Abstract
Mammalian cells respond to UV radiation by signaling cascades leading to activation of transcription factors, such as activated protein 1, NFκB, and p53, a process known as the “UV response.” Nuclear factor of activated T cells (NFAT) was first identified as an inducible nuclear factor in immune response and subsequently found to be expressed in other tissues and cells. To date, however, the regulation and function of NFAT in tissues and cells, other than the immune system, are not well understood. In this study, we demonstrate that UV radiation activates NFAT-dependent transcription through a calcium-dependent mechanism in mouse epidermal JB6 cell lines, as well as in the skin of NFAT-luciferase reporter transgenic mice. Exposure of JB6 cells to UV radiation leads to the transactivation of NFAT in a dose-dependent manner. A23187 had a synergistic effect with UV for NFAT induction, whereas pretreatment of cells with nifedipine, a calcium channel blocker, dramatically impaired the NFAT activity induced by either UV or UV plus A23187. Calcium-dependent activation of NFAT by UV was further confirmed by an in vivo study using NFAT-luciferase reporter transgenic mice. These results demonstrated that UV radiation is a strong activator for skin NFAT transactivation through calcium-dependent pathways, suggesting that NFAT activation may be a part of the UV response.
The nuclear factor of activated T cells (NFAT)1 was originally described as a transcriptional factor expressed in activated T cells but not resting T cells (1–4). The induction of NFAT in T cells required a calcium-activated signaling path-way and was blocked by cyclosporin A (CsA) and FK506 (5–11). Over the last decade, studies from several laboratories have indicated that the preexisting/cytoplasmic component of NFAT was a mixture of proteins belonging to a novel family of transcription factors (12–14). The first member of the family (NFATp, later renamed NFAT1) was purified from cytoplasmic extracts of a murine T cell cloned by affinity chromatography using the distal NFAT site of murine IL-2 promoter (9, 15) and cloned from murine (Ar-5) and human (Jurkat) T cell cDNA libraries (15, 16). Other distinct proteins belonging to the same family, such as NFATc, NFAT3, and NFAT4, were isolated and cloned (17–20). There are three functional domains in NFAT family proteins: the Rel similarity domain, which is responsible for the DNA binding activity and interaction with AP-1; the NFAT homology region, which regulates the intracellular localization; and the transcriptional activation domain (21). The activation of NFAT in T cells includes dephosphorylation, nuclear translocation, and increase in affinity for DNA binding (5). Stimuli that elicit calcium mobilization result in rapid dephosphorylation of NFAT proteins and their translocation to the nucleus; the dephosphorylated proteins show increased affinity for DNA binding (5).
Growing evidence indicates that NFAT is not only a T cell-specific transcriptional factor but is also expressed in a variety of lymphoid cells and in nonlymphoid tissue (5, 22), including heart, testis, brain, ovary, small intestine, prostate, colon, muscle, placenta, lung, and kidney, as well as skin (5, 22). However, the regulation and function of NFAT in these tissues remains unclear. In this study, we investigated the activation of NFAT by UV radiation using mouse epidermal JB6 cells and NFAT-luciferase transgenic mice.
MATERIALS AND METHODS
Plasmids and Reagents
Cytomegalovirus-neo vector plasmid and NFAT-luciferase reporter plasmid were constructed as previously reported (4, 23–25); fetal bovine serum (FBS) and Eagle’s minimal essential medium (MEM) were from BioWhittaker; LipofectAMINE was from Life Technologies, Inc.; phosphocholine (PCho) was from Sigma; C2-ceramide was from Biomol; TNF-α was from Roche Molecular Biochemicals; EGF was from Collaborative Research; luciferase assay substrate was from Promega; cyclosporin A was from Alexis Biochemicals; and Fluo-3 and Pluronic F-127 were from Molecular Probes.
Cell Culture
The JB6 P+ mouse epidermal cell line Cl 41 and its transfectant, Cl 41 NFAT mass1, were cultured in monolayers at 37 °C and 5% CO2 using Eagle’s minimal essential medium containing 5% fetal calf serum, 2 mm l-glutamine, and 25 µg of gentamicin/ml (26, 27).
Generation of Stable Co-transfectants
JB6 Cl 41 cells were cultured in a six-well plate until they reached 85–90% confluence. One µg of cytomegalovirus-neo vector, with or without 12 µg of NFAT-luciferase reporter plasmid DNA and 15 µl of LipofectAMINE reagent, was used to transfect each well in the absence of serum. After 10–12 h, the medium was replaced with 5% FBS MEM. Approximately 30–36 h after the beginning of the transfection, the cells were digested with 0.033% trypsin, and cell suspensions were plated into 75-ml culture flasks and cultured for 24–28 days with G418 selection (400 µg/ml). Stable transfectants were identified by measuring basal level of luciferase activity. Stable transfectant Cl 41 NFAT mass1 was established and cultured in G418-free MEM for at least two passages before each experiment.
Assay for NFAT Activity in Vitro
Confluent monolayers of Cl 41 NFAT mass1 were trypsinized, and 8 × 103 viable cells suspended in 100 µl of 5% FBS MEM were added into each well of a 96-well plate. Plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% gaseous air. Twelve to 24 h later, cells were starved by replacing medium with 0.1% FBS MEM for 12–24 h prior to exposure of UV radiation. The cells were then exposed to UV radiation for the indicated times and dosages. The cells were extracted with lysis buffer, and luciferase activity was measured as described previously (28).
NFAT-Luciferase Reporter Transgenic Mice
3× NFAT-luciferase reporter transgenic mice were originally established by Rincon and Flavell (4). These transgenic mice carry the 3× NFAT-luciferase reporter. The offspring mice were screened by testing both the basal level and A23187-induced level of luciferase activity for the presence of the NFAT luciferase reporter gene. Males and females were housed separately in solid-bottomed polycarbonate cages on ventilated animal racks (~4–5 mice/cage, individualized by incisions in the ears) in temperature-, humidity-, and yellow light-controlled conditions. Food and water were available ad libitum, and the dorsal skin of the mice was shaved every week during the experiment period.
Intracellular Free Calcium Assay
The intracellular free calcium was measured using calcium-sensitive Fluo-3 dye according to the manufacturer’s recommendations (Molecular Probes Inc.). JB6 P+ cells cultured on coverslips were loaded 45 min prior to the experiment with the tetracarboxylate Fluo-3 AM ester dye to a final concentration of 4 µM dissolved in Me2SO containing 20% of the nonionic detergent Pluronic® F-127 to increase cellular dye uptake. The coverslips were washed three times with Ca2+-free PBS and then were placed in a quartz cuvette containing Ca2+-free PBS, temperature controlled to 37 °C ± 0.1 °C (Neslab, RTE-111, Neslab Instruments, Portsmouth, NH). The changes in the fluorescence intensity at 530 nm (excitation at 500 nm) as a result of intracellular free calcium changes were recorded with a SPEX Fluoromax instrument (Instruments S.A., Inc., Edina, NJ). For all experiments, a basal level of free intracellular Ca2+ was recorded prior to all treatments for each coverslip. The ionophore A23187 in Me2SO was directly added to the cuvette, and for the UVC stimulation, the coverslip was removed from the cuvette, treated with UVC radiation, and placed back in the same cuvette. The data recording during the UVC stimulation was halted. The coverslip was removed from the cuvette for 2 min (equal to the UVC stimulation) and then placed back in the cuvette for control experiments.
Indirect Immunofluorescence Staining
Cells were grown on coverslips to 40–60% confluency and were fixed with precooled 100% methanol for 15 min at −20 °C. The cells were incubated with the rabbit anti-NFATc1 (K-18) antibody (Santa Cruz Biotechnology) for 1 h in 37 °C. The coverslips were then washed for three periods of 10 min with PBS and counter-stained for 30 min with Alexa 488-labeled goat anti-rabbit IgG (Molecular Probes Inc., Eugene, OR) and washed for another three periods of 10 min with PBS. The coverslips were mounted in ProLong antifade kit (Molecular Probes Inc.) and examined with a Leica microscope (Leica, Heidelberg, Germany).
Assay of NFAT Activity in Vivo
The NFAT-luciferase transgenic mice were identified, grouped, and housed as described above. Two weeks after grouping, both basal level and A23187-induced level of luciferase activity were measured by punch skin biopsy. Two weeks after the last punch biopsy, the mice were exposed to UVB (10 KJ/m2), A23187 (0.5 µM/mouse), or UVB (10 KJ/m2) plus A23187 (0.5 µM/ mouse). The mice were punch-biopsied to measure luciferase activity at the times indicated. For investigation of inhibitory effects by nifedipine on NFAT activation, 40 µM nifedipine dissolved in 300 µl of acetone/day was applied to the mice topically for 5 days. The last of the five topical doses of nifedipine was given 3 h prior to exposure to UVB (10 KJ/m2) or A23187 (0.5 µM/mouse). Eighteen hours after treatment, the mice were punch-biopsied to determine the effect of nifedipine on induced NFAT transactivation in the epidermis. The luciferase activity of punch-biopsied epidermis was measured as described previously (4, 29). The relative NFAT activity was presented relative to basal level of luciferase activity of each mouse.
Statistical Analysis
The significance of the difference in the NFAT activity was determined with Student’s t test.
RESULTS
Induction of NFAT Transactivation in Mouse Epidermal JB6 Cells by UV Radiation
To study the regulation of NFAT transcription activity in skin cells, we generated a NFAT-luciferase reporter stable transfectant by co-transfecting the NFAT-luciferase reporter plasmid and cytomegalovirus-neo plasmid into the mouse epidermal JB6 cell line, Cl 41. The results observed from the stable transfectant showed that either UVB or UVC radiation could induce marked activation of NFAT in JB6 cells (Fig. 1). The activation of NFAT by UV radiation appears to occur in a dose-dependent manner (data not shown). The maximum induction of NFAT activity by UV radiation occurred between 18 and 24 h after cells were exposed to UV radiation (Fig. 2b). Because previous studies reported that EGF and TNF-α receptors, PCho and sphingomyelinase may be involved in UV-induced signal transduction pathways leading to activation of AP-1 or NFκB (30–33), we exposed the Cl 41 NFAT mass1 cells to EGF, TNF-α, PCho, and C2-ceramide. However, no induction of NFAT activity was observed in cells stimulated with EGF (10 ng/ml), TNF-α (25 units/ml), PCho (5 mM), or C2-ceramide (20 µM) (Fig. 1). These results demonstrate that UV radiation is a specific stimulus for NFAT transactivation in mouse epidermal JB6 cells, suggesting that signal transduction pathways leading to transactivation of NFAT in the “UV response” may not involve EGF receptors, TNF-α receptors, phosphocholine, or C2-ceramide.
FIG. 1. Induction of NFAT-dependent transcription by UV radiation in JB6 cells.
8 × 103 Cl 41 NFAT mass1 cells were seeded into each well of 96-well plates. After being cultured at 37 °C overnight, the cells were starved for 12 h by replacing medium with 0.1% FBS MEM. Then, the cells were treated with either UVB (4 KJ/m2 with filter), UVC (60 J/m2), EGF (10 ng/ml), TNF-α (25 units/ml), PCho (5 mM), or C2-ceramide (20 µM). After being cultured for 18 h, the luciferase activity was measured. Each bar indicates the mean and S.D. of four repeat assay wells. The results are presented as relative NFAT-dependent transcription activity.
FIG. 2. Synergistic effect of A23187 on UV-induced NFAT activity JB6 cells.
8 × 103 Cl 41 NFAT mass1 cells suspended in 5% FBS MEM were added to each well of 96-well plates. After being cultured at 37 °C overnight, the cells were starved by replacing the medium with 0.1% FBS MEM for 12 h. The cells were treated with UVB, UVC, A23187, UVB + A23187, or UVC + A23187 at the doses indicated. The cells were harvested after being cultured for 18 h (a) or for the time indicated (b). The NFAT activity was measured by luciferase activity assay. The results are presented as relative NFAT-dependent transcription activity. Each bar indicates the mean and S.D. of assays from triplicate wells.
Synergistic Effect of A23187 on UV-induced NFAT Activation in JB6 Cells
Ionomycin reportedly has a synergistic effect with 12-O-tetradecanoylphorbol-13-acetate for NFAT transactivation in T and B cells, whereas ionomycin or 12-O-tetradecanoylphorbol-13-acetate alone does not induce NFAT transactivation (4). To test the effect of Ca2+ ionophore on UV-induced NFAT activation in mouse epidermal cells, we incubated Cl 41 NFAT mass1 cells with either A23187 alone or UV radiation plus A23187. The results showed that A23187 (1 µM) has a high synergistic effect on UV-induced NFAT activation (Fig. 2), whereas administration of A23187 alone results in minimal induction of NFAT activity (Fig. 2a). These data suggest that calcium mobilization in JB6 cells is not the only pathway leading to NFAT activation in response to UV radiation.
UV Radiation Induces Calcium Release and Has a Synergistic Effect on A23187-induced Calcium Release
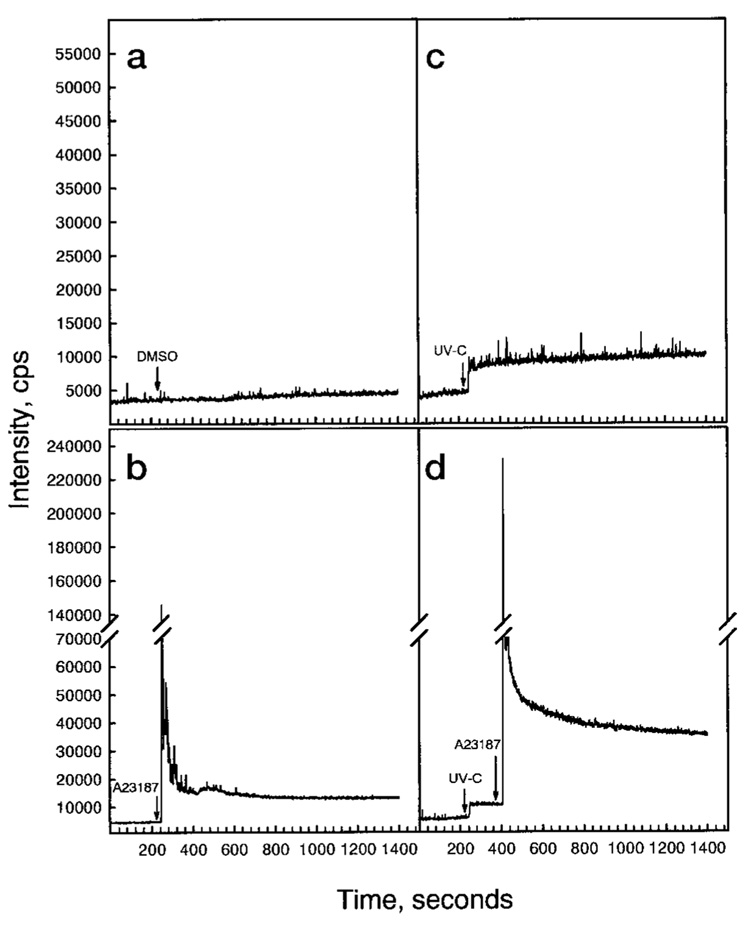
To assess the alteration of intracellular free calcium after cells were exposed to UVC radiation, the calcium-sensitive Fluo-3 dye was used. The results are shown in Fig. 3. Treatment of cells with either ionophore A23187 or UVC leads to a rapid increase of intracellular intracellular free calcium (Fig. 3). UVC radiation also has a strong synergistic effect on A23187-induced calcium release (Fig. 3d). These data support the notion that calcium mobilization is involved in UV-induced NFAT transactivation.
FIG. 3. Induction of Ca2+ release by UV radiation.
The JB6 Cl 41 cells were cultured on coverslips to 40–60% confluency. The cells were loaded with and were allowed to take up the tetracarboxylate Fluo-3 AM ester dye (4 µM) for 45 min. The cells were washed with Ca2+-free PBS three times. The cells were treated with 0.1% Me2SO (negative control) (a), A23187 (1 µM) (b), UVC (60 J/m2) (c), or UVC plus A23187 (d). The intracellular free Ca2+ was recorded as described under “Materials and Methods.”
UV-induced NFAT Activation Is Dependent on Calcium Mobilization in JB6 Cells
To determine the role of calcium-dependent pathways in UV-induced NFAT activation, we investigated the effect of nifedipine, a calcium channel blocker, on UV-induced NFAT activation. Blocking calcium channel activity by pretreatment of JB6 cells with nifedipine resulted in inhibition of UV-induced or UV plus A23187-induced calcium release (Fig. 4) and NFAT activation (Fig. 5). These data suggested that activation of NFAT by UV radiation and the synergistic effect of A23187 on UV-induced activation of NFAT are both through calcium mobilization-dependent pathways.
FIG. 4. Blocking of UV-induced Ca2+ release by nifedipine.
The JB6 Cl 41 cells were cultured and loaded with tetracarboxylate Fluo-3 AM ester dye as in Fig. 3. The cells were washed with Ca2+-free PBS three times and incubated with nifedipine (40 µM) for 30 min. Then, the cells were treated with 0.1% Me2SO (a), A23187 (1 µM) (b), UVC (60 J/m2+) (c), or UVC plus A23187 (d), and the intracellular free Ca2+ level was recorded.
FIG. 5. Inhibition of UV-induced NFAT activity by pretreatment of cells with nifedipine.
8 × 103 JB6 Cl 41 NFAT mass1 cells suspended in 5% FBS MEM were added to each well of 96-well plates. After being cultured at 37 °C overnight, the cells were starved by replacing the medium with 0.1% FBS MEM for 12 h. Then, the cells were first treated with 40 µM nifedipine for 1 h and were then exposed to A23187 (1 µM), UVB (4 KJ/m2), UVB (4 KJ/m2) + A23187 (1 µM), UVC (60 J/m2), or UVC (60 J/m2) + A23187 (1 µM). After being cultured for 18 h, the cells were harvested, and the NFAT activity was measured by luciferase activity assay. The results are presented as relative NFAT-dependent transcription activity. Each bar indicates the mean and S.D. of assays from triplicate wells.
Blocking UV-induced NFAT Transactivation by CsA
Previous studies demonstrated that in T cells the major NFAT activation pathway appears through calcium/calmodulin-dependent phosphatase calcineurin (5, 6). To test the role of calcineurin in UV-induced NFAT-dependent transcription activity in mouse epidermal JB6 cells, CsA, a pharmacological inhibitor of the phosphatase calcineurin, was used. Pretreatment of cells with CsA resulted in a dramatic inhibition of NFAT transactivation induced by either UV radiation or UV + A23187 (Fig. 6). These data revealed that activation of calcineurin is required for UV-induced NFAT activation, suggesting that UV radiation activates the NFAT transcription activity through the pathway that is similar to that in T cells.
FIG. 6. Blocking UV-induced NFAT transactivation by cyclosporin A.
JB6 Cl 41 NFAT mass1 cells were seeded to each well of 96-well plates and cultured until 90% confluent. The cells were then first treated with 1 µM cyclosporin for 1 h and then were exposed to A23187 (1 µM), UVB (4 KJ/m2), UVB + A23187, UVC (60 J/m2) or UVC + A23187. After being cultured for 18 h, the cells were harvested, and the NFAT activity was measured by luciferase activity assay.
UV Radiation Triggers Translocation of NFAT Protein to Nuclear in JB6 Cells
To investigate whether the activation of NFAT by UV radiation involved the nuclear translocation of NFAT protein, we used the indirect immunofluorescence staining for the analysis of the NFAT protein localization. As shown in Fig. 7, exposure of cells to UVC radiation triggered the translocation of NFAT protein from cytosol to the nucleus. These data suggest that UV radiation induced NFAT transactivation through NFAT protein translocation.
FIG. 7. UV radiation-induced NFAT translocation to the nucleus.
JB6 Cl 41 cells were cultured on coverslips to 40–60% confluency. The cells were (right column) or were not (left column) exposed to UVC (60 J/m2) radiation and then were fixed and labeled with rabbit anti-NFAT antibody by using Alexa 488-labeled goat anti-rabbit antibody as the second antibody.
Induction of NFAT-dependent Transactivation by UV Radiation in Mouse Skin of NFAT-Luciferase Reporter Transgenic Mice
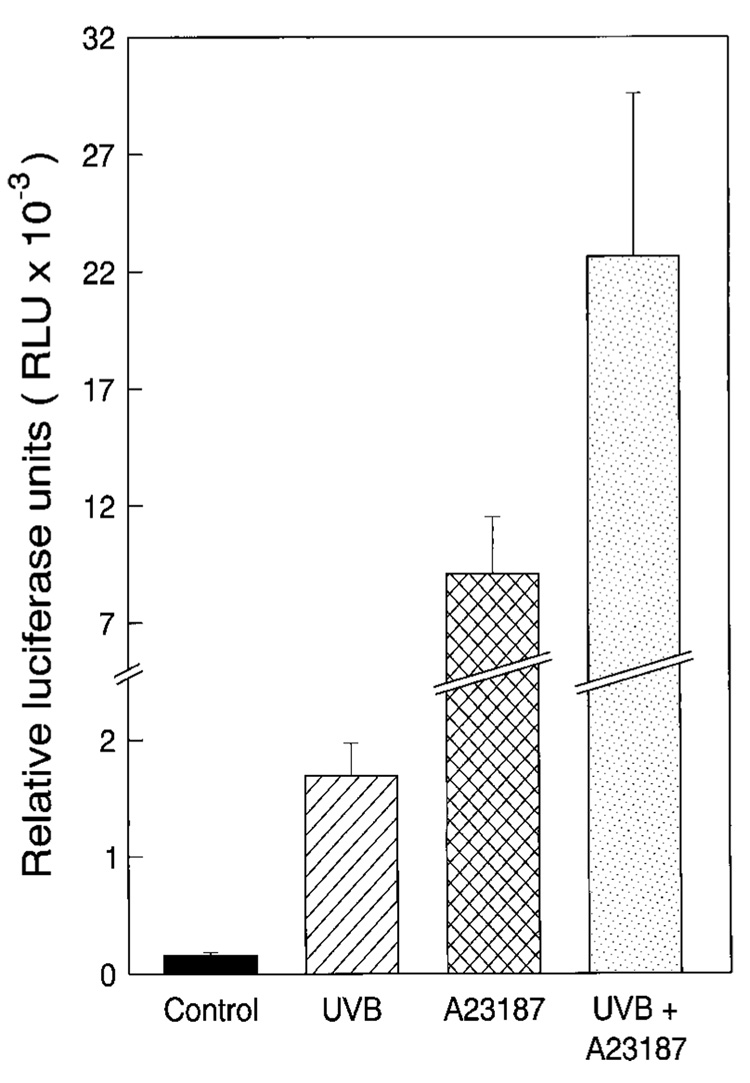
To investigate the regulation of NFAT induction activity by UV radiation in mouse skin, we exposed NFAT-luciferase reporter transgenic mice to either A23187 (0.5 µM/mouse), UVB radiation (10 KJ/m2), or A23187 plus UVB radiation. In agreement with findings in JB6 cells, UVB radiation resulted in more than a 10-fold increase of NFAT-dependent transcription in mouse skin (Fig. 8). Administration of both UVB and A23187 resulted in a large synergistic effect on NFAT induction (~140-fold). Furthermore, application of A23187 alone to skin also caused marked activation of NFAT (Fig. 8), which was further confirmed by time course studies (data not shown). Interestingly, similar levels of augmented UV-induced NFAT activity were observed in mouse skin treated with either A23187 and UV simultaneously or A23187 at 96 h prior to UV radiation (data not shown), whereas skin samples from mice at 96 h after exposure to A23187 did not show any induction of NFAT activity (data not shown). Considering data from the JB6 cell study, our results not only demonstrate that UV radiation is a strong activator for NFAT activation, in vitro and in vivo, but also suggested that there are some differences for NFAT activation between the epidermal cell culture model and the mouse skin in vivo model.
FIG. 8. UV radiation induced the transactivation of NFAT in NFAT-luciferase reporter transgenic mice.
The NFAT-luciferase transgenic mice were described under “Materials and Methods.” One week after grouping, the mice were exposed to UVB (10 KJ/m2), A23187 (0.5 µm/mouse), or UVB (10 KJ/m2) + A23187 (0.5 µM/mouse). Eighteen hours after treatment, the dorsal skin of the mice was punch-biopsied using biopsy punch (1.5 mm, Acuderm, Inc., Ft. Lauderdale, FL). The luciferase activity of punch-biopsied epidermis was measured as described previously (28) after adding 100 µl of lysis buffer overnight at 4 °C. The results are presented as relative luciferase units.
Blocking of UV-induced NFAT Activation by Nifedipine In Vivo
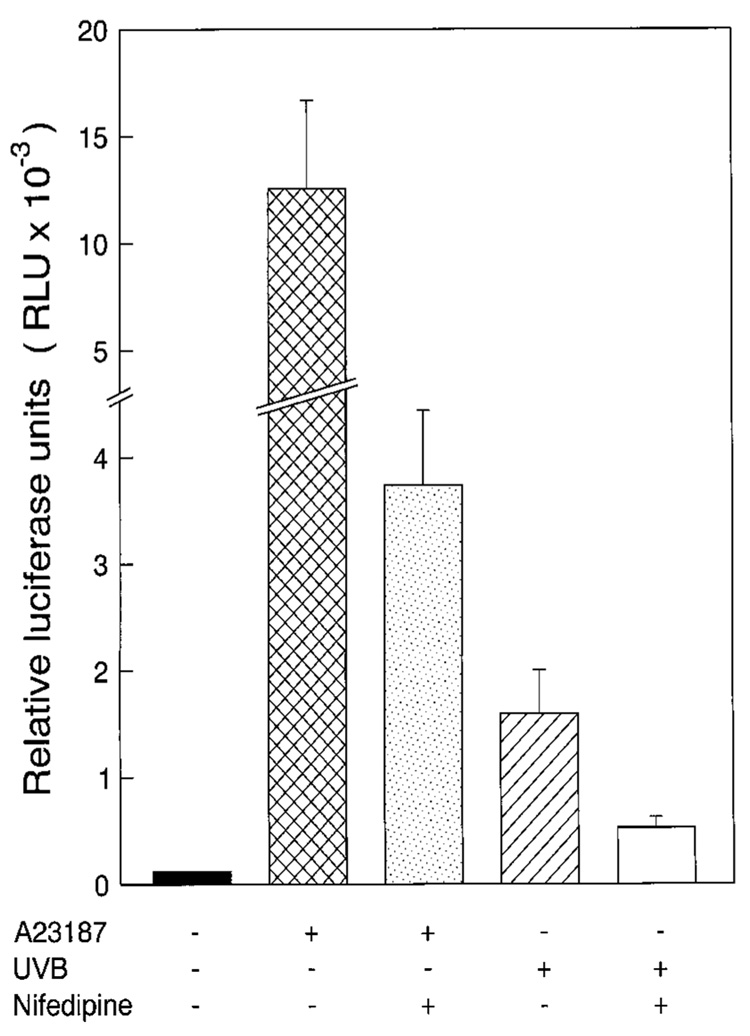
To determine whether calcium-dependent pathways are required for UV-induced NFAT activation in vivo, we also investigated the influence of nifedipine on UV-induced NFAT activity in NFAT-luciferase transgenic mice (Fig. 9). Topical application of nifedipine (40 µM/mouse) on mouse skin resulted in dramatic inhibition of A23187- and UVB-induced NFAT-dependent transactivation (Fig. 9) (p < 0.05). These results strongly demonstrate that UV-induced activation of NFAT is mediated by a calcium-dependent pathway.
FIG. 9. Blocking of UV-induced NFAT activity by nifedipine in vivo.
The NFAT-luciferase transgenic mice were randomly grouped. The mice were treated five times over 5 days as indicated with nifedipine (40 µM/mouse/day) dissolved in 300 µl of acetone. The last of the five topical doses of nifedipine was given 3 h prior to exposure to UVB (10 KJ/m2) or A23187 (0.5 µM/mouse). The negative control mice, A23187 group mice, and UVB group mice received the acetone alone. Eighteen hours after exposure, the mice were punch-biopsied to look at the effect of nifedipine on induced NFAT transactivation in the epidermis. The luciferase activity of punch-biopsied epidermis was measured as described previously (4, 29).
DISCUSSION
Expression of the antigen-regulated, cyclosporin A-sensitive NFAT is not restricted to lymphoid cells as once thought but is now known to be expressed in other cells and organs such as skin (22). The function and regulation of NFAT in skin, however, remain unknown. In this report, we investigated the possible involvement of NFAT in the UV response by using JB6 cells and NFAT-luciferase transgenic mice. Exposure of JB6 cells or skin of transgenic mice to UV radiation caused marked increases of NFAT-dependent transcription in a dose-dependent manner, whereas other stimuli, such as EGF, TNF-α, PCho, and ceramide, did not induce NFAT activation. Co-stimulation studies showed that A23187 augments the NFAT-mediated transcription synergistically in response to UV radiation both in vitro and in vivo. Furthermore, pretreatment of cells with nifedipine or cyclosporin A results in impairment of NFAT transactivation induced by either UV radiation or UV plus A23187. These results strongly suggest that UV radiation is a specific inducer for skin NFAT transactivation and that induction of NFAT in skin by UV radiation occurs through a calcium-and CsA-dependent pathway. Collectively, these results suggest that NFAT may be a new transcription factor involved in the UV response.
NFAT isoforms are expressed in different tissues, as it has been reported that NFAT1 and NFAT2 mRNAs have been detected in brain, heart, skeletal muscle, testis, placenta, pancreas, small intestine, prostate, and colon, as well as in skin tumors (5, 22). NFAT expression or NFAT-derived transactivation has also been described in several types of nonlymphoid cells, including mast cells (34), endothetial cells (35), neuronal cells (36), vascular smooth muscle cells (37), and liver-derived Chang (CHL) cells (38). In this study, we found that exposure of epidermal cells or mouse skin to UV radiation resulted in marked activation of NFAT-dependent transcription. These results are consistent with previous findings that NFAT mRNA is detectable in skin (22).
In T cells, transactivation of NFAT is regulated tightly in response to elevations of both intracellular calcium ion (Ca2+) and diacylglycerol following activation of phospholipase C (5). Increased intracellular calcium stimulates the production of calmodulin (5). It is believed that binding of calmodulin to a region near the C terminus of calcineurin displaces the auto-inhibiting domain and exposes the calcineurin active site (5). Activated calcineurin dephosphorylates the cytoplasmic NFAT proteins, leading to NFAT nuclear translocation (5, 6, 39). NFAT forms a heteromeric transcriptional co-activator complex with AP-1 that co-induces NFAT-dependent transactivation (5). It is also reported that phosphorylation of NFAT is regulated by several protein kinases, including glycogen synthase kinase 3 and c-Jun NH2-terminal kinase2 (5, 40–42). With the exception of antigen receptor on lymphocytes, there are only a few reports regarding NFAT activation induced by PDGF and hepatitis B virus×protein in vascular smooth muscle cells and liver-derived CHL cells, respectively (37, 38). Also, little is known about regulation and signaling of NFAT activation in skin. This report showed that UV radiation resulted in marked activation of NFAT-mediated transcription through CsA-sensitive and calcium-dependent pathway in both epidermal culture cells and transgenic mouse skin. The observation that A23187 is a stronger inducer of NFAT in mouse skin but a weaker inducer in cultured cells suggests that additional signaling systems in vivo are responsible for NFAT activation induced by A23187. Differential basal levels of AP-1 activity between in vitro culture cells and in vivo transgenic mouse skin, which may be caused by responsiveness of the mouse to its environment, may be one of the additional signals.
UV in solar light elicits a number of biological effects in skin, including pigmentation, erythema, skin cell death, and inflammation (43). Of particular concern is the role of UV radiation as a major etiologic factor in the development of human cancer (43). Exposure of cells to UV radiation leads to a large number of physiological changes in cells, such as activation of transcription factors AP-1, NFκB, and p53 (44–46), referred to as the UV response. Generally, this UV response serves to protect the cells (44). The initial signal triggering the UV response is in large part independent of DNA damage, but rather appears to be mediated by a membrane-associated component of the Ras pathway with activation of mitogen-activated protein kinases (45–49). This idea is supported by our findings that atypical protein kinase C is a mediator of UV-induced activation of extracellular signal-regulated kinases and AP-1 activity (48) and that sphingomyelinase is required for UV-induced c-Jun NH2-terminal kinase activation (33). However, others argue that UV-induced activation of mitogen-activated protein kinases may also have a DNA damage signal component (50). Recently, Bender et al. (51) reported that UV-induced activation of NFκB occurs through both DNA damage-dependent and-independent pathways. In the case of p53, activating signals clearly involve both DNA damage signals and other nongenotoxic stress pathways (52). It is known that p53 function is regulated at the levels of transcription, translocation, stabilization, association with other proteins, and phosphorylation (44). Regulation of p53 at transcription level may be due to DNA damage signal, whereas phosphorylation, translocation and association with other proteins are mainly through nongenotoxic signal transduction pathways. p53 phosphorylation has been found to be mediated by several cellular kinases, including CKI, CKII, protein kinase A, cyclin-dependent kinase7, extracellular signal-regulated kinases, and c-Jun NH2-terminal kinases (44). Recently, we reported that P38 kinase is a key mediator for mouse p53 protein phosphorylation at serine 389 in cell response to UV radiation (44). In the present study, we found that UV radiation resulted in strong activation of NFAT through a calcium-dependent mechanism in mouse skin. It is known that NFAT is a transcription factor playing an essential role in IL-2 gene expression (4) and that binding sites for NFAT have also been found within the promoter regions of cytokines, including granulocyte macrophage colony-stimulating factor, TNF-α, IL-3, IL-4, IL-5, and IL-8 (38, 41). Previous studies indicated that expression of IL-8, TNF-α and other cytokines is associated with initiating and controlling effective immune and inflammatory responses and may be related to cancer development (38, 41). Therefore, we suggest that NFAT is a new important transcription factor involved in UV response and that NFAT may be involved in UV-induced skin cancer promotion. This idea is supported by previous findings that NFAT expression in skin tumor is much higher than in normal skin tissue (22) and that CsA and FK506, the two most commonly used inhibitors for NFAT activation, by specific targeting and blocking the activation of Ca2+-dependent phosphatase calcineurin, have strong antitumor promotion activity (53, 54). In this study, we also found that CsA has strong inhibitory effects on UV-induced NFAT transactivation. In conclusion, UV radiation is a strong inducer of NFAT activation in mouse epidermal cells and skin. Transactivation of NFAT in mouse skin by UV radiation may play an important part in the UV response, which may be associated with UV radiation-induced inflammatory and tumor promotion through NFAT-mediated expression of inflammation cytokines, such as IL-2 and TNF-α. Furthermore, these results provide a possible mechanism of antitumor promotion activity of CsA and FK506.
Acknowledgments
We thank Dr. Douglas Bibus for critical reading and Andria Percival for secretarial assistance.
Footnotes
This work was supported by National Institutes of Health Grants CA77646, CA81064 and GM45928, the Academy of Finland, and the Hormel Foundation.
The abbreviations used are: NFAT, nuclear factor of activated T cells; AP-1, activated protein 1; EGF, epidermal growth factor; MEM, Eagle’s minimal essential medium; FBS, fetal bovine serum; PCho, phosphocholine; TNF, tumor necrosis factor; PBS, phosphate-buffered saline; IL, interleukin; CsA, cyclosporin A.
This paper is available on line at http://www.jbc.org
REFERENCES
- 1.Durand DB, Shaw JP, Bush MR, Replogle RE, Belageje R, Crabtree GR. Mol. Cell. Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serfling E, Barthelmäs R, Pfeuffer I, Schenk B, Zarius S, Swoboda R, Mercurio F, Karin M. EMBO J. 1989;8:465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw J-P, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- 4.Rincon M, Flavell RA. Mol. Cell. Biol. 1997;17:1522–1534. doi: 10.1128/mcb.17.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao A, Luo C, Hogan PC. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 6.Clipstone NA, Crabtree GR. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 7.Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan WF, Corthesy B, Bram RJ, Crabtree GR. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 9.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curram T, Rao A. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 11.Mattila PS, Ullman KS, Fiering S, Emmel EA, McCutcheon M, Crabtree GR, Herzenberg LA. EMBO J. 1990;9:4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree GR, Clipstone NA. Annu. Rev. Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 13.Jain J, Loh C, Rao A. Curr. Opin. Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 14.Serfling E, Avots A, Neumann M. Biochim. Biophys. Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 15.McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, Burgeon E, Lane WS, Lambert JN, Curran T, Verdine GL, Rao A, Hoga PG. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 16.Luo C, Burgeon E, Carew JA, Badalian TM, McCaffrey PG, Lane WS, Hogan PG, Rao A. Mol. Cell. Biol. 1996;16:3955–3966. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoey T, Sun Y-L, Williamson K, Xu X. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 18.Masuda E, Naito Y, Tokummitsu H, Campbell D, Saito F, Hannum C, Arai K-I, Arai N. Mol. Cell. Biol. 1995;15:2697–2706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho SN, Thomas DJ, Timmerman LA, Li X, Francke U, Crabtree GR. J. Biol. Chem. 1995;270:19898–19907. doi: 10.1074/jbc.270.34.19898. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Takeuchi A, Sharma S. J. Biol. Chem. 1996;271:29014–29021. doi: 10.1074/jbc.271.34.20914. [DOI] [PubMed] [Google Scholar]
- 21.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Cell. Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 22.Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 23.Rincon M, Flavell RA. Mol. Cell. Biol. 1996;16:1074–1084. doi: 10.1128/mcb.16.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Ma W-Y, Colburn N, Dong Z. Proc. Natl. Acad. Sci. U. S. A. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Ma W-Y, Ryan CA, Dong Z. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11957–11962. doi: 10.1073/pnas.94.22.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Ma W-Y, Li J, Hecht SS, Dong Z. Cancer Res. 1998;58:4102–4106. [PubMed] [Google Scholar]
- 27.Huang C, Ma W-Y, Dong Z. Mol. Cell. Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Ma W-Y, Dawson MI, Rincon M, Flavell RA, Dong Z. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Ma W-Y, Hanenberger D, Cleary MP, Bowden GT, Dong Z. J. Biol. Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- 30.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 31.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 32.Dong Z, Huang C, Ma W-Y, Kiss Z. Oncogene. 1998;17:1845–1853. doi: 10.1038/sj.onc.1202084. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Ma W, Ding M, Bowden GT, Dong Z. J. Biol. Chem. 1997;272:27753–27757. doi: 10.1074/jbc.272.44.27753. [DOI] [PubMed] [Google Scholar]
- 34.Weiss DL, Hural J, Tara D, Timmerman LA, Henkel G, Brown MA. Mol. Cell. Biol. 1996;16:228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cockerill GW, Bert AG, Ryan GR, Gamble JR, Vadas MA, Cockerill PN. Blood. 1995;86:2689–2698. [PubMed] [Google Scholar]
- 36.Ho AM, Jain J, Rao A, Hogan PG. J. Biol. Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 37.Boss V, Abbott KL, Wang X-F, Pavlath GK, Murphy TJ. J. Biol. Chem. 1998;273:19664–19671. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]
- 38.Lara-Pezzi E, Armesilla AL, Majano PL, Redono JM, Lopez-Cabrera M. EMBO J. 1998;17:7066–7077. doi: 10.1093/emboj/17.23.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nature. 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 40.Shibasaki F, Price ER, Milan D, McKeon F. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 41.Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 42.Tsatsanis C, Patriotis C, Tsichlis PN. Oncogene. 1998;17:2609–2618. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]
- 43.Matsui MS, DeLeo VA. Carcinogenesis. 1990;11:229–234. doi: 10.1093/carcin/11.2.229. [DOI] [PubMed] [Google Scholar]
- 44.Huang C, Ma W-Y, Maxiner A, Sun Y, Dong Z. J. Biol. Chem. 1999;274:12229–12235. doi: 10.1074/jbc.274.18.12229. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmed MF, Avruch J, Woodgett JR. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 46.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 47.Smith ML, Fornace AJ., Jr Proc. Natl. Acad. Sci. U. S. A. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C, Ma W-Y, Bowden GT, Dong Z. J. Biol. Chem. 1996;271:31262–31268. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- 49.Huang C, Ma W-Y, Dong Z. Oncogene. 1997;14:1945–1954. doi: 10.1038/sj.onc.1201056. [DOI] [PubMed] [Google Scholar]
- 50.Adler V, Fuchs SY, Kim J, Kraft A, King MP, Pelling J, Ronai Z. Cell Growth Differ. 1995;6:1437–1446. [PubMed] [Google Scholar]
- 51.Bender K, Gottlicher M, Whiteside S, Rahmsdorf HJ, Herrlich P. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartwell LH, Kastan MB. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 53.Singh RK, Gutman M, Reich R, Bar-Eli M. Cancer Res. 1995;55:3669–3674. [PubMed] [Google Scholar]
- 54.Jiang H, Yamamoto S, Nishikawa K, Kato R. Carcinogenesis. 1993;14:67–71. doi: 10.1093/carcin/14.1.67. [DOI] [PubMed] [Google Scholar]