with a standard brevetoxin mix containing PbTx-2 and PbTx-3, obtained from the Center for Marine Science, UNC Wilmington, NC.
3. Results
3.1. Experimental exposure of omnivorous fish to toxic shellfish
A subset of hard clams used in the exposure studies was analyzed by LC-MS prior to exposures and was found to contain PbTx-3 and metabolites. Brevetoxin concentration by ELISA was 1800 ng g−1, and toxicity assessed by mouse bioassay was 30 mouse units (MU) per 100 g. No brevetoxins were detected by ELISA in the control clams.
While feeding toxic hard clams for 2 consecutive weeks, none of the pinfish or croakers died or exhibited any obvious signs of adverse effects. For comparison with the planktivorous fish exposures, toxin concentrations measured in liver and remaining viscera were averaged by tissue weight and are referred to as viscera. Higher concentrations of brevetoxins were measured on day 14 compared to day 7 of the exposures (Fig. 1), with the maximum average levels in pinfish (1412±151 ng g−1 in muscle and 2233±442 ng g−1 in viscera, n = 3) exceeding those measured in croakers (955±73 ng g−1 in muscle and 1990±166 ng g−1 in viscera, n = 3). The highest levels measured in an individual fish were 2675 ng g−1 in viscera and 1540 ng g−1 in muscle in a pinfish on the final day of the exposure period (day 14). When fish were switched to a diet of nontoxic clams, brevetoxin concentrations dropped but remained detectable in both muscle and viscera after 2 weeks of feeding on the nontoxic diet. Brevetoxins were not detected in fish on day 0 (before exposure) or in any of the control fish.
Fig. 1.
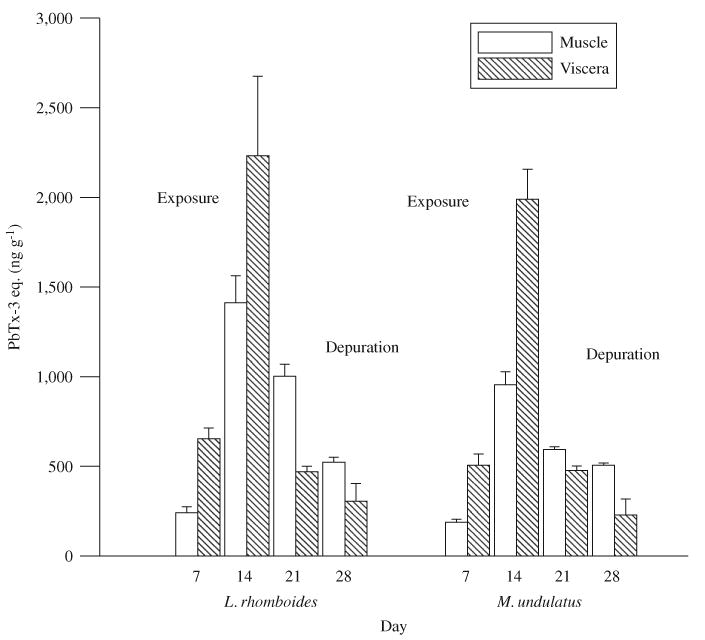
Brevetoxin concentrations measured in muscle and viscera of pinfish (L. rhomboides) and croakers (M. undulatus) fed brevetoxin-contaminated clams (day 1–14) followed by noncontaminated clams (day 15–28). Error bars indicate standard deviation between individual fish (n = 3).
3.2. Experimental exposure of planktivorous fish to K. brevis culture
During the exposure of juvenile striped mullet to K. brevis cultures (500–4000 cells ml−1), microscopic observations of the exposure media at 6 and 24 h verified that K. brevis cells remained intact. ELISA analyses of the exposure media after 6 and 24 h confirmed that greater than 95% of brevetoxin measured in the water was associated with the K. brevis cells (Fig. 2a). Under these conditions, all fish survived and quickly accumulated brevetoxins in both muscle and viscera. After 6 h, brevetoxins were measurable in all fish with concentrations ranging from 25 to 231 ng g−1 in muscle and 120–335 ng g−1 in viscera (data not shown). Brevetoxin concentrations in fish tissues increased with increasing K. brevis cell densities and with the duration of exposure—reaching an average of 333 ng g−1 in muscle and 616 ng g−1 in viscera after 24 h (Fig. 2a). In viscera, this increase was linear with increasing cell concentration (r2 = 0.998), while in muscle the increase was only linear up to 2000 cells ml−1 (r2 = 0.91), with no further increase at the highest cell density. Brevetoxins were not detected in any of the control fish.
Fig. 2.
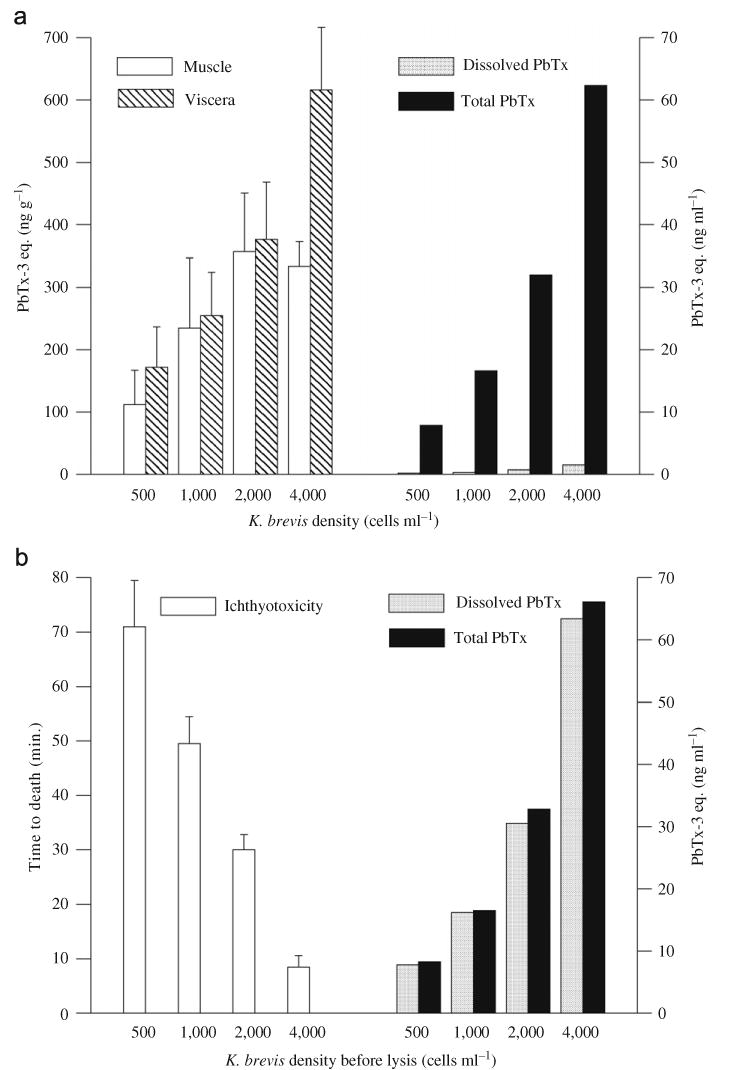
(a) Brevetoxin concentrations in muscle and viscera of juvenile striped mullet after 24 h exposure to K. brevis cell cultures at indicated cell densities, and total and dissolved concentrations of brevetoxins measured in exposure media. (b) Time to the death of juvenile striped mullet exposed to identical densities of lysed K. brevis cells, and total and dissolved concentrations of brevetoxins measured in exposure media. For (a) and (b), error bars indicate standard deviation between individual fish.
In parallel experiments, the ichthyotoxicity of dissolved brevetoxins was confirmed when K. brevis cells were lysed by sonication before addition to the aquaria (Fig. 2b). In this case, the fish did not accumulate toxins but died quickly (7–80 min), and the time to death correlated with the number of lysed cells (r2 = 0.99). In both experiments, the total brevetoxin concentrations in the aquaria were nearly identical (ranging from 8 to 66 ng ml−1) and were proportional to the initial K. brevis cell densities (Figs. 2a and b).
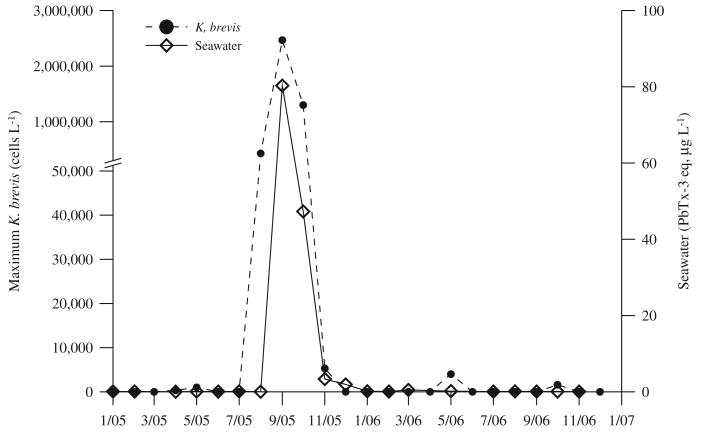
3.3. Field results
Maximum K. brevis cell densities observed in St. Joseph Bay each month from January 2005 through December 2006 are shown in Fig. 3. No K. brevis cells at bloom densities were observed in samples collected in St. Joseph Bay between January and July 2005; and only background levels were present in a few of the samples collected in April and May 2005. In August 2005, a southwest Florida K. brevis bloom was transported into the Florida panhandle, and elevated cell densities were first observed in St. Joseph Bay in a sample collected on August 31, 2005. In September 2005, K. brevis cell concentrations in the bay reached as high as 2.5 million-cells l−1 (Fig. 3). K. brevis cell densities remained elevated in the bay until late October. In mid-November 2005, maximum densities of 5300 K. brevis cells l−1 were observed. Subsequent to this date, no K. brevis were found until May 2006, when very low levels (maximum of 4000 cells l−1) were noted at only a few sites. Brevetoxin concentrations measured in St. Joseph Bay water samples ranged from <1 to 80 μg PbTx l−1 during the red tide bloom in September 2005, and were often measurable but low (typically <1 μg l−1) in the absence of a K. brevis bloom (Fig. 3).
Fig. 3.
Maximum Karenia brevis cell densities and brevetoxin concentrations measured in seawater samples collected throughout St. Joseph Bay between January 2005 and December 2006.
From February 2005 to November 2006, 184 fish representing 38 species of varying diets and trophic groups were collected from St. Joseph Bay and analyzed for brevetoxins. Species collected and maximum brevetoxins concentrations measured in muscle, liver and stomach contents are listed in Table 1, which includes 101 fish collected during the spring of 2004. Species included small planktivorous thread herring (Opisthonema oglinum) and sardines (Harengula jaguana), large piscivorous bluefish (Pomatomus saltatrix) and seatrout (Cynoscion nebulosus), as well as demersal and benthic omnivorous species such as pinfish (L. rhomboides) and catfish (Ariopsis felis).
Table 1.
Maximum brevetoxin concentrations measured in fish collected from St. Joseph Bay between March 2004 and November 2006
| Species | Common name | Main diet | N | Maximum [PbTx-3 eq. (ng g−1)] | ||
|---|---|---|---|---|---|---|
| Muscle | Liver | GI contents | ||||
| Sardinella auritaa,b | Spanish sardine | Plankton | 6 | 581 | <ld | 137 |
| Mugil cephaluesa | Striped mullet | Plankton | 9 | 40 | 6682 | 393 |
| Harengula jaguana | Scaled sardine | Plankton | 13 | 52 | 2472 | 2839 |
| Opisthonema oglinum | Atlantic thread herring | Plankton | 21 | 54 | 473 | 508 |
| Remora remora | Remora | Plankton | 1 | <ld | 131 | 384 |
| Lagodon rhomboidesa | Pinfish | Herbivore | 45 | 129 | 1453 | 911 |
| Hyporhamphus meekia | American halfbeak | Invertebrates | 5 | 124 | 1977 | 188 |
| Chilomycterus schoepfia | Striped burrfish | Benthic invert. | 1 | 27 | 571 | 138 |
| Acanthostracion quadricornis | Scrawled cowfish | Benthic invert. | 2 | 20 | 228 | 52 |
| Bairdiella chrysoura | Silver perch | Benthic invert. | 1 | <ld | 246 | 37 |
| Orthopristis chrysoptera | Pigfish | Benthic invert. | 3 | <ld | 277 | 105 |
| Syngnathus scovelli | Gulf pipefish | Benthic invert. | 1 | <ld | <ld | <ld |
| Archosargus probatocephalus | Sheepshead | Benthic invert. | 5 | 18 | 3709 | 613 |
| Haemulon plumierii | White grunt | Benthic invert. | 1 | <ld | 19 | <ld |
| Lobotes surinamensis | Tripletail | Benthic invert. | 1 | <ld | <ld | <ld |
| Ariopsis felis | Hardhead sea catfish | Benthic invert. | 2 | 12 | 1408 | 27 |
| Bagre marinus | Gafftopsail catfish | Benthic invert. | 1 | <ld | 1312 | <ld |
| Leiostomus xanthurus | Spot | Benthic invert. | 27 | 186 | Viscera 386c | |
| Menticirrhus americanus | Southern kingfish | Benthic invert. | 3 | <ld | 22 | 8 |
| Menticirrhus littoralis | Gulf kingfish | Benthic invert. | 2 | <ld | Viscera 31c | |
| Menticirrhus saxatilis | Northern kingfish | Benthic invert. | 7 | <ld | Viscera 77c | |
| Micropogonias undulatus | Atlantic croaker | Benthic invert. | 6 | <ld | 51 | 57 |
| Urophycis floridana | Southern codling | Benthic invert. | 4 | 60 | Viscera 285c | |
| Prionotus tribulus | Bighead sea robin | Benthic invert. | 1 | <ld | <ld | <ld |
| Elops saurusa | Ladyfish | Piscivore | 5 | 11 | 76 | 49 |
| Cynoscion nebulosusa | Spotted seatrout | Piscivore | 16 | 414 | 5133 | 3955 |
| Lutjanus griseusa | Mangrove snapper | Piscivore | 10 | 52 | 321 | 615 |
| Mycteroperca microlepisa | Gag grouper | Piscivore | 6 | 116 | 6034 | 720 |
| Opsanus betaa | Gulf toadfish | Piscivore | 1 | 69 | 7 | 400 |
| Lutjanus campechanusa | Red snapper | Piscivore | 3 | 102 | 16483 | 664 |
| Paralichthys albiguttaa | Gulf flounder | Piscivore | 5 | 65 | 528 | 742 |
| Carangoides bartholomaei | Yellow jack | Piscivore | 1 | <ld | 218 | <ld |
| Caranx crysos | Blue runner jack | Piscivore | 1 | <ld | 478 | <ld |
| Pomatomus saltatrix | Bluefish | Piscivore | 18 | 45 | 190 | 131 |
| Scomberomorus maculatus | Spanish mackerel | Piscivore | 28 | 24 | 221 | 11 |
| Strongylura marina | Atlantic needlefish | Piscivore | 1 | <ld | <ld | <ld |
| Centropristis striata | Black sea bass | Piscivore | 10 | <ld | 52 | 206 |
| Cynoscion arenarius | Sand seatrout | Piscivore | 1 | <ld | 468 | 14 |
| Lutjanus synagris | Lane snapper | Piscivore | 1 | <ld | <ld | 16 |
| Oligoplites saurus | Leatherjacket | Piscivore | 4 | <ld | 109 | 33 |
| Paralichthys lethostigma | Southern flounder | Piscivore | 2 | <ld | 8 | <ld |
| Synodus foetens | Inshore lizardfish | Piscivore | 4 | <ld | 96 | 12 |
<ld, below level of detection.
At least one specimen collected during September or November 2005 (i.e. during or just after K. brevis bloom).
No liver or GI contents analyzed from K. brevis bloom period.
Fish collected only in 2004, muscle and viscera (organs and GI tract) analyzed.
The average brevetoxin concentrations measured by ELISA in muscle, liver and stomach contents for each collection are shown in Fig. 4, and the prevalence of brevetoxins, expressed as the percentage of brevetoxin-positive samples (by ELISA) of each tissue type, is shown in Fig. 5. Although no K. brevis bloom was noted in the bay until September 2005, brevetoxins were detected in more than 69% of fish collected in February and June 2005 (Fig. 5). In general, average levels were highest during and immediately following the red tide in September and November 2005, after which they slowly decreased (Fig. 4).
Fig. 4.
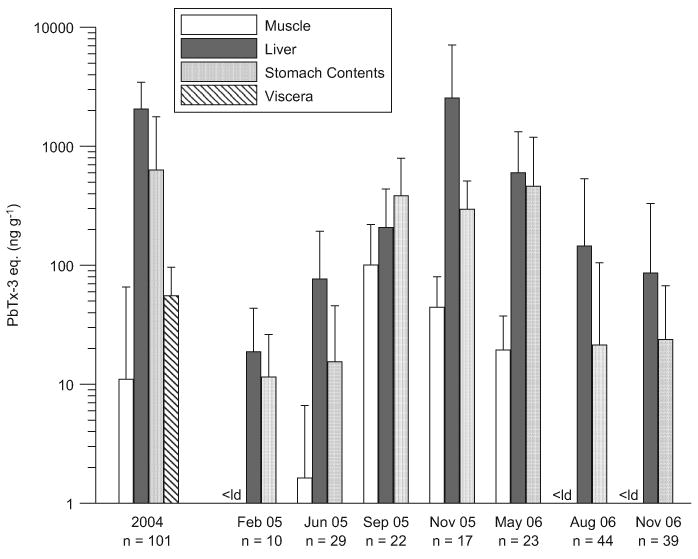
Average concentrations of brevetoxins measured in fish (all species) collected throughout St. Joseph Bay between 2004 and 2006. In 2005 and 2006, fish collections were performed during 2–3 day sampling trips. Error bars indicate standard deviation.
Fig. 5.
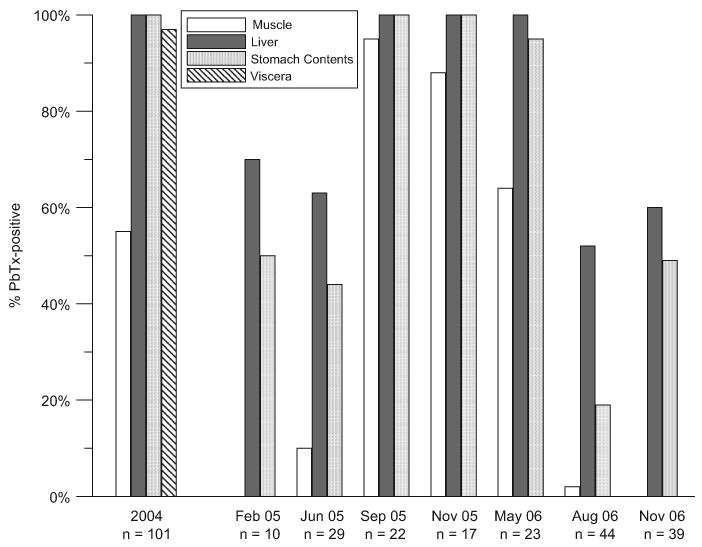
Prevalence of brevetoxin contamination in fish (all species) collected throughout St. Joseph Bay between 2004 and 2006. Prevalence is expressed as the percentage of fish containing detectable levels of brevetoxins (>5–10 ng g−1).
In February 2005, no toxin was measured in the muscle of any fish. In June, 10% of the fish tested contained low brevetoxin levels (13–20 ng g−1) in the muscle. During the K. brevis bloom in September 2005, brevetoxins were measurable in the muscle of 96% of the fish tested, with concentrations ranging from 9 to 581 ng g−1. In November 2005 after the K. brevis bloom, brevetoxin was found in the muscle of 88% of the fish collected (n = 17), with a maximum concentration of 116 ng g−1 measured in a gag grouper (Mycteroperca microlepis). By May 2006, both the proportion of positive muscle samples (64%, n = 23) and the concentrations measured in the muscle had decreased. With the exception of one hardhead sea catfish (A. felis), brevetoxin was not found in the muscle of any fish collected in August or November 2006. Overall, the highest brevetoxin concentration we measured in muscle (581 ng g−1) was from a Spanish sardine (Sardinella aurita) collected during the September 2005 red tide.
Liver samples consistently yielded the greatest proportion of brevetoxin-positive results (Figs. 4 and 5). In February 2005, brevetoxin concentrations in fish livers ranged from not detectable (<5–10 ng g−1) in 30% of the fish collected to 81 ng g−1. Average liver brevetoxin concentrations increased in each collection reaching peak levels in November 2005, just after the red tide ended. The highest liver concentration measured was 16,483 ng g−1 in a red snapper (Lutjanus campechanus) collected in November 2005. In May 2006, the average concentration in the livers had decreased but was still high (598 ng g−1), and brevetoxin was detected in 100% of the livers tested. In August and November 2006, the averages continued to decline, but brevetoxin was still found in the livers of more than half of the fish collected.
Brevetoxin concentrations in stomach contents displayed the greatest variability. In February and June 2005, brevetoxins were measured in the stomach contents of approximately half of the fish collected (Fig. 5) and at only low levels, with maximum concentrations of 43 and 144 ng g−1, respectively, both measured in pinfish (L. rhomboides). In September and November 2005, brevetoxins were measured in all fish stomach contents. Surprisingly, the average concentrations in stomach contents in fish collected in September and November 2005 and May 2006 were not significantly different from each other (Kruskal–Wallis one-way analysis of variance by ranks followed by a Dunn's multiple comparisons test, 95% significance level). The highest level we measured in 2005 and 2006 was 2839 ng g−1 in the stomach of a scaled sardine (H. jaguana) collected in May 2006. However, the highest level reported in Table 1 (3955 ng g−1) is from a spotted seatrout (C. nebulosus) collected in 2004.
3.4. Toxin profiles in fish
For both types of experiments (fish exposures to toxic clams and to K. brevis cultures), LC-MS analyses revealed that the fish had accumulated the same toxins to which they were exposed (Table 2). The toxin profile observed in the hard clams was almost identical to that observed in the liver of both pinfish and croakers, consisting principally of brevetoxin metabolites (cys-PbTx-2 and OxCys-PbTx-2) and, to a lesser extent, PbTx-3 (approximately 30% of the total amount of toxins). For planktivorous fish, the relative proportion of the toxins (PbTx-2>PbTx-3>PbTx-6>PbTx-9) observed in viscera was almost identical to that observed in the K. brevis culture (PbTx-2>PbTx-3,-9>PbTx-6>brevenal). Interestingly, brevenal, the brevetoxin antagonist produced by K. brevis was not detected in the viscera of these fish despite its presence in the K. brevis culture.
Table 2.
Brevetoxin composition in fish compared to their food sources in experimental exposures (K. brevis, toxic hard clams) and in selected fish species collected from St. Joseph Bay
| Species | Common name | Tissue type | Brevetoxins detected by HPLC-MS |
|---|---|---|---|
| Karenia brevisa | Florida red tide | PbTx-2>PbTx-3,-9>PbTx-6>brevenal | |
| Mugil cephalusa | Striped mullet | Viscera | PbTx-2>PbTx-3>PbTx-6>PbTx-9 |
| Mercenaria sp.a | Hard clam | Whole | cys-PbTx-2>PbTx-3>OxCys-PbTx-2 |
| Lagodon rhomboidesa | Pinfish | Liver | cys-PbTx-2>PbTx-3>OxCys-PbTx-2 |
| Micropogonias undulatusa | Atlantic croaker | Liver | cys-PbTx-2>PbTx-3>OxCys-PbTx-2 |
| Brevoortia sp.b,c | Menhaden | Muscle/viscera | PbTx-3>PbTx-2>cys-PbTx-2 |
| Cynoscion nebulosusb | Spotted seatrout | Liver | cys-PbTx-2>PbTx-3 |
| Pomatomus saltatrixb | Bluefish | Liver | cys-PbTx-2>PbTx-3 |
| Ariopsis felis | Hardhead catfish | Liver | cys-PbTx-2 |
| Hyporhamphus meeki | American halfbeak | Liver | cys-PbTx-2 |
| Cynoscion nebulosus | Spotted seatrout | Liver | cys-PbTx-2 |
From experimental exposures.
Fish collected in 2004.
Menhaden recovered from bottlenose dolphin stomach contents.
Toxin profiles determined for fish collected live from St. Joseph Bay were mainly metabolized brevetoxins (Table 2). No brevetoxins could be identified by LC-MS in extracts from four additional fish (two pinfish, L. rhomboides; one scaled sardine, H. jaguana; and one Atlantic thread herring, O. oglinum). These sample extracts were less concentrated (150–850 ng ml−1 by ELISA) than those listed in Table 2 for which profiles could be obtained. The lack of any detectable brevetoxins may be the result of insufficient sensitivity of our LC-MS method, the duration of sample storage prior to LC-MS analysis (6–30 months at −20 °C) or the presence of unidentified brevetoxin metabolites.
4. Discussion
4.1. Pathways for accumulation of brevetoxins in fish
Our experimental results demonstrate that fish can accumulate ichthyotoxic brevetoxins when exposed through their diet, and that toxins can be transferred through the food web by fish feeding on toxic prey such as toxic shellfish and K. brevis cells. However, fish in red tide-endemic areas are exposed to brevetoxins through other prey or forage items as well, including zooplankton, fish and seagrass with associated epiphytes (Fig. 6), all of which have the potential to accumulate brevetoxins (Tester et al., 2000; Flewelling et al., 2005). The presence of small planktivorous contaminated fish (clupeids) in the stomachs of larger piscivorous fish (sea trout and hardhead sea catfish) from St. Joseph Bay pointedly illustrated the transfer of brevetoxins to additional trophic levels in the fish food web.
Fig. 6.
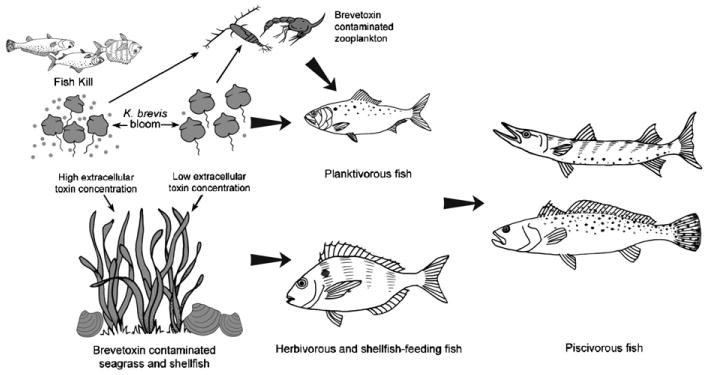
Potential sources of brevetoxins and trophic transfer pathways in the fish food web.
From our field-caught fish, the highest brevetoxin level observed in muscle was 581 ng g−1 in planktivorous Spanish sardines (Table 1). During our experiments, the levels of brevetoxin accumulation resulting from exposure of planktivorous striped mullet to K. brevis were lower than this and lower than those achieved when fish fed on toxic shellfish, but the exposure duration for the former was restricted to 24 h due to the need to prevent lysing of the K. brevis cells (no filtration or aeration), while the latter was 2-weeks long. While brevetoxin concentration in the viscera of experimentally exposed mullet increased linearly with cell concentration, brevetoxin concentration in muscle increased linearly only up to 2000 cells ml−1, no longer increasing at the highest level. This could reflect a saturation effect or more likely a change in the kinetics of accumulation since higher levels were measured in the fish exposed to toxic shellfish as well as in field specimens. Transfer of brevetoxins into fish during similar short-term exposures to K. brevis cultures was also demonstrated by Woofter et al. (2005) who noted immediate uptake of brevetoxin with measurable blood levels as soon as 1 h after exposing juvenile striped mullet to K. brevis. Over the course of the 24-h experiment, maximum blood levels were found after 8–12 h of exposure, with a drop to 50% of maximum levels after 24 h. Tissue concentrations were not determined, but slow elimination was observed with brevetoxin measurable in blood several days after exposure.
Although problematic, longer exposure studies with planktivorous fish and K. brevis are required to assess to full potential of planktivorous fish to accumulate brevetoxins. Undigested menhaden recovered from the stomach contents of bottlenose dolphins during the 2004 mortality event indicate that such potential far exceeds what we observed in our experiments. These fish contained excessive levels of brevetoxins, with concentrations reaching 33,200 ng g−1 in viscera and 1500 ng g−1 in muscle (Flewelling et al., 2005). The toxins present were mainly PbTx-3 and PbTx-2 with lesser amounts of cys-PbTx-2 (Table 2; Flewelling et al., 2005).
Tester et al. (2000) clearly demonstrated vectorial transport of brevetoxins from copepods to fish in short-term experiments (2–25 h). In these experiments, they exposed three species of copepod to various concentrations of K. brevis cultures. Cope-pod mortality was low and no comments on a change in the behavior of the copepods were noted. Juvenile fish that were fed these toxic copepods accumulated PbTx-2 and PbTx-3 in both the muscle and viscera. It is noteworthy that copepod grazing experiments have reported differences in copepod response to K. brevis exposure, with adverse effects noted in some cases (Huntley et al., 1986, 1987; Turner and Tester, 1997), but not others (Turner and Tester, 1989; Tester et al., 2000). While these studies may have illustrated species differences amongst copepods in their response to brevetoxin exposure, dissolved vs. intracellular brevetoxin concentrations of the exposure media were not considered. The rapid recovery of copepods when transferred out of water containing K. brevis reported by both groups of investigators may suggest that the adverse effects that have been noted on copepods are at least partially due to exposure to dissolved toxins.
4.2. Persistence of brevetoxins in fish
After a 2-week experimental exposure to brevetoxic shellfish, pinfish and croakers were switched to a diet of nontoxic clams and depuration was further monitored for 2 weeks. Even after 2 weeks without exposure, muscle tissue and viscera still contained 40% and 15% of the maximum observed concentrations, respectively. Brevetoxin profiles in pinfish and croaker measured after 2 weeks exposure reflected those of the toxic clams. However, conclusions regarding metabolism of brevetoxins in fish cannot be made based on these experiments since the shellfish used in the exposure experiments already contained metabolized brevetoxins as well as PbTx-3.
During the red tide from September to October 2005, there were reports of massive fish kills in St. Joseph Bay. Decomposed fish were still piled along the shores even in November 2005. Nevertheless, apparently unaffected live fish were collected from the bay during the red tide bloom in September and immediately following the bloom in November 2005. However, our sample size during and immediately following the K. brevis bloom (17–22 fish per collection) was approximately half the sample size of collections 1 year later (39–43 fish per collection). While the average muscle and stomach contents dropped between September and November 2005, average liver concentrations rose (Fig. 4), possibly reflecting the accumulation of brevetoxin in those fish that were not killed during the 2-month bloom.
Since it would be difficult to assess the persistence of brevetoxin contamination in fish collected from southwest Florida where K. brevis blooms occur almost annually and can last for several months (Steidinger et al., 1998b), this study was carried out in the Florida panhandle where red tides historically occur less frequently. Given the occurrence of only one 2-month K. brevis bloom during the 2 years of our collections, our data may suggest that, although the average levels of brevetoxins are higher during or just after red tide event, brevetoxins continue to be present in the livers of fish for more than a year after the cessation of a bloom. Brevetoxins are known to accumulate in shellfish where they can persist at levels dangerous for human consumers for several weeks after a K. brevis bloom has ended (Morton and Burklew, 1969; Pierce et al., 2006), and in some species, for several months (Steidinger et al., 1998a). Although PbTx-3 is largely eliminated from shellfish within weeks after a red tide has ended, brevetoxin metabolites (identical to those identified here in fish) have been found to persist for months (Plakas et al., 2002, 2004; Pierce et al., 2006). However, the surprising levels of brevetoxins measured in the stomach contents of the majority of fish, especially in planktivorous species (scaled sardines and Atlantic thread herring), collected in May 2006 when no red tide bloom was present in St. Joseph Bay, suggest there were undetected sources of brevetoxins. With the exception of low levels of K. brevis observed in two samples from the mouth of the bay (333–4000 cells l−1), no other potential brevetoxin-producing organisms were seen and only very low levels of brevetoxins were measured in any St. Joseph Bay water samples. The low levels of K. brevis found only at the mouth of the bay may be evidence of a bloom that remained undetected outside of the bay. Additionally, chronic exposure of omnivorous and piscivorous fish to toxins in food sources is likely since brevetoxins are known to persist in the environment (e.g. in shellfish and seagrass communities) for extensive period of time after red tides. Regardless of the source, the brevetoxins in the stomach contents of these fish would certainly contribute to the persistence of toxins we noted in the livers over the next several months.
4.3. Brevetoxins and ciguatoxins
Even before ciguatoxins and brevetoxins were identified, chemically characterized and their mode of action understood, their similarity was recognized by the symptoms they induced following ingestion (McFarren et al., 1965). However, while ciguatoxins are known to accumulate in fish by dietary transfer up the food chain (Legrand, 1991), brevetoxin-producing K. brevis blooms (Florida red tides) characteristically result in massive fish kills (Steidinger et al., 1973; Landsberg, 2002). The ichthyotoxicity of brevetoxins is their most obvious property; it has been recognized since 1844 (Ingersoll, 1882) and was originally used to guide the fractionation and the purification of these toxins leading to the elucidation of their structures (Baden et al., 1981). Curiously, ciguatoxins, like brevetoxins, are also extremely toxic to fish. In fact, under identical exposure conditions when toxins were dissolved in seawater, ciguatoxins were more potent to fish than brevetoxins (Lewis, 1992). A more recent study also demonstrates that ciguatoxins have a greater affinity than brevetoxins on fish sodium channels (Dechraoui et al., 2006). Nonetheless, this toxicity does not prevent the accumulation of ciguatoxins in fish tissue by dietary transfer. Our experimental exposures as well as the results obtained from fish collected live in Florida demonstrate that fish also have the potential to accumulate high levels of brevetoxins in their tissues without obvious adverse effects.
The experimental exposure of planktivorous fish to K. brevis cells may illustrate the importance of exposure route to the toxins and their consequences. When we exposed planktivorous mullet to intact K. brevis cells with only low concentrations of brevetoxins dissolved in the water, the fish survived, fed on K. brevis and quickly accumulated toxins in their tissues. However, when K. brevis cells were lysed, the release of brevetoxins caused rapid fish death, probably from acute absorption of fatal concentrations of dissolved toxins across the gills (Abbott et al., 1975; Baden, 1988). These results support the hypothesis that the apparent dissimilarity regarding the typical impact of ciguatoxins and brevetoxins on fish (accumulation vs. massive fish kills) does not result from differences in their ichthyotoxicity, but rather reflects important differences in the physiology and ecology of the two toxin-producing dinoflagellates. G. toxicus, the source of ciguatoxins and their precursors, is an armored epibenthic dinoflagellate (Adachi and Fukuyo, 1979) that does not typically produce planktonic blooms. This species lives in close association with macrophytes, corals, sediments and other substrates associated with coral reefs in tropical and subtropical waters (Bagnis et al., 1979; Anderson and Lobel, 1987). Ciguatoxins have been shown experimentally to adversely affect fish (Davin and Kohler, 1986; Davin et al., 1988; Kohler et al., 1989; Gonzalez et al., 1994), and were postulated in association with tropical reef fish mortalities (Landsberg, 1995), but these toxins are not typically associated with fish kills. We suggest that the concentrations of dissolved ciguatoxins that would be lethal to fish are prevented from occurring in situ due to insufficient cell densities, minimal extracellular toxin and rapid dilution of toxin in the water column. Fish are primarily exposed to ciguatoxins by food web transfer. In contrast, although less toxic than ciguatoxins, lethal ichthyotoxic brevetoxin concentrations commonly occur in seawater because K. brevis is an unarmored and easily lysed planktonic bloom-forming dinoflagellate (Steidinger and Baden, 1984). Blooms can cover tens of thousands of square kilometers of ocean (Vargo et al., 1987) with cells densities commonly exceeding 1 million cells l−1 (Steidinger et al., 1998b) and even documented up to 1.1 billion cells l−1 (Trebatoski, 1988). Dense blooms can result in the release of high concentrations of dissolved brevetoxins by lysed or dying cells throughout the water column, and routinely cause massive fish kills in southwest Florida (Landsberg, 2002).
As is the case for ciguatoxins, the biological mechanism allowing accumulation of brevetoxins after ingestion without toxic effects remains unidentified. It has been suggested by Lewis (1992) that the capacity of fish to accumulate ichthyotoxins without adverse effects could be restricted and may impose an upper limit to the levels of ciguatoxins carried by fish, thus contributing to the low incidence of human fatality associated with ciguatera. Although there may be an upper limit to the dosage of brevetoxin that fish can tolerate by ingestion, we could not assess that in our experiments.
While we draw parallels regarding the biological pathways that allow for the accumulation of ichthyotoxic brevetoxins and ciguatoxins in healthy fish, the implications for human health of brevetoxin and ciguatoxin accumulation in fish are very different. Brevetoxins are far-less toxic than ciguatoxins. As a comparison, the established action limit for brevetoxin levels in shellfish is 20 MU per 100 g of shellfish meat (USFDA, 2005). Using a conversion factor of 4 μg per MU based on the LD50 (i.p.) of PbTx-2 and -3 as determined by Baden and Mende (1982), this action limit would correspond to 80 μg per 100 g or 800 ng g−1. For ciguatoxins, levels as low as 0.1–1 μgkg−1 or 0.1–1 ng g−1 are believed to be toxic for humans (Lehane and Lewis, 2000). Based on the levels of brevetoxins we have measured, the risk of acute poisoning in humans following consumption of fish fillets appears to be highly unlikely.
Additionally, it has been shown that as ciguatoxins and their precursors, gambiertoxins, are transferred through the fish food web, they are bioaccumulated and metabolized into more potent compounds, resulting in higher trophic level fish presenting greater risks to human health (Lewis and Holmes, 1993; Lewis et al., 2000). It is not known how fish metabolize brevetoxins. Brevetoxin composition determined in a limited number of live-caught fish revealed mainly metabolized brevetoxins identical to those found in shellfish, but the toxicity of these metabolites is not well known. Although they are generally believed to be less toxic (Naar et al., 2004), other metabolites identified from shellfish have been clearly implicated as causative factors for NSP (Morohashi et al., 1999; Poli et al., 2000; Plakas et al., 2004; Pierce et al., 2006). Studies on the toxicity of known brevetoxin metabolites are lacking, with many brevetoxin metabolites remaining unidentified and their toxicity uncharacterized. Until more information is available on the full suite of brevetoxin metabolites, the potential health risk they present cannot be conclusively assessed.
5. Conclusions
Through experimental exposures we have demonstrated that fish have the potential to accumulate brevetoxins in their muscle and, at higher levels, their viscera when feeding on toxic prey; and our data from live-caught fish confirm that they do, in fact, accumulate brevetoxins in the wild. During a K. brevis bloom, brevetoxins were detected in the muscle and liver of 95% and 100% of the fish tested, respectively.
We have presented an overview of the levels of brevetoxins that we observed in fish in St. Joseph Bay during 2005 and 2006. Many of the species tested were not caught during the bloom in the fall of 2005, and in several cases only one fish per species was tested. For these reasons, our results are not sufficiently robust for a species comparison, or for drawing any conclusions regarding the ability for brevetoxins to accumulate in particular fish species. Such topics are the focus of a separate study. However, we have demonstrated that the brevetoxin accumulation in fish we observed in St. Joseph Bay during the 2004 dolphin mortality was not a unique occurrence, and that the presence of brevetoxin in fish tissues can persist for an extended period of time.
Despite the almost annual occurrence of red tide in the eastern Gulf of Mexico, and with the exception of one anecdotal case involving consumption of whole fish (NRC, 1999), human intoxications from eating fish caught during K. brevis red tides have not been documented. In this study, we observed levels of brevetoxins in the muscle of fish at all trophic levels rise significantly, but not to dangerous levels, during a relatively brief red tide. However, our analyses of less than 300 fish from the Florida panhandle, an area far-less impacted by K. brevis blooms than the southwest coast of Florida, cannot rule out the potential that this could occur. Current larger-scale monitoring of brevetoxin concentrations in fish muscle from areas in southwest Florida that are more frequently impacted by red tide will help address this issue. Nevertheless, it is clear that brevetoxin concentrations in the liver and digestive tracts of fish can become excessively high and would likely result in acute intoxication if consumed. Differing from other populations from around the world, people in the US do not typically consume whole fish or their digestive organs, thus reducing the risk of exposure to harmful levels of brevetoxin. However, our results reveal an obvious threat to marine birds and mammals that feed directly on whole live fish prey—a threat that was clearly realized during the bottlenose dolphin mortality in 2004 (NMFS, 2004; Flewelling et al., 2005).
The persistence of brevetoxins and brevetoxin metabolites in fish tissues as well as shellfish over extended periods of time suggests that marine organisms in red tide endemic areas are routinely exposed to these compounds. Little information is available on their toxicity and nothing is known about how chronic, low-level exposure to brevetoxins may impact human or animal health. The growing number of brevetoxin metabolites that are being identified, together with the increasing evidence for widespread distribution and persistence of brevetoxins in the marine environment, supports the need for human and environmental risk assessments of chronic or long-term exposure to these toxins.
Acknowledgments
We thank Dr. Tomas Lankford and Dr. Carmelo Thomas (UNCW) for their assistance with exposure experiments and experimental K. brevis cell counts, Melissa Smith (UNCW) for artistic representation of brevetoxin transfer in the fish food web, Karen Atwood and Sheila O Dea (FWRI) for laboratory assistance, Earnest Truby (FWRI) for K. brevis cell counts and the Florida DEP St. Joseph Bay Aquatic Preserve staff for collection of monthly water samples. This work was supported by funds from the CDC and Florida Department of Health (Expanding Existing Surveillance Systems to Include Pfiesteria, Other Harmful Algal Blooms and Marine Toxins in Florida, Grant # U50-CCU423360-01), NOAA Oceans and Human Health Initiative (Grant # NA05NOS4781246), the State of Florida and the NIEHS (Grant # PO1ES010594-06A1).
Footnotes
Ethical statement: The results presented in this manuscript are originals and are not being considered elsewhere. Regarding the use of vertebrate animals as described in the manuscript, experiments were performed according to the animal use policy (approved IUCAC protocol) at the corresponding author's institution.
References
- Abbott BC, Siger A, Spigelstein M. Toxins from the blooms of Gymnodinium breve. In: LoCicero VR, editor. Proceedings of the First International Conference on Toxic Dinoflagellate Blooms; Wakefield: Massachusetts Science and Technology Foundation; 1975. pp. 355–366. [Google Scholar]
- Adachi R, Fukuyo Y. The thecal structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. et sp. nov. collected in a ciguatera-endemic area. Bull Jpn Soc Sci Fish. 1979;45:67–71. [Google Scholar]
- Anderson DM, Lobel PS. The continuing enigma of ciguatera. Biol Bull. 1987;172:89–107. [Google Scholar]
- APHA. Recommended Procedures for the Examination of Sea Water and Shellfish. fourth. American Public Health Association; Washington, DC: 1970. Subcommittee on laboratory methods for the examination of shellfish Method for the bioassay of Gymnodinium breve toxin(s) in shellfish; pp. 61–66. [Google Scholar]
- Baden DG. Public health problems of red tides. In: Tu AT, editor. Handbook of Natural Toxins, vol 3: Marine Toxins and Venoms. Marcel Dekker, Inc.; New York: 1988. pp. 259–277. [Google Scholar]
- Baden DG, Mende TJ. Toxicity of two toxins from the Florida red tide marine dinoflagellate, Ptychodiscus brevis. Toxicon. 1982;20:457–461. doi: 10.1016/0041-0101(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Baden DG, Mende TJ, Block RE. Two similar toxins isolated from Gymnodinium breve. In: Taylor DL, Seliger HH, editors. Toxic Dinoflagellate Blooms. Elsevier; New York: 1979. pp. 327–334. [Google Scholar]
- Baden DG, Mende TJ, Lichter W, Wellham L. Crystallization and toxicology of T34: a major toxin from Florida's red tide organism (Ptychodiscus brevis) Toxicon. 1981;19:455–462. doi: 10.1016/0041-0101(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Baden DG, Fleming LE, Bean JA. Marine toxins. In: DeWolf FA, editor. Handbook of Clinical Neurology: Intoxications of the Nervous System. Part H. Natural Toxins and Drugs. Elsevier Press; Amsterdam: 1995. pp. 141–175. [Google Scholar]
- Bagnis R, Kuberski T, Laugier S. Clinical observation on 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067–1073. doi: 10.4269/ajtmh.1979.28.1067. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierce R, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden DG. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environ Health Perspect. 2005;113:638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin WT, Jr, Kohler CC. Effects of ciguatera toxins on the bluehead. Trans Am Fish Soc. 1986;115:908–912. [Google Scholar]
- Davin WT, Jr, Kohler CC, Tindall DR. Ciguatera toxins adversely affect piscivorous fishes. Trans Am Fish Soc. 1988;117:374–384. [Google Scholar]
- Dechraoui MY, Naar J, Pauillac S, Legrand AM. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon. 1999;37:125–143. doi: 10.1016/s0041-0101(98)00169-x. [DOI] [PubMed] [Google Scholar]
- Dechraoui MY, Wacksman JJ, Ramsdell JS. Species selective resistance of cardiac muscle voltage gated sodium channels: characterization of brevetoxin and ciguatoxin binding sites in rats and fish. Toxicon. 2006;48:702–712. doi: 10.1016/j.toxicon.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Dickey RW, Plakas SM, Jester ELE, El Said KR, Johannessen JN, Flewelling LJ, Scott P, Hammond DG, Dolah FMV, Leighfield TA, Dachraoui MYB, Ramsdell JS, Pierce RH, Henry MS, Poli MA, Walker C, Kurtz J, Naar J, Baden DG, Musser SM, White KD, Truman P, Miller A, Hawryluk TP, Wekell MM, Stirling D, Quilliam MA, Lee JK. Multi-laboratory study of five methods for the determination of brevetoxins in shellfish tissue extracts. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL, USA: 2004. pp. 300–302. [PMC free article] [PubMed] [Google Scholar]
- Flewelling LJ, Naar JP, Abbott JP, Baden DG, Barros NB, Bossart GD, Bottein MYD, Hammond DG, Haubold EM, Heil CA, Henry MS, Jacocks HM, Leighfield TA, Pierce RH, Pitchford TD, Rommel SA, Scott PS, Steidinger KA, Truby EW, Dolah FMV, Landsberg JH. Red tides and marine mammal mortalities. Nature. 2005;435:755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Brusle J, Crespo S. Ultrastructural alterations of cabrilla sea bass Serranus cabrilla liver related to experimental Gambierdiscus toxicus (dinoflagellate) ingestion. Dis Aquat Organ. 1994;18:187–193. [Google Scholar]
- Halstead BW. Poisonous and Venomous Marine Animals of the World. Darwin Press; Princeton: 1978. [Google Scholar]
- Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, Mackenzie L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J Phycol. 2004;40:165–179. [Google Scholar]
- Huntley ME, Sykes PF, Rohan S, Marin V. Chemically-mediated rejection of dinoflagellate prey by the copepod Calanus pacificus and Paracalanus parvus: mechanism, occurrence and significance. Mar Ecol Prog Ser. 1986;28:105–120. [Google Scholar]
- Huntley ME, Ciminiello P, Lopez MDG. Importance of food quality in determining development and survival of Calanus pacificus (Copepoda: Calanoida) Mar Biol. 1987;95:103–113. [Google Scholar]
- Ingersoll E. On the fish mortality in the Gulf of Mexico. Proc US Natl Mus. 1882;4:74–80. [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart GD, Baden DG. Literature review of Florida red tide: implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CC, Paleudis GA, Tindall DR. Behavioral abnormalities displayed by ocean surgeon following consumption of ciguatoxigenic dinoflagellates. Proc Assoc Island Mar Labs Carrib. 1989;22:34. [Google Scholar]
- Landsberg JH. Tropical reef-fish disease outbreaks and mass mortalities in Florida, USA: what is the role of dietary biological toxins? Dis Aquat Organ. 1995;22:83–100. [Google Scholar]
- Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10:113–390. [Google Scholar]
- Legrand AM. Les toxines de la ciguatera. In: Fremy JM, editor. Proceedings of the Symposium on Marine Biotoxins; Paris, France: CNEVA, Godin; 1991. pp. 53–59. [Google Scholar]
- Lehane L, Lewis RJ. Ciguatera: recent advances but the risk remains. Int J Food Microbiol. 2000;61:91–125. doi: 10.1016/s0168-1605(00)00382-2. [DOI] [PubMed] [Google Scholar]
- Lewis RJ. Ciguatoxins are potent ichthyotoxins. Toxicon. 1992;30:207–211. doi: 10.1016/0041-0101(92)90474-j. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Holmes MJ. Origin and transfer of toxins involved in ciguatera. Comp Biochem Physiol C. 1993;106:615–628. doi: 10.1016/0742-8413(93)90217-9. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Molgó J, Adams DJ. Ciguatera toxins: pharmacology of toxins involved in ciguatera and related fish poisonings. In: Botana LM, editor. Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection. Marcel Dekker, Inc.; New York: 2000. pp. 419–447. [Google Scholar]
- Lombet A, Bidard JN, Lazdunski M. Ciguatoxin and brevetoxins share a common receptor-site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987;219:355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- McFarren EF, Tanabe H, Silva FJ, Wilson WB, Campbell JE, Lewis KH. The occurrence of a ciguatera-like poison in oysters, clams, and Gymnodinium breve cultures. Toxicon. 1965;3:111–123. doi: 10.1016/0041-0101(65)90005-x. [DOI] [PubMed] [Google Scholar]
- Morohashi A, Satake M, Naoki H, Kaspar HF, Oshima Y, Yasumoto T. Brevetoxin B4 isolated from green-shell mussels Perna canaliculus, the major toxin involved in neurotoxic shellfish poisoning in New Zealand. Nat Toxins. 1999;7:45–48. doi: 10.1002/(sici)1522-7189(199903/04)7:2<45::aid-nt34>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Morton RA, Burklew MA. Florida Shellfish Toxicity Following Blooms of the Dinoflagellate Gymnodinium breve. Florida Department of Natural Resources—Marine Research Laboratory; St. Petersburg: 1969. p. 26. (Technical Series No. 60). [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling LJ, Steidinger KA, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar J, Kubanek J, Weidner AL, Flewelling LJ, Bourdelais A, Steidinger K, Baden DG. Brevetoxin depuration in shellfish via production of non-toxic metabolites: consequences for seafood safety and the environmental fate of brevetoxins. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL, USA: 2004. pp. 488–490. [PMC free article] [PubMed] [Google Scholar]
- NMFS. Interim Report on the bottlenose dolphin (Tursiops truncatus) unusual mortality event along the panhandle of Florida, March–April 2004. National Oceanic and Atmospheric Administration, National Marine Fisheries Service and the Florida Fish and Wildlife Conservation Commission; 2004. [Google Scholar]
- NRC. From Monsoons to Microbes: Understanding the Ocean's Role in Human Health. Ocean Studies Board; Commission on Geosciences, Environment, and Resources; National Research Council (US), National Academy Press; Washington, DC: 1999. Harmful algal blooms; pp. 59–70. [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Hamel SL, Kirkpatrick B, Cheng YS, Zhou Y, Irvin CM, Naar J, Weidner A, Fleming LE, Backer LC, Baden DG. Brevetoxin composition in water and marine aerosol along a Florida beach: assessing potential human exposure to marine biotoxins. Harmful Algae. 2005;4:965–972. [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Plakas SM, Granade HR, Jester ELE, Said KRE, Dickey RW, Steidinger KA, Scott PS, Flewelling LJ, Wright JLC. Comparison of methods for determination of brevetoxins and their metabolites in NSP-toxic bivalved molluscs. In: Henshilwood K, Deegan B, McMahon T, Cusack C, Keaveney S, Silke J, O'Cinneide M, Lyons D, Hess P, editors. Proceedings of the Fifth International Conference on Molluscan Shellfish Safety; Galway Ireland. June 14–18, 2004; Rinville, Oranmore, Galway, Ireland: The Marine Institute; 2006. pp. 37–42. [Google Scholar]
- Plakas SM, el-Said KR, Jester EL, Granade HR, Musser SM, Dickey RW. Confirmation of brevetoxin metabolism in the Eastern oyster (Crassostrea virginica) by controlled exposures to pure toxins and to Karenia brevis cultures. Toxicon. 2002;40:721–729. doi: 10.1016/s0041-0101(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Plakas SM, Wang Z, Said KRE, Jester ELE, Granade HR, Flewelling L, Scott P, Dickey RW. Brevetoxin metabolism and elimination in the Eastern oyster (Crassostrea virginica) after controlled exposures to Karenia brevis. Toxicon. 2004;44:677–685. doi: 10.1016/j.toxicon.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986;30:129–135. [PubMed] [Google Scholar]
- Poli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Neurotoxic shellfish poisoning and brevetoxin metabolites: a case study from Florida. Toxicon. 2000;38:981–993. doi: 10.1016/s0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- Steidinger KA. Some taxonomic and biologic aspects of toxic dinoflagellates. In: Falconer I, editor. Algal Toxins in Seafood and Drinking Water. Academic Press; Limited, London: 1993. pp. 1–28. [Google Scholar]
- Steidinger KA, Baden DG. Toxic marine dinoflagellates. In: Spector D, editor. Dinoflagellates. Academic Press; Orlando: 1984. pp. 201–261. [Google Scholar]
- Steidinger KA, Burklew MA, Ingle RM. The effects of Gymnodinium breve toxin on estuarine animals. In: Martin DF, Padilla GM, editors. Marine Pharmacognosy. Academic Press; New York: 1973. pp. 179–202. [Google Scholar]
- Steidinger KA, Carlson P, Baden D, Rodriguez C, Seagle J. Neurotoxic shellfish poisoning due to toxin retention in the clam Chione cancellata. In: Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; 1998a. pp. 457–458. [Google Scholar]
- Steidinger KA, Vargo GA, Tester PA, Tomas CR. Bloom dynamics and physiology of Gymnodinium breve with emphasis on the Gulf of Mexico. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Springer; Heidelberg: 1998b. pp. 133–153. [Google Scholar]
- Steidinger KA, Wolny JL, Haywood AJ. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. Nova Hedwigia; in press. [Google Scholar]
- Tester PA, Turner JT, Shea D. Vectorial transport of toxins from the dinoflagellate Gymnodinium breve through copepods to fish. J Plankton Res. 2000;22:47–61. [Google Scholar]
- Trebatoski B. Observations on the 1986–1987 Texas red tide (Ptychodiscus brevis) Report 88-02. Texas Water Commission; Austin: 1988. [Google Scholar]
- Trainer VL, Baden DG. An enzyme immunoassay for the detection of Florida red tide brevetoxins. Toxicon. 1991;29:1387–1394. doi: 10.1016/0041-0101(91)90126-c. [DOI] [PubMed] [Google Scholar]
- Turner JT, Tester PA. Zooplankton feeding ecology: copepod grazing during an expatriate red tide. In: Cosper EM, Bricelj VM, Carpenter EJ, editors. Coastal and Estuarine Studies, 35. Novel Phytoplakton Blooms. Causes and Impacts of Recurrent Brown Tides and Other Unusual Blooms. Springer; Berlin: 1989. pp. 359–374. [Google Scholar]
- Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 1997;42:1203–1214. [Google Scholar]
- USFDA. National Shellfish Sanitation Program Guide for the Control of Molluscan Shellfish. US Food and Drug Administration, Center for Food Safety and Applied Nutrition; Washington, DC: 2005. [Google Scholar]
- Vargo GA, Carder KL, Gregg W, Heil E, Shanley E, Steidinger KA, Haddad KD. The potential contribution of primary production by red tides to the west Florida shelf ecosystem. Limnol Oceanogr. 1987;32:762–767. [Google Scholar]
- Woofter RT, Brendtro K, Ramsdell JS. Uptake and elimination of brevetoxin in blood of striped mullet (Mugil cephalus) after aqueous exposure to Karenia brevis. Environ Health Perspect. 2005;113:11–16. doi: 10.1289/ehp.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto T, Nakajima I, Bagnis R, Adachi R. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull Jpn Soc Sci Fish. 1977;43:1021–1026. [Google Scholar]