Abstract
Brevetoxins (PbTxs) are highly potent trans-syn polyether neurotoxins produced during blooms of several species of marine dinoflagellates, most notably Karenia brevis. These neurotoxins act on voltage-sensitive sodium channels prolonging the active state. During red tides, the commercial fishing and tourism industries experience millions of dollars of lost revenue. Human consumption of shellfish contaminated with PbTxs results in neurotoxic shellfish poisoning (NSP). Additionally, blooms of K. brevis are potentially responsible for adverse human health effects such as respiratory irritation and airway constriction in coastal residents. There is little information regarding the full range of potential toxic effects caused by PbTxs. Recent evidence suggests that PbTxs are genotoxic substances. The purpose of this study was to determine if PbTxs could induce chromosomal aberrations and inhibit cellular proliferation in CHO-K1-BH4 cells, and if so, could the damage be negated or reduced by the PbTx antagonist brevenal. Results from the chromosomal aberrations assay demonstrated that PbTxs are potent inducers of CHO-K1-BH4 chromosome damage. Results from the inhibition of cellular proliferation assays demonstrated that PbTxs inhibit the ability of CHO-K1-BH4 cells to proliferate, an effect which can be reduced with brevenal.
Keywords: Brevetoxin, Brevenal, Cell proliferation, Chromosomal aberration, Neurotoxic shellfish poisoning, Genotoxic
1. Introduction
Harmful algal bloom (HAB) toxins are numerous, chemically complex and diverse, and produced by a variety of organisms under poorly understood environmental stimuli through even less understood molecular mechanisms. Some information is available concerning acute toxicities in humans and animals. The ingestion of tainted shellfish or finfish is responsible for human acute toxicity and the extent of the symptoms is related to the dosage of toxin ingested (Poli et al., 2000). It is known that the HAB toxins generally disrupt intracellular ion concentrations of sodium, calcium, or potassium with each toxin having a high affinity for a specific receptor site. For example, the ingestion of saxitoxin or its congeners produced by the organism Alexandrium sp. causes paralytic shellfish poisoning (PSP) (Schantz et al., 1957). Saxitoxin and all of its derivatives (at least 21 forms have been described) bind specifically to the voltage-gated sodium channel in peripheral neurons and inhibits activation in response to a membrane-depolarizing event (Kulagina et al., 2004). While acute HAB toxicities often manifest as neurological effects, other effects via alternative mechanisms have been noted. Okadaic acid produced by the dinoflagellates Dinophysis and Prorocentrum causes diarrhetic shellfish poisoning (DSP). This toxin inhibits protein phosphatases, which have a wide variety of cellular functions including regulation of cell division, regulation of phosphate metabolism, regulation of many intracellular enzymes and other proteins and smooth muscle function (Vieytes et al., 1997).
Brevetoxins (PbTxs) are cyclic trans-syn polyether compounds produced during red tide events by the marine dinoflagellates Karenia brevis and Chatonella antiqua in the Gulf of Mexico, off the coasts of Florida and North Carolina, and other coastal areas around the world. The toxins produced by these organisms are a serious problem to human health as well as the commercial fishing industry. Red tides are a cause of massive fish kills resulting in millions of dollars in lost revenue. A study by Anderson et al. (2000) estimated an average of over 18 million dollars lost to the commercial fishing industry due to red tides alone over a 5-year period. In addition, there have been unusually high mortality rates of threatened marine species including manatees, bottlenose dolphins, and sea turtles, which have coincided with red tide events (Trainer and Baden, 1999). Avian and fish species which reside in coastal areas are also negatively affected during red tides (Fairey et al., 2001). The tourism and recreation industry of costal areas is also negatively affected by red tides through the closure of beaches and other establishments located within the area (Anderson et al., 2000).
Humans can be exposed to PbTxs through two main routes, ingestion of contaminated shellfish and via aerosols from coastal seaspray. Shellfish acquire PbTxs through filter feeding which then becomes sequestered within their tissues (Gordon et al., 2001; LePage et al., 2002). PbTxs are tasteless, odorless, lipid soluble, and heat and acid stable. Human ingestion of PbTx tainted shellfish results in neurotoxic shellfish poisoning (NSP). Upon consumption of PbTx contaminated shellfish, neurological and gastrointestinal effects occur. The symptoms, which may last for several days, include paresthesia, ataxia, vertigo, myalgia, thermal dysthesia, diarrhea, pupil dilation, bradycardia diarrhea, and mild to severe headache. Exposure to PbTxs in aerosolized form causes airway irritation (Music et al., 1973), asthma attacks, and airway and bronchoconstriction in sheep (Abraham et al., 2004). This type of physiological response evoked by aerosolized PbTxs may occur at very low exposure concentrations in sensitive populations such as asthmatics, individuals with chronic obstructive pulmonary disease, and individuals inflicted with other types of pulmonary disease. The cause of the enhanced pulmonary responses in sensitive people caused by aerosolized PbTxs has yet to be determined.
The bronchoconstriction, respiratory irritation, severe congestion, and airway constriction resulting from PbTx in aerosolized form can most likely be attributed to an immune response caused by the degranulation of mast cells (Backer et al., 2003). While the neurotoxic effects of PbTxs are well documented, not much is known about PbTx immunotoxicity. PbTxs exert their neurotoxic effect by acting upon subunit 5 of voltage-gated Na+ channels at nanomolar concentrations, causing the channel to remain in the open state for a prolonged amount of time (Jeglitsch et al., 1997; Kulagina et al., 2004; Lombet et al., 1987; Trainer and Baden, 1999). Brevenal, a naturally produced PbTx antagonist, is believed to antagonize PbTxs at the active site on the Na+ channel in rat brain synaptosomes (Bourdelais et al., 2004).
Previous experiments with human lymphocytes demonstrate PbTxs damage lymphocyte DNA and raises questions about potential genotoxicity (Sayer et al., 2005). In order to further study mammalian genotoxicity, we hypothesized that PbTxs may cause chromosomal aberrations in CHO-K1-BH4 cells. Since chromosomes are crucial for gene expression, damaging the chromosomes could detrimentally affect an organism.
To further classify the mechanism of PbTx-induced cell death, we hypothesize PbTxs may prevent or inhibit the ability of CHO-K1-BH4 cells to replicate. If PbTx does inhibit the ability of CHO-K1-BH4 cells to proliferate, could brevenal antagonize the effect of the toxin? PbTxs from different sources present in Karenia brevis were tested for their ability to induce toxicity in CHO-K1-BH4 cells. Exposure outcomes, including genotoxic and non-genotoxic events that lead to cancer or organ toxicity in mammalian cells and tissues were assessed. Damaged chromosomes may result in gene mutation, chromosome aberration, and modulation of gene regulation, which have been associated with carcinogenesis (Clayson et al., 1994; Perera et al., 1991). The results from these studies will help define the types of morphologic changes associated with HAB toxin exposure.
2. Methods
2.1. Inhibition of cellular proliferation assay
The inhibition of cell proliferation evaluated the ability of PbTx to inhibit cell proliferation in CHO-K1-BH4 cells. CHO-K1-BH4 cells were chosen for their robust nature and ease of use. After the incubation period, cells were evaluated for their ability to proliferate by comparing the number of cells found in treated wells compared to the number of cells in untreated (control) wells.
2.1.1. Cell maintenance and PbTx preparation
Dr. G. Charles of The Dow Chemical Company, Midland, MI, generously donated first passage CHO-K1-BH4 cells. The cells were grown in HAMS F12 culture media containing HEPES buffer, 5% fetal bovine serum, 1% fungicide, and 1% penicillin. 75 cm2 flasks containing 15 ml culture media were used to grow the cells (100 μl of cells were used per passage). Cells were allowed to grow for approximately 7 days at 37 °C in 5% CO2. By the end of the cellular growth phase flasks were at least 80% confluent.
PbTx-2 (FW 895.1 lot # 082K1237) and PbTx-9 (FW 899.1 lot # 102K1347) both from the marine dinoflagellate Karenia brevis were purchased from Sigma. The Center for Marine Science at The University of North Carolina at Wilmington (UNCW) provided PbTx-2, PbTx-3, and brevenal. PbTxs were prepared as follows: Sigma PbTx-2 and UNCW PbTxs 2 and 3 (500 μg) were dissolved in 80% ethanol (EtOH) and appropriately diluted to make 10−4, 10−6, 10−9, 10−12, and 10−15 M concentrations using Hanks balanced salt solution (HBSS) without calcium and magnesium (IGN lot # 1810486). Sigma PbTx-9 (10 μg) was dissolved in 80% ethanol and diluted using HBSS to make 10−6, 10−9, 10−12, and 10−15 M concentrations. Brevenal (FW 656.4) was also used to evaluate the potential for PbTx antagonism. Brevenal was dissolved in 80% ethanol and diluted into 1.0 μg/ml and 0.1 μg/ml concentrations using HBSS. Bourdelais et al. (2004, 2005) described purification of the UNCW PbTxs and brevenal.
2.1.2. PbTx and brevenal treatment
Six-well plates were used for PbTx treatment purposes. Each 6-well plate contained one type of PbTx (Sigma PbTx-2 or 9, or UNCW PbTx-2 or 3), and each well had a different concentration of PbTx. One well on each plate was designated as a control well. Culture media (2 ml) and 100,000 cells were added to each well. PbTx at 10−4, 10−6, 10−9, 10−12, and 10−15 M concentrations were administered to five different wells (except for Sigma PbTx-9 in which case the initial concentration used was 10−6 M). After PbTx treatment, plates were returned into the incubators at 37 °C in 5% CO2 for 48 h.
2.1.3. Determination of cellular proliferation
At the end of the treatment phase, 6-well plates were removed from the incubators. Media was aspirated; cells were trypsinized and resuspended in 1 ml culture media. Each well was counted using the coulter counter (Beckman Coulter Z2 Cell and Particle Counter: 6605700). On any 6-well plate the percentage of proliferating cells in treatment wells was compared to the percentage of proliferating cells in the control well of that plate. In this experiment, 100% of the cells found in control wells were considered capable of proliferating (Morita et al., 1992). The experiment was conducted in triplicate. In preliminary experiments, the viability of adherent cells, and the loss of viability of aspirated cells was determined by trypan blue exclusion.
2.1.4. Cell proliferation with brevenal
The effect of brevenal on CHO-K1-BH4 cells exposed to PbTx was also investigated. UNCW PbTx-2 at concentrations of 10−4, 10−5, 10−6, and 10−7 M were used. Three 6-well plates were used, each containing a different treatment group. The wells of different plates had either 10−4, 10−5, 10−6, and 10−7 M UNCW PbTx-2, 10−4, 10−5, 10−6, and 10−7 M UNCW PbTx-2 with 1.0 μg/ml brevenal per well (1.5 × 10−3 M), or 10−4, 10−5, 10−6, and 10−7 M UNCW PbTx-2 with 0.1 μg/ml brevenal per well (1.5 × 10−4 M). The experiment was conducted in triplicate.
2.1.5. Determination of the IC50
Identification of the IC50 was also undertaken. Previous results indicated the IC50 value ranged between 10−6 and 10−9 M. Thus PbTx concentrations used in this trial were 10−6.5, 10−7, 10−7.5, and 10−8 M. One 6-well plate was used using UNCW PbTx-2. Cell growth, treatment, and analysis were conducted as previously described.
2.1.6. Statistical analysis
Control and treated groups were compared using a one way analysis of variance and differences among means were determined by using the least significant difference test (LSD test), p < 0.05. Means and standard errors were calculated (Enterprise Guide, SAS Statistical Software, Cary, NC).
2.2. Chromosomal aberrations assay
CHO-K1-BH4 cells were grown and maintained using the parameters set in the inhibition of cellular proliferation experiment. UNCW PbTx-2 was dissolved in 80% ethanol and diluted to 10−7 M using HBSS. The positive control stock, mitomycin C (MMC), was made using 500 μg MMC (Sigma # M0503) in 4 ml culture media. The working solution was then diluted 1:100 using culture media.
Hundred thousand cells were used per treatment group. Three wells of a 6-well plate were used for treating the cells (Charles et al., 2002). One well was a negative control (untreated), one well was a positive control (30 μl MMC), and the third well contained 10−7 M UNCW PbTx-2. After treating the cells, the plates were returned to the incubator for 48 h at 37 °C in 5% CO2.
Following the 48-h treatment period, the 6-well plate was removed from the incubator. Five microlitres per milliliter demecolcine solution was added to each well and the plate was incubated for an additional 3 h.
Following the 3-h demecolcine treatment phase, the plates were taken out of the incubator and placed in a laminar flow hood. The cells were trypsinized, resuspended in fresh media and placed in a 15 ml centrifuge tube (Charles et al., 2002) and centrifuged.
The pellets of each treatment group were resuspended in 4.5 ml 0.075 M KCl solution. Following a 15-min period, 0.5 ml of a fixative solution (3:1 methanol/acetic acid) was added and mixed within each tube. Tubes were centrifuged again at 1200 RPMs for 10 min. The supernatant was aspirated and 5 ml of fixative solution was added (Biswas et al., 1997). The pellet was resuspended in the 5 ml of the fixative solution and the suspension was left for 20 min. Following the 20-min period, tubes were centrifuged and the supernatant aspirated and 5 ml of a fixative solution added and the resuspended pellet was left for 30 min. Following the 30-min period, the tubes were centrifuged and the supernatant was aspirated away until approximately 0.5 ml of fixative and the pellet remained in the bottom of the centrifuge tubes. The pellet was resuspended in the remaining 0.5 ml fixative solution.
2.2.1. Affixing and staining cells on slides
Glass slides were chilled overnight in distilled water at 4 °C. Using a Pasteur pipette, the fixative/cell suspension was dripped onto a slide. Once a single fixative/cell suspension sample was dripped onto a slide, the chromosomes were spread by gently blowing on the slide. After each drop and chromosome spread, the slide was quickly wafted over a candle flame in an effort to affix the sample in place on the slide. Approximately 10 drops per treatment group were administered per slide. Once the samples were affixed to the slides, they were allowed to dry for approximately 10 min (Charles et al., 2002) and then GIEMSA stain solution was applied. The solution consisted of 9 ml GURR buffer (Gibco # 10582-013) mixed with 1 ml GIEMSA stain (Sigma # G-3032). The stain was applied to the slides for 10 min. Following the staining process the stain was removed from the slides (by pouring it away) and gently rinsed with distilled water. Slides were allowed to dry overnight.
2.2.2. Scoring chromosomal aberrations
The slides were viewed at 64× on a light microscope using an oil emersion technique to enhance image detail (Carl Zeiss, Inc., # 47-30-9901, West Germany). Treatment groups were unknown to the scorer. A green lens was placed over the light source, to enhance the purple-stained chromosomes. Only cells undergoing metaphase were viewed, and only those metaphase cells where all the individual chromosomes could be distinguished were scored. Scoring of chromosomes consisted of gaps, breaks, or other clearly distinguishable aberrations (Rooney and Czepulkowski, 1986; Savage, 1999). While many types of aberrations were present, the main objective of the experiment was to determine if a chromosome had an aberration. Fifty distinguishable chromosomes were counted for each of three replicates per treatment group.
2.2.3. Statistical analysis
Control and treated groups were compared using the Mann–Whitney U-test (Wilcoxon two sample test), p < 0.05 (Sokal and Rohlf, 2003).
3. Results
3.1. Inhibition of cellular proliferation
Following the treatment phase cells that had died during PbTx treatment were considered unable to proliferate and aspirated away. The remaining adherent cells were considered able to proliferate and were trypsinized, resuspended, and counted. The number of cells counted in each treatment well was compared to a control well. Hundred percent of the cells in the control well were considered able to proliferate. Thus, the data is presented in terms of percentage of cells in treated wells compared to the control well. Brevenal alone, at 1.0 μg/ml and 0.1 μg/ml concentrations, had no significant effect on the ability of cells to proliferate compared to controls (data not shown).
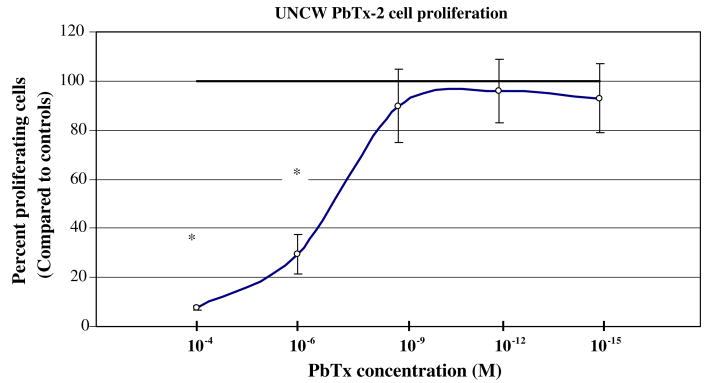
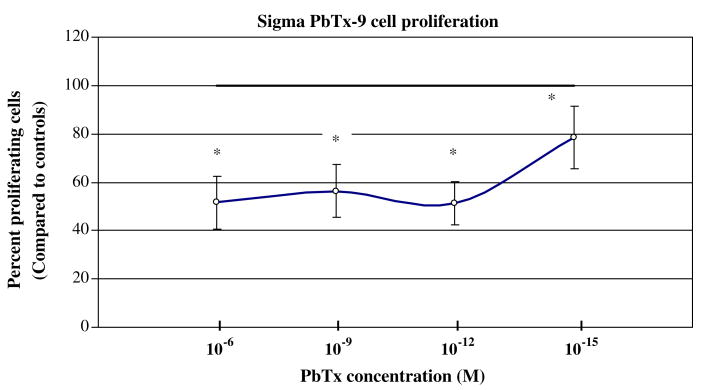
All types of PbTx were able to inhibit cellular proliferation of CHO-K1-BH4 cells by more than 50% at high molar concentrations (10−4 M). At concentrations higher than 10−9 M the effect of the toxin varied among the PbTx types. The dose/response curve of UNCW PbTx-2 (Fig. 1a) and UNCW PbTx-3 (Fig. 1b) leveled off after 10−9 M and were not significantly different from controls. At 10−12 M, the Sigma PbTx-2 (Fig. 1c) declined to approximately 55% of cells capable of proliferation. This decrease at 10−12 M also occurred with Sigma PbTx-9 (Fig. 1d). In all instances, at concentrations of 10−6 M and higher, PbTx was able to inhibit the ability of CHO-K1-BH4 cells to proliferate by 50% or more.
Fig. 1a.
The effect of UNCW PbTx-2 on CHO-K1-BH4 cells at different concentrations. CHO cells were incubated with different concentrations of PbTx for 48 h. At the end of the treatment period, the cells were harvested and counted using a coulter counter. Dead cells were no longer able to proliferate and aspirated away when the media was removed prior to trypsinization. The asterisk indicates a significant reduction in cell proliferation at p < 0.05.
Fig. 1b.
The effect of UNCW PbTx-3 on CHO-K1-BH4 cells at different concentrations (see (a) for description).
Fig. 1c.
The effect of Sigma PbTx-2 on CHO-K1-BH4 cells at different concentrations (see (a) for description).
Fig. 1d.
The effect of Sigma PbTx-9 on CHO-K1-BH4 cells at different concentrations (see (a) for description).
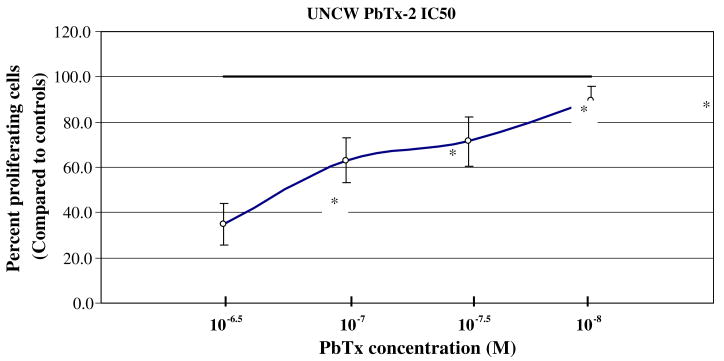
The concentration at which 50% of CHO-K1-BH4 cells are unable to proliferate (IC50) was also determined. From the previous UNCW PbTx-2 dose/response curve it appeared that the IC50 was between 10−6 and 10−9 M. The IC50 was found to be 10−6.75 M (Fig. 2).
Fig. 2.
The lethal concentration at which 50% of CHO cells were unable to proliferate using UNCW PbTx-2 was evaluated. The ability of CHO cells to proliferate was compared to controls. The asterisk indicates a significant reduction in cell proliferation at p < 0.05.
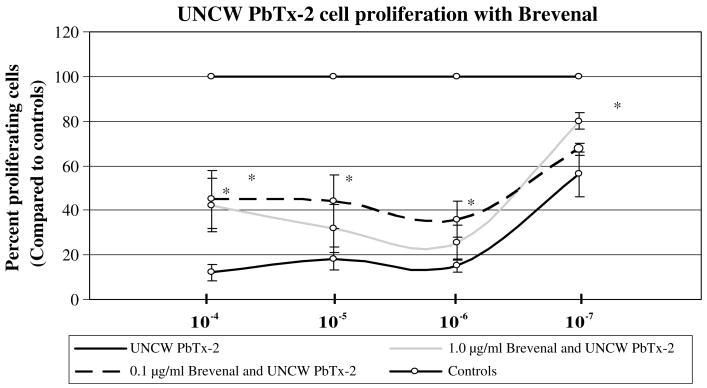
In a previous study, results from the single-cell gel electrophoresis assay demonstrated that brevenal was able to reduce the amount of DNA damage generated by PbTx. One type of PbTx was used (UNCW PbTx-2) and brevenal at two different concentrations were used (1.0 μg/ml and 0.1 μg/ml). Brevenal increased the number of proliferating cells by over 20% in both brevenal treated groups (Fig. 3).
Fig. 3.
The effect of brevenal on the ability of CHO-K1-BH4 cells to proliferate. Two concentrations of brevenal were used, 1.0 μg/ml and 0.1 μg/ml, and were compared with UNCW PbTx at equal molar treatment concentrations. The asterisk indicates a significant reduction in cell proliferation at p < 0.05.
3.2. Chromosomal aberrations assay
While there are many types of chromosomal aberrations, only gaps and breaks, were officially categorized in this experiment. A third category, classified as ‘other’ contained all other types of chromosomal anomalies. These other anomalies included: aligned and displaced terminal fragments; dicentric and centric interchanges; proximal, distal, and sister non-unions; symmetrical and asymmetrical interchanges; symmetrical and asymmetrical intra-changes; monocentric and dicentric triradial breaks.
While inherent chromosome breakages do occur, a frequency of more than one aberration in 20 metaphase cells would be considered a high aberration occurrence for non-treated cells. The negative control group of CHO-K1-BH4 cells (Table 1a) contained few chromosomal aberrations. In contrast, the positive control group (Table 1b) MMC induced a very high occurrence of chromosomal defects with 114 damaged chromosomes found among 3,152 pairs examined. The PbTx treated group (Table 1c) had numerous abnormalities and irregularities found among those chromosomes. Compared to controls, the PbTx treated group contained significant damage that could not be considered inherent chromosomal defects. Examples of the chromosomal aberrations from each group are in Figs. 4–6.
Table 1a.
Chromosomal aberrations scored when CHO-K1-BH4 cells untreated
| Treatment day | Controls | Gaps | Breaks | Other |
|---|---|---|---|---|
| Number of chromosome pairs counted (in 50 cells) | ||||
| 1 | 1054 | 1 | ||
| 2 | 1039 | 1 | ||
| 3 | 1090 | 1 | ||
| Totals | 3183 | 1 | 1 | 1 |
| Total number of damaged chromosome pairs: 3 | Percentage of chromosomes damaged: 0.09% | |||
| Average number of undamaged chromosomes per break | ||||
| 1 chromosome break in 1061 chromosome pairs | ||||
| 1 chromosome break per 50 metaphase cells | ||||
Table 1b.
Chromosomal aberrations scored when CHO-K1-BH4 cells were treated with mitomycin C
| Treatment day | MMC treatment | Gaps | Breaks | Other |
|---|---|---|---|---|
| Number of chromosome pairs counted (in 50 cells) | ||||
| 1 | 1038 | 19 | 10 | 33 |
| 2 | 1049 | 25 | 12 | 33 |
| 3 | 1065 | 25 | 14 | 48 |
| Totals | 3152 | 69 | 36 | 114 |
| Total number of damaged chromosome pairs: 219a | Percentage of chromosomes damaged: 6.9% | |||
| Average number of undamaged chromosome pairs per break | ||||
| 1 chromosome break in 14.39 chromosome pairs | ||||
| 1 chromosome break per 0.685 metaphase cells | ||||
Indicates significance compared to controls.
Table 1c.
Chromosomal aberrations scored when CHO-K1-BH4 cells were treated with 10−7 M UNCW PbTx-2
| Treatment day | PbTx treatment | Gaps | Breaks | Other |
|---|---|---|---|---|
| Number of chromosome pairs counted (in 50 cells) | ||||
| 1 | 1031 | 5 | 4 | 12 |
| 2 | 1050 | 5 | 2 | 12 |
| 3 | 1050 | 5 | 1 | 8 |
| Totals | 3131 | 15 | 7 | 32 |
| Total number of damaged chromosome pairs: 54a | Percentage of chromosomes damaged: 1.7% | |||
| Average number of undamaged chromosomes per break | ||||
| 1 chromosome break per 57.9 chromosome pairs | ||||
| 1 chromosome break per 2.77 metaphase cells | ||||
Indicates significance compared to control and MMC treated groups.
Fig. 4.

Undamaged CHO-K1-BH4 cell chromosomes. Negative control CHO-K1-BH4 cell chromosomes were undamaged and had a low incidence of inherent chromosomal damage. 0.09% of negative control CHO-K1-BH4 cell chromosomes had aberrations.
Fig. 6.

10−7 M UNCW PbTx-2 treated CHO-K1-BH4 cell chromosomes. CHO-K1-BH4 cell chromosomes treated with 10−7 M UNCW PbTx-2 contained a significant number of aberrations compared to control (untreated) CHO-K1-BH4 cell chromosomes. While the number of aberrations was not as frequent as MMC treated chromosomes, 1.7% of PbTx treated CHO-K1-BH4 cells contained chromosomal aberrations. Arrows indicate aberrations.
PbTx at a concentration of 10−7 M negatively affects chromosomes and induces chromosomal aberrations. While only 0.09% of negative control CHO-K1-BH4 cells exhibited chromosomal defects, 1.7% of PbTx treated groups contained aberrations, approximately a 18-fold increase in frequency. Based on the Mann–Whitney U-test (p < 0.05), the difference in the frequency of aberrations found between the control and PbTx treated CHO-K1-BH4 cells was significant.
4. Discussion
Among the consequences of damaging genetic material are the potential for mutagenicity and/or disruption of cell signaling processes such as those involved with cell cycling, proliferation, migration, differentiation, plasticity, inflammation, immunomodulation, apoptosis, cell survival, and developmental processes (Clayson et al., 1994).
In addition to being toxic to neurons and the GI tract, PbTxs also cause airway constriction, bronchoconstriction, and may induce asthma attacks (Abraham et al., 2004; Asai et al., 1982; Backer et al., 2003). Asthma is a hypersensitivity to an allergen or foreign substance that can initiate the immune response (Schuster et al., 2000). The harmful effects of PbTx on the respiratory system trigger an immune response that may be highly sensitive to asthmatics, individuals with chronic obstructive pulmonary disease, and those inflicted with other types of pulmonary disease. The concentrations of marine toxins found during red tide events have not been precisely measured. However, the concentrations are sufficient to cause massive fish kills, death of marine mammals, and death of sea birds. More importantly, the concentrations are sufficient to cause respiratory irritation and to induce asthma attacks in people near the seashore. In previous experiments using the single-cell gel electrophoresis assay, DNA damage was measured in human lymphocytes to determine the extent of single and double DNA strand breaks upon exposure to PbTx (Sayer et al., 2005). DNA damage was apparent upon PbTx exposure and each PbTx subtype tested caused human lymphocyte DNA damage of similar magnitude. As expected, 10−8 M PbTx caused greater damage than 10−12 M PbTx. Still, the 10−12 M PbTx, caused significant DNA damage compared to controls. Both 10−8 and 10−12 M PbTx concentrations were potent inducers of DNA damage in normal human lymphocytes, yet this damage was fully antagonized by brevenal. Thus, disruption of lymphocyte function could severely impair the ability of an organism to eliminate foreign antigens. If the immune system is impaired, the organism will not likely survive.
Cell proliferation experiments revealed that PbTx inhibited the ability of cells to proliferate by 50% at concentrations of approximately 10−6 M. While there was considerable variability among the different types of PbTx, the general trend of the dose/response curve was high cell death at high PbTx concentrations and low cell death at low PbTx concentrations. At lower concentrations, there was considerable variability between types of PbTx. UNCW PbTx-2 and 3 displayed a typical movement towards 100% cell proliferation. It is interesting to note the different sources of PbTx-2 (from both UNCW and Sigma) demonstrated IC50s that differed by roughly two orders of magnitude. We believe the differences in the two sources are related to differences in the original organisms used and the extraction process. It is not unexpected to see such differences when the material source is different. We have no means to compare the chemistry of the materials.
Cell proliferation experiments revealed that PbTx inhibited the ability of cells to proliferate by 50% at concentrations of approximately 10−6 M. While there was considerable variability among the different types of PbTx, the general trend of the dose/response curve was high cell death at high PbTx concentrations and low cell death at low PbTx concentrations. At lower concentrations, there was considerable variability between types of PbTx.
The PbTx antagonist, brevenal, prevented the full toxic potential of PbTx in the cellular proliferation assays. Treatment of CHO-K1-BH4 cells with brevenal prior to the addition of PbTx at very toxic concentrations significantly reduced cell death caused by PbTx alone. More importantly, brevenal increased the number of cells capable of cell proliferation. The cell proliferation study showed an increased number of proliferating cells at each PbTx treatment concentration in at least one of the two brevenal concentrations used in the assay. Therefore, brevenal appears to reduce the toxic effect of PbTx and increases the number of CHO-K1-BH4 cells capable of proliferation.
UNCW PbTx-2 (10−7 M) induced chromosomal aberrations in CHO cells while spontaneous chromosomal damage occurred rarely in negative controls. The positive control, mitomycin C, caused a high incidence of chromosomal aberrations. Mitomycin C is an alkylating agent that cross-links with DNA, causes strand breaks, and inhibits repair. Mitomycin C treated chromosomes displayed several types of aberrations such as interchanges and terminal fragments. PbTx caused significantly greater numbers of chromosomal aberrations compared to controls. A 18-fold increase in the frequency of aberrations was found between the PbTx and negative control groups. While gaps and breaks occurred within the PbTx treated groups, there were frequently terminal fragments. In several instances in PbTx and MMC groups it appeared as though a terminal fragment from one chromosome had paired up with an adjacent chromosome. The information provided by the chromosomal aberrations experiment suggests PbTx was capable of inducing significant chromosomal abnormalities at 10−7 M.
Organisms involved in harmful algal blooms (HABs) produce a variety of toxins, often in high amounts. These toxins include: ciguatoxins, maitotoxins, saxitoxin, scaritoxins, okadaic acid, and palytoxins (in addition to other toxins). Different toxins may be produced during the same HAB event. Many of these toxins bind to cellular or nuclear membrane ion channels in eukaryotic cells causing ion transport disruptions (Ito et al., 2003; Rein and Barrone, 1999). PbTxs bind to voltage-gated sodium channels on voltage gated cells (neurons) at nanomolar concentrations but their activity on non-voltage gated cells and nuclear membranes is unknown. It has also been shown that PbTxs induce a concentration dependent increase in cytosolic Ca2+ in cerebellar neurons (LePage et al., 2002). It is possible that brevetoxin and brevenal may bind to nuclear receptors. Binding of PbTxs to the nuclear membrane receptors in CHO-K1-BH4 cells and other cell types could lead to ion concentration disturbances, especially Ca2+, in the nucleus. This would result in damage to the nuclear DNA through Ca2+ activation of endonucleases or alterations in the tertiary structure of the DNA. Brevenal may work to inhibit or antagonize brevetoxin-induced genotoxic damage by blocking brevetoxin's effect on nuclear receptors and therefore nuclear ion concentrations.
PbTx acting on voltage-gated Na+ channels in neuronal membranes prolongs the active state, and most likely disrupts the Na+ ion concentration gradient.
The information obtained herewith indicates PbTxs inhibit the ability of CHO-K1-BH4 cells to proliferate and cause chromosomal aberrations. The harmful effects on mammalian cells found in the chromosomal aberration assays suggest that exposure to PbTxs may cause genotoxic effects at concentrations around 10−7 M. Furthermore, data from the cellular proliferation assay indicates PbTxs inhibit CHO-K1-BH4 cell proliferation at concentrations as low as 10−12 M. Brevenal, the PbTx antagonist, increases the number of mammalian cells able to proliferate. The results also indicate PbTxs increase the rate of cell death and genetically alter chromosomes. Thus, exposure to PbTxs may cause detrimental genetic abnormalities, such as uncontrolled cell proliferation and differentiation, and increase the rate of apoptosis. This indicates PbTxs are genotoxic materials, which may be associated with somatic, and germ cell mutations. These data taken together suggests that PbTxs are potential carcinogens.
Fig. 5.

Mitomycin C treated CHO-K1-BH4 cell chromosomes. Mitomycin C treated CHO-K1-BH4 cells exhibited a large number of chromosomal abnormalities. 6.9% of all MMC treated chromosomes contained abnormalities. Arrows indicate aberrations.
Acknowledgments
Supported by the North Carolina Agromedicine Center and USDA/CSREES. The PbTxs and the brevenal were provided under NIEHS Grant P01 ES10594. The experiments conducted are in accordance with IRB regulations (UMCIRB# 03-0290). This material is based upon work supported in whole or part by the North Carolina Biotechnology Center.
Abbreviations
- PbTx
brevetoxin
- DSP
diarrhetic shellfish poisoning
- HAB
harmful algal bloom
- IC50
inhibitory concentration at which 50% of cells are unable to proliferate
- LSD
least significant difference
- MMC
mitomycin C
- NSP
neurotoxic shellfish poisoning
References
- Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am J Respir Crit Care Med. 2004;10:1164. doi: 10.1164/rccm.200406-735OC. rccm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Kaoru Y, White AW. Estimated annual economic impacts from harmful algal blooms (HABs) in the United States. Woods Hole Oceanographic Institution Technical Report 2000 [Google Scholar]
- Asai S, Krzanowski JJ, Anderson WH, Martin DF, Polson JB, Lockey RF, Bukantz SC, Szentivanyi A. Effects of toxin of red tide, Ptychodiscus brevis, on canine tracheal smooth muscle: a possible new asthma-triggering mechanism. J Allergy Clin Immunol. 1982;69:418–428. doi: 10.1016/0091-6749(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harm Algae. 2003;2:19–28. [Google Scholar]
- Biswas S, Talukder G, Sharma A. Selenium salts and chromosome damage. Mut Res. 1997;390:201–205. doi: 10.1016/s1383-5718(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Jacocks HM, Wright JLC, Bigwarfe PM, Baden DG. A new polyether ladder compound produced by the dinoflagellate Karenia brevis. J Nat Prod. 2005;68:2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JL, Carsi J, Baden DG. Brevenal is a natural inhibitor of Brevetoxin action in sodium channel receptor binding assays. Cell Mol Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles GD, Linscombe VA, Tornesi B, Mattsson JL, Gollapudi BB. An in vitro screening paradigm for extracts of whole foods for detection of potential toxicants. Food Chem Toxicol. 2002;40:1391–1402. doi: 10.1016/s0278-6915(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Clayson DB, Mehta R, Iverson F. International commission for protection against environmental mutagens and carcinogens: oxidative DNA damage-the effects of certain genotoxic and operationally non-genotoxic carcinogens. Mutat Res. 1994;317:25–42. doi: 10.1016/0165-1110(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Fairey ER, Shuart NG, Busman M, Moeller PDR, Ramsdell JS. Biomonitoring brevetoxin exposure in mammals using blood collection cards. Environ Health Perspect. 2001;109:717–720. doi: 10.1289/ehp.01109717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Kimm-Brinson KL, Padnos B, Ramsdell JS. Acute and delayed thermoregulatory response of mice exposed to brevetoxin. Toxicon. 2001;39:1367–1374. doi: 10.1016/s0041-0101(01)00092-7. [DOI] [PubMed] [Google Scholar]
- Ito K, Toyoda I, Higashiyama M, Uemura D, Sato MH, Yoshimura SH, Ishii T, Takeyasu K. Channel induction by palytoxin in yeast cells expressing Na+, K+-ATPase or its chimera with sarco/endoplasmic reticulum Ca2+-ATPase. FEBS Lett. 2003;543:108–112. doi: 10.1016/s0014-5793(03)00418-6. [DOI] [PubMed] [Google Scholar]
- Jeglitsch G, Rein K, Baden DG, Adams DJ. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J Pharmacol Exp Ther. 1997;284:516–525. [PubMed] [Google Scholar]
- Kulagina NV, O'Shaughnessy TJ, Ma W, Ramsdell JS, Pancrazio JJ. Pharmacological effects of the marine toxins, brevetoxin and saxitoxin, on murine frontal cortex neuronal networks. Toxicon. 2004;44:669–676. doi: 10.1016/j.toxicon.2004.07.023. [DOI] [PubMed] [Google Scholar]
- LePage KT, Baden DG, Murray TF. Brevetoxin derivatives act as partial agonists at neurotoxin site 5 on the voltage gated Na+ channel. Brain Res. 2002;959:120–127. doi: 10.1016/s0006-8993(02)03737-x. [DOI] [PubMed] [Google Scholar]
- Lombet A, Bidard JN, Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987;219:355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Morita T, Nagaki T, Fukuda I, Okumura K. Clastogenicity of low pH to various cultured mammalian cells. Mutat Res. 1992;272:223–226. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- Music SI, Howell JT, Brumback CL. Red tide, its public health implications. JFMA. 1973;60:27–29. [PubMed] [Google Scholar]
- Perera MIR, Betschart JM, Virji MA, Katyal SL, Shinozuka H. Free radical injury and liver tumor promotion. Toxicol Path. 1991;15:51–59. doi: 10.1177/019262338701500106. [DOI] [PubMed] [Google Scholar]
- Poli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Neurotoxic shellfish poisoning and brevetoxin metabolites: a case study from Florida. Toxicon. 2000;38:981–993. doi: 10.1016/s0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- Rein KS, Barrone J. Polyketides form dinoflagellates: origins, pharmacology and biosynthesis. Comp Biochem Physio B. 1999;124:117–131. doi: 10.1016/s0305-0491(99)00107-8. [DOI] [PubMed] [Google Scholar]
- Rooney DE, Czepulkowski BH. Human Cytogenetics: A Practical Approach. IRL Press; Washington, DC: 1986. [Google Scholar]
- Savage JR. An introduction to chromosomal aberrations. Atlas of Genetics and Cytogenetics in Oncology and Haematology 1999 [Google Scholar]
- Sayer AN, Hu Q, Bourdelais AJ, Baden DG, Gibson JE. The effect of brevenal on brevetoxin-induced DNA damage in human lymphocytes. Arch Toxicol. 2005;79:683–688. doi: 10.1007/s00204-005-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz EI, Mold JD, Stranger DW, Shavel J, Reil FJ, Bowden JP, Lynch JM, Wyler RS, Riegel B, Sommer H. J Am Chem Soc. 1957;79:5230–5235. [Google Scholar]
- Schuster M, Tschernig T, Krug N, Pabst R. Lymphocytes migrate from the blood into the bronchoalveolar lavage and lung parenchyma in the asthma model of the brown Norway rat. Am I Respir Crit Care Med. 2000;161:558–566. doi: 10.1164/ajrccm.161.2.9812021. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. W.H. Freedman and Company; Washington, DC: 2003. pp. 428–429. [Google Scholar]
- Trainer VL, Baden DG. High affinity binding of red tide neurotoxins to marine mammal brain. Aqua Toxicol. 1999;46:139–148. [Google Scholar]
- Vieytes MR, Fontal OI, Leira F, Baptista JMV, Botana LM. A fluorescent microplate assay for diarrheic shellfish toxins. Anal Biochem. 1997;248:258–264. doi: 10.1006/abio.1997.2127. [DOI] [PubMed] [Google Scholar]