Abstract
Background
It is unclear whether reducing antibiotic prescriptions can reduce rates of resistance once resistance becomes prevalent. We attempted to determine whether reduced antibiotic consumption, which is observed yearly in children during the warm season, is associated with a reduction in antibiotic resistance in pneumococcal acute otitis media (AOM).
Methods
Antibiotic prescriptions and resistance were measured prospectively during 1999−2003 in 2 demographically distinct populations: Jewish and Bedouin children (aged <5 years) in southern Israel. Associations were assessed using seasonally clustered logistic regression models.
Results
The study included 236,466 prescriptions and 3609 pneumococcal isolates. Prescription rates decreased during the warm months by 36% and 15% in Jewish and Bedouin children, respectively (P < .001 for the season). Among Jewish children, higher resistance rates were observed during the cold than the warm months (P < .001 for each antibiotic). This difference remained significant after adjustment for age, ethnic group, study year, history of antibiotic use, and serotype. The difference was not observed in Bedouin children.
Conclusions
Rapid seasonal decline in resistant AOM-causing pneumococci occurred only in Jewish children, among whom a marked prescribing seasonality was noted, and not in Bedouin children, among whom prescription was less seasonal. The rapid seasonal decrease in resistance associated with markedly reduced antibiotic use suggests that drug-resistant pneumococci may pay a fitness cost.
During the last 20 years, a dramatic increase in antibiotic resistance among pneumococci has been observed and linked to increasing antibiotic consumption [1-4]. Several studies have suggested that reduction in antibiotic consumption leads to a reduction in the antibiotic resistance of Streptococcus pneumoniae in the community [2, 5-7]. However, other studies could not demonstrate such an effect [8-10].
The relationship between antibiotic consumption and resistance is complex. Some antimicrobial agents select resistant pneumococcal strains more effectively than others [8, 11-15]. Moreover, the rate of the decrease in resistance expected after a decrease in antibiotic use depends on both the fitness cost of resistance for the bacteria and the extent of the reduction in consumption [16, 17]. If fitness cost is minimal or if the reduction in antibiotic use is small, antibiotic resistance may continue increasing, albeit perhaps more slowly, or it may decline gradually. However, if resistance exacts a strong fitness cost (with respect to transmissibility) on the bacteria, then a quick and significant decrease in resistance would be expected after a major decrease in antibiotic consumption [18]. A recent study in rats suggested that penicillin resistance in S. pneumoniae, particularly high-level resistance, carries a substantial fitness cost [19]. Another recent study showed a fitness cost of fluoroquinolone-resistant mutants of S. pneumoniae [20]. A complicating factor is the presence of dual and multiple resistance, because reduction mainly in the use of a single antibiotic may not result in the expected reduction in resistance, because of coselection of resistance caused by the continued use of other antibiotics [18].
Surveillance of antibiotic prescriptions in children <5 years old has been ongoing in southern Israel since 1998. We observed yearly higher antibiotic prescription rates during the cold months (October through March) than during the warm months (April through September). We hypothesized that macrolide and β-lactam resistance in S. pneumoniae carries a substantial fitness cost, as suggested in experimental models. This would be observable as a yearly decline in the proportion of resistant isolates during the warm months, when antimicrobial prescribing is reduced.
Therefore, our objectives were to (1) determine the seasonal prescription patterns of commonly used oral antibiotics in young children and (2) compare the seasonal antibiotic prescribing patterns with the seasonal resistance patterns of pneumococci isolated from middle ear fluid (MEF) during acute otitis media (AOM).
METHODS
Setting
In southern Israel (the Negev region), Jewish and Bedouin populations live side by side. The socioeconomic conditions and the lifestyles of the 2 groups differ, but both have access to the same medical services. The Jewish population is mainly urban, whereas the Bedouin population, formerly desert nomads, is in transition to a Western lifestyle [21]. Contact between children of the 2 populations is rare. The Bedouin population is characterized by overcrowding, lower levels of education, lower income, and larger family size than the Jewish population [21]. Hospitalization rates for respiratory and other infectious diseases are higher among Bedouins [22].
All children in the area are born in a single hospital, where they also receive all emergency services and are hospitalized. More than 60% of children in the Negev region are medically insured in the largest health plan in Israel, the General Health Insurance Plan (GHIP). No change in policy occurred during the study period regarding referral of children with AOM to the pediatric emergency department with recommendations regarding tympanocentesis. No pneumococcal conjugate vaccines were introduced in the country during the study period.
Measuring antibiotic prescription rates
Data were gathered from 7 large GHIP pediatric primary care clinics, where all prescriptions are computerized: 5 in urban Jewish centers (yearly mean, 6163 children <5 years old; range, 5407−6937) and 2 in Bedouin townships (yearly mean, 6636 children <5 years old; range, 5269−7219). This represented ∼20% of the region's children <5 years old. All antibiotic prescriptions were recorded during 1998−2003. Oral antimicrobials were grouped as (1) amoxicillin plus amoxicillin-clavulanate, (2) oral cephalosporins (cefaclor, cephalexin monohydrate, and cefuroxime axetil), or (3) azithromycin. The use of phenoxymethylpenicillin, sulfa-containing agents, and erythromycin was negligible, and thus these drugs were excluded from the analysis by drug group.
Bacterial isolates and data from children with AOM
All MEF samples obtained from patients treated at the hospital and >90% of those obtained in the community were cultured at the Clinical Microbiology Laboratory of the Soroka Medical Center. The proportion of MEF samples obtained in the hospital was 80% among Jewish and 70% among Bedouin children. No significant differences were observed between years and seasons. All MEF isolates from 1999−2003 were included. The AOM diagnosis was made by pediatricians, family physicians, or otolaryngologists. Demographic and clinical information was prospectively obtained from culture-positive children. For each culture-positive episode, we recorded culture date, patient's age, ethnicity, previous AOM episodes, and recent history of antibiotic treatment. Data were obtained from medical charts and the child's physician or parents, as appropriate.
Bacteriology
Swabs of MEF aspirates were placed in MW173 Amies transport medium (Transwab; Medical Wire and Equipment), plated immediately on trypticase agar medium containing 5% sheep blood and 5 μg/mL gentamicin and on chocolate agar, and incubated at 35°C for 48 h in a 5% enriched CO2 atmosphere.
Identification, serotyping, and antimicrobial susceptibility testing of S. pneumoniae isolates was performed as described elsewhere [8]. Isolates with a penicillin MIC ≥0.1 μg/mL were considered to be penicillin-nonsusceptible S. pneumoniae (PNSP). Isolates with a penicillin MIC ≥1.0 μg/mL were analyzed separately. Nonsusceptibility to ≥3 antibiotic classes was considered to be multidrug resistance.
Data analysis
An episode was defined as AOM after a pathogen-free interval of ≥30 days for isolation of identical pneumococcal serotype or any interval for different serotypes. Episodes were identified by the presence of a MEF sample. Only 1 isolate was counted per episode. Seasons were defined a priori as follows: cold months, October through March; warm months, April through September.
Data were analyzed with SPSS (version 12.0) and STATA (version 9) software for Windows. Prescriptions were compared between groups or seasons by use of 2-tailed t tests. Resistance was compared between seasons or by month by use of logistic regression models that accounted for clustering by period. A period was defined as a contiguous block of warm or cold months, assuming that observations are correlated within a period but independent across periods. In multivariable analyses, these logistic regressions were also adjusted for age, previous antibiotic treatment, and AOM history. Robust variance estimators were used [23]. Differences for which P < .05 were considered to be statistically significant.
RESULTS
A total of 236,466 prescriptions (149,589 in Bedouin and 86,877 in Jewish children) were recorded. The yearly trends in antibiotic prescription patterns in the 2 populations from 1998 through 2003 have been described elsewhere [8]. The mean annual prescription rate per child was 2.35 for Jewish and 3.75 for Bedouin children [8].
Seasonality of antibiotic prescription rates
A significant decrease in overall antibiotic prescription rates (per 1000 children aged <5 years each year) during the warm months was noted in both populations (P ≤ .005 for the difference in rates between the cold and warm months) (figure 1). This pattern was consistent across all study years. The mean ± SD overall monthly antibiotic prescription rate was 291 ± 77 during the cold months and 222 ± 77 during the warm months, representing a 24% reduction in prescription rate during the warm months (P < .001). This contrast between seasons was more pronounced in Jewish children than in Bedouin children— 241 ± 62 versus 155 ± 32 during the cold versus warm months, respectively, in Jewish children, a 36% reduction in prescription rates (P < .001); and 341 ± 55 versus 290 ± 39, respectively, in Bedouin children, a 15% reduction (P < .001).
Figure 1.

Monthly distribution of antibiotic prescriptions for Bedouin and Jewish children <5 years old in southern Israel during 1998−2003. A and B, Monthly antibiotic prescription rates for Jewish (A) and Bedouin (B) children. C and D, Mean monthly antibiotic prescription rates (for individual months averaged across the entire study period) for Jewish (C) and Bedouin (D) children.
Amoxicillin was the most commonly prescribed antibiotic in both populations, followed by amoxicillin-clavulanate. These 2 drugs were prescribed at higher rates for Bedouin children (mean ± SD monthly rate, 189 ± 37 and 59 ± 13, respectively) than for Jewish children (123 ± 29 and 28 ± 12, respectively) (P < .001 for the comparison between populations). In both populations, amoxicillin and amoxicillin-clavulanate prescription rates decreased significantly during the warm months. The mean monthly rates during the cold versus the warm months were 184 ± 53 versus 119 ± 29, respectively, in Jewish children and 273 ± 45 versus 223 ± 34 in Bedouin children (P < .001 for both). Cephalosporin prescription rates were higher among Bedouin (38 ± 9) than among Jewish (13 ± 5) children (P < .001). In Jewish children, the seasonal pattern of cephalosporin prescriptions paralleled those of amoxicillin and amoxicillin-clavulanate: 15 ± 5 during the cold months versus 11 ± 3 during the warm months (P < .001). In contrast, in Bedouin children, the rates were similar during the cold and warm months: 37 ± 8 versus 41 ± 10 (P = .055).
The overall prescription rates for azithromycin, introduced only in 1998, were significantly higher in Jewish (23 ± 13) than in Bedouin (17 ± 9) children (P < .001). Among Jewish children, the seasonal pattern for azithromycin prescriptions paralleled that of other antibiotics, with a mean monthly rate of 26 ± 16 during the cold months versus 13 ± 8 during the warm months (P < .001). In contrast, prescription rates for azithromycin in Bedouin children did not show seasonal variation: 15 ± 11 during the cold months versus 13 ± 9 during the warm months (P = .253).
Seasonality of antibiotic resistance
Of 11,022 AOM episodes recorded during the study, S. pneumoniae was recovered for 3609 cases, with 1401 and 2205 isolates from Jewish and Bedouin children, respectively. The patient's ethnicity was not recorded in 3 cases, and antibiotic susceptibility was not tested in 37 isolates. Of the nonpneumococcal cases, 2984 had other pathogens, and 4429 were culture negative. Of all AOM episodes during the cold months, the proportions of S. pneumoniae, those with other pathogens, and those that were culture negative were 32.7%, 27.1%, and 40.2%, respectively. The respective proportions during the warm months (n = 4064) were 32.5%, 24.4%, and 43.2%.
In Jewish children, the proportion of isolates with a penicillin MIC ≥1.0 μg/mL was significantly higher during the cold months than during the warm months (43% vs. 29%; odds ratio [OR], 1.86 [95% confidence interval {CI}, 1.37−2.52]; P < .001). The respective figures for erythromycin resistance were 29% versus 20% (OR, 1.56 [95% CI, 1.15−2.12]; P < .005); for multidrug resistance, they were 24% versus 15% (OR, 1.73 [95% CI, 1.25−2.39]; P < .001) (figures 2 and 3). The differences remained significant in our multivariable model that accounted for temporal clustering and that adjusted for age, previous antibiotic treatment, and AOM history (figure 3). In contrast, in Bedouin children, differences were smaller and not statistically significant; the proportion of isolates with a penicillin MIC ≥1.0 μg/mL during the cold months was 26%, versus 21% during the warm months (OR, 1.30 [95% CI, 0.93−1.92]; P = .122). The respective figures for erythromycin resistance were 16% versus 17% (OR, 0.91 [95% CI, 0.52−1.63]; P = .78); for multidrug resistance, they were 20% versus 19% (OR, 1.05 [95% CI, 0.71−1.54]; P = .082).
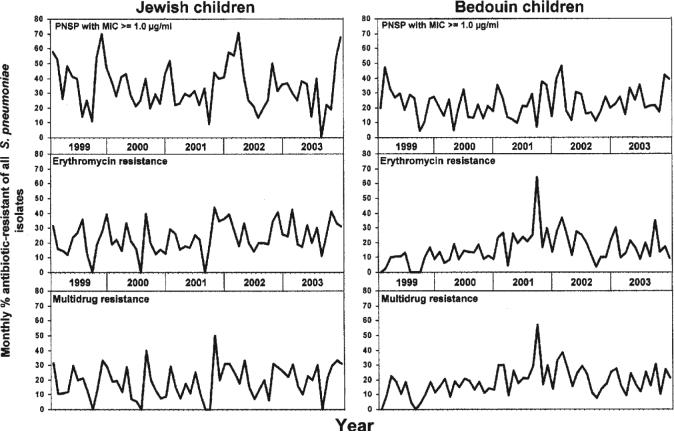
Figure 2.
Monthly proportions of antibiotic-resistant Streptococcus pneumoniae (of all S. pneumoniae isolates) obtained during acute otitis media episodes from Bedouin and Jewish children aged <5 years in southern Israel from 1999 through 2003. PNSP, penicillin-nonsusceptible S. pneumoniae.
Figure 3.
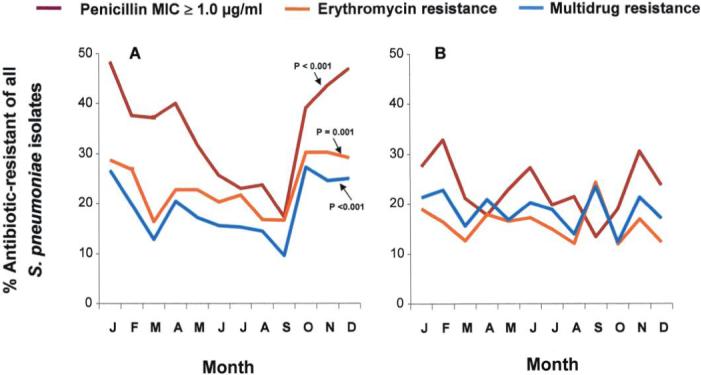
Proportions of antibiotic-resistant Streptococcus pneumoniae (of all S. pneumoniae isolates) obtained during acute otitis media episodes from Bedouin and Jewish children aged <5 years in southern Israel from 1999 through 2003. P values refer to hypothesis tests of an association between the odds of resistance and season (October through March vs. April through September) and were derived from multivariable logistic models. A, Isolates from Jewish children (n = 1401). B, Isolates from Bedouin children (n = 2205).
Association between seasonality of prescriptions and resistance
We hypothesized that the seasonality of resistance resulted from differences in antibiotic use. To test this hypothesis, we again used multivariable logistic models accounting for temporal clustering and adjusting for age, individual antibiotic use, and AOM history (figure 4). Among Jewish children, each monthly increase in 10 prescriptions/1000 children was associated with a 1.05-fold increase in the odds of penicillin resistance during that month (95% CI, 1.03−1.07; P < .001). The corresponding OR for erythromycin resistance was 1.04 (95% CI, 1.02−1.05; P < .001), for multidrug resistance, it was 1.04 (95% CI, 1.02−1.06; P < .001). In contrast, among Bedouin children, no significant correlation was found for penicillin (OR, 1.01 [95% CI, 0.98−1.04]; P = .56), erythromycin (OR, 1.01 [95% CI, 0.97−1.04]; P = .71), or multidrug resistance (OR, 1.01 [95% CI, 0.97−1.06]; P = .65).
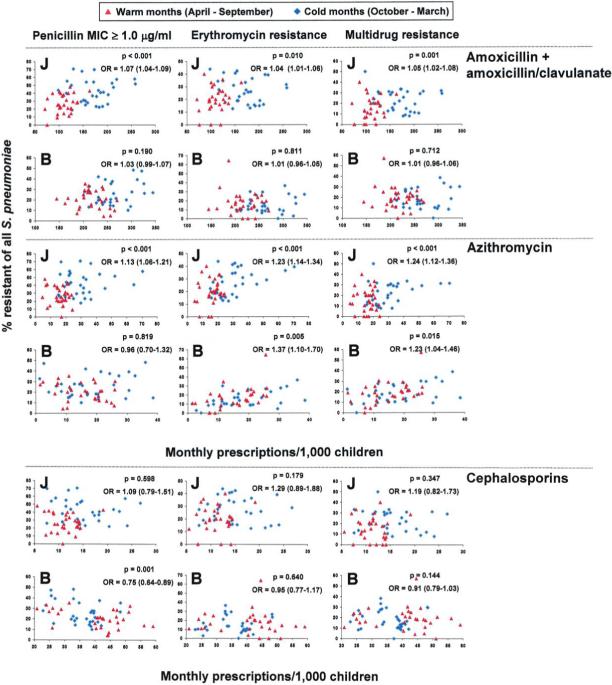
Figure 4.
Relationship between prescription rates and proportions of antibiotic-resistant Streptococcus pneumoniae (of all S. pneumoniae isolates) in children from January 1999 through December 2003. For each month, the prescription rates of amoxicillin plus amoxicillin-clavulanate, azithromycin, and oral cephalosporins are plotted in relation to the respective resistance rates. The 60 months of the study period were divided into warm months (April through September, in red) and cold months (October through March, in blue). P values refer to hypothesis tests of an association between the odds of resistance and season (October through March vs. April through September) and were derived from multivariable logistic models. Odds ratios (ORs) with 95% confidence intervals (in parentheses) for increases of 10 prescriptions/1000 child-months are also provided. J, Jewish children; B, Bedouin children.
We further explored the relationship between prescription rates of antibiotic subgroups (amoxicillin plus amoxicillinclavulanate, azithromycin, and oral cephalosporins) and antibiotic resistance, using similar multivariable models. In figure 4, the monthly prescription rate is plotted against the overall proportion of resistant isolates. Each graph also provides ORs, 95% CIs, and P values for the increase in the odds of resistance associated with an increase in prescription rates of 10 prescriptions/1000 child-months. Prescription rates for amoxicillin plus amoxicillin-clavulanate were associated with penicillin, erythromycin, and multidrug resistance in Jewish but not in Bedouin children. Azithromycin prescription rates were associated with penicillin, erythromycin, and multidrug resistance in Jewish children. In Bedouin children, azithromycin prescription rates were associated with erythromycin and multidrug resistance but not with penicillin resistance. The prescription rates for cephalosporins, which were the lowest among the Jewish children, were not significantly associated with resistance rates for any of the categories analyzed. Among Bedouin children, cephalosporin prescription rates (which peaked during the warm months, in contrast to prescription rates for all other antibiotics) showed an inverse relationship with penicillin resistance rates and were not associated with erythromycin or multidrug resistance.
To ensure that the seasonal pattern of pneumococcal antibiotic resistance could not be attributed to a seasonal shift in pneumococcal serotypes distribution, monthly proportions of antibiotic resistance patterns within 3 selected serotypes in each population were analyzed (figure 5). For this analysis, we selected serotypes that were common and had sufficient numbers of both penicillin-susceptible and penicillin-resistant S. pneumoniae. In Jewish children, serotypes 14, 19F, and 6B were analyzed. In Bedouin children, serotypes 14, 19F, and 23F were analyzed. In Jewish children, higher proportions of serotypes 14, 6B, and 19F with a penicillin MIC ≥1.0 μg/mL were observed during the cold months than during the warm months. The proportions of nonsusceptible isolates for serotype 14 were 79% versus 66% (OR, 1.93 [95% CI, 1.24−3.00]; P = .004); for sero-type 6B, 33% versus 20% (OR, 1.94 [95% CI, 0.60−6.30]; P = .269); and for serotype 19F, 54% versus 37% (OR, 1.99 [95% CI, 0.78−5.90]; P = .152). Among Bedouin children, in whom seasonality in pneumococcal resistance was in general significantly less pronounced than in Jewish children, the proportions of serotype 14, 23F, and 19F isolates with a penicillin MIC ≥1.0 μg/mL did not vary significantly between seasons. For serotype 14, the respective proportions were 50% versus 47% (OR, 1.13 [95% CI, 0.59−2.13]; P = .718); for serotype 23F, 56% versus 49% (OR, 1.32 [95% CI, 0.70−2.52]; P = .391); and for serotype 19F, 40% versus 34% (OR, 1.29 [95% CI, 0.88−1.91]; P = .194).
Figure 5.
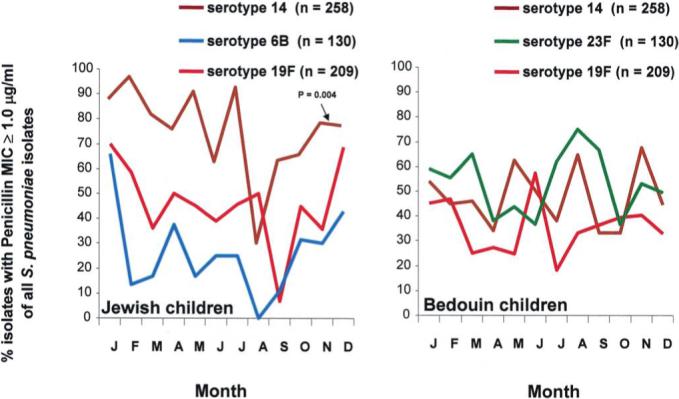
Seasonal distribution of common Streptococcus pneumoniae isolates that were penicillin nonsusceptible with a MIC ≥1.0 μg/mL (as a percentage of all S. pneumoniae isolates) obtained during acute otitis media episodes from Bedouin and Jewish children <5 years old in southern Israel from 1999 through 2003. P values refer to hypothesis tests of an association between the odds of resistance and season (October through March vs. April through September) and were derived from multivariable logistic models.
In theory, seasonality in resistance rates could be explained by an increased number of children presenting with nonresponsive AOM during the cold months, thus simply representing a selection bias toward more recently treated patients during the cold months. To examine this possibility, we selected Jewish children aged 6−29 months (the population showing the highest seasonal variation and the highest resistance rates; n = 977). These children were divided into those who (1) had not received antibiotics during the preceding month (n = 272), (2) had received antibiotics during the preceding month but not at the time of culture (n = 459), and (3) were still receiving antibiotics at the time of culture (n = 246). The proportions of antibiotic-resistant S. pneumoniae by month (grouped for all 5 study years) were then plotted (figure 6). Overall, the highest resistance rate was observed in children receiving antibiotics at the time of the culture (representing treatment failure), followed by those who had received antibiotics during the month preceding the culture. For PNSP (for both penicillin MIC ≥0.1 μg/mL and MIC ≥1.0 μg/mL), seasonality was shown in children currently or recently treated with antibiotics. The same trend was found in those who had not received antibiotics during the preceding month, but this trend did not reach statistical significance. However, for both erythromycin and multidrug resistance rates, seasonality was demonstrated only in children who had not received antibiotics during the preceding month or had received antibiotics during the preceding month but not at the time of culture.
Figure 6.
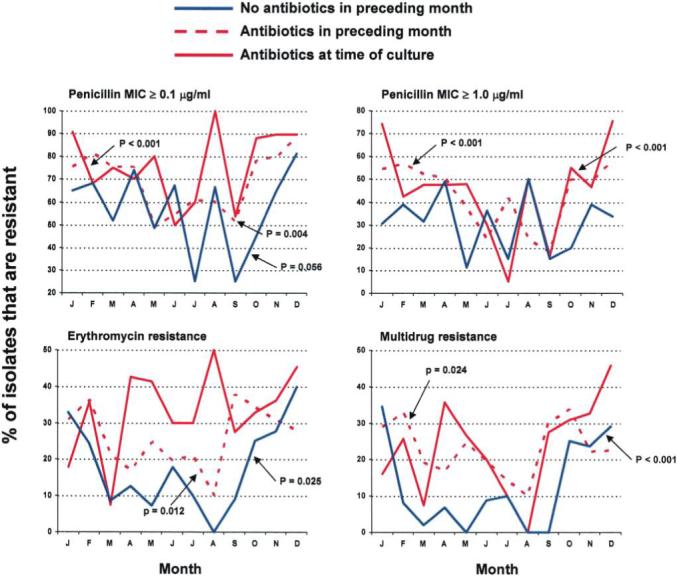
Monthly antibiotic resistance rates of Streptococcus pneumoniae isolates from middle ear fluid of Jewish children aged 6−29 months presenting with acute otitis media (AOM) from January 1999 through December 2003. Each point presents the summation of all isolates for the specific month during the entire 5-year period. P values refer to hypothesis tests of an association between the odds of resistance and season (October through March vs. April through September) and were derived from multivariable logistic models adjusted for history of AOM.
DISCUSSION
Seasonality in antibiotic prescription rates is common and is more accentuated in countries with high antibiotic use [24]. In the present study, we were able to relate oral antibiotic prescription rates in children aged <5 years in the community to antibiotic resistance rates in S. pneumoniae that caused AOM. Furthermore, we showed that the yearly seasonal reduction in antibiotic prescriptions during the warm months was significantly associated with a marked reduction in antibiotic resistance rates among these pneumococcal isolates. This change remained significant in most instances after adjustment for age, AOM history, and previous or current antibiotic use. Furthermore, the seasonal variation in resistance was also found within the prevalent serotypes. Several points strengthen our results: (1) we conducted this study over 5 successive years and found a similar pattern each year; (2) we used large numbers of prescriptions and isolates derived from 2 different databases; and (3) the relationship between prescription rates and antibiotic use was measured in 2 distinct populations (Jews and Bedouins), one of which has more pronounced seasonal variation than the other in both prescribing and resistance rates, strengthening the evidence for an association between seasonality in antibiotic use and resistance.
The comparison between Jewish and Bedouin children provided some important observations. First, Bedouin children consumed more amoxicillin, amoxicillin-clavulanate, and cephalosporins than Jewish children, but this difference was not associated with a higher rate of penicillin resistance among Bedouin children.
Second, azithromycin, introduced only in 1998, has rapidly gained an important place in the treatment of pediatric respiratory tract infections. Azithromycin and oral cephalosporins were used at lower rates than amoxicillin and amoxicillin-clavulanate. However, recent studies, including data from our own region, show that the use of oral cephalosporins and azithromycin in children is probably a major driver of macrolide, penicillin, and multidrug resistance among S. pneumoniae, especially if these drugs are introduced when dual penicillin-macrolide is already prevalent in the community [8, 25-29]. It is plausible that oral cephalosporins and azithromycin promote the carriage and spread of antibiotic-resistant S. pneumoniae because of their pharmacokinetic and pharmacodynamic characteristics [30]. The absence of a significant cold-month peak in the penicillin resistance rate among S. pneumoniae isolates for Bedouin children (in whom significant winter peaks were demonstrated only for amoxicillin and amoxicillin-clavulanate use) supports this hypothesis.
Among Bedouin children, the reverse seasonality of oral cephalosporin prescription rates—peaking in the summer (probably because of the increased rate of skin and soft-tissue infections), in contrast to patterns for all other drugs— did not have a demonstrable effect on seasonal pneumococcal resistance. This was probably because of the opposite seasonal pattern of the drugs used more than cephalosporins.
Because AOM peaks during the cold months (as a consequence of increased viral infections, an important predisposition for the development of AOM), it is plausible that a high rate of nonresponsive AOM cases might be seen during the cold months, thus artificially increasing the proportion of antibiotic-resistant pneumococcal AOM. We tested this potential bias in Jewish children aged 6−29 months (the population showing the highest seasonal variation). The same trend for seasonality in resistance was found in children not receiving antibiotics. Furthermore, for azithromycin, the seasonality was found only in children not receiving antibiotics during the last month. These findings strongly suggest that the increase in resistance during cold months is unrelated to a potential enrichment by treatment failures.
Although the rapid increase in pneumococcal resistance rates in relation to the increase in antibiotic consumption during the cold months was expected, the rapid reduction in resistance rates during the warm months in relation to the reduction of antibiotic use could not be inferred from previous observations. Mathematical models suggest that a key parameter determining resistance rates for community-acquired infections is the fitness cost of antimicrobial resistance, defined as the difference in transmissibility between drug-susceptible and drug-resistant pathogens in the absence of antibiotic treatment [18, 31]. In the present case, if antibiotic resistance imposed a substantial cost on S. pneumoniae transmissibility, we would expect that, once antibiotic pressure is reduced, antibiotic-susceptible S. pneumoniae organisms would rapidly replace resistant ones in the population [18].
The specific costs of antibiotic resistance are not yet known for S. pneumoniae, and it is not clear whether resistance to different drugs (e.g., penicillin and macrolide resistance) conferred through different mechanisms has similar effects on transmissibility. Laboratory studies suggest that antibiotic-resistant S. pneumoniae may pay some fitness cost [20, 32, 33]. A recent study conducted in infant rats demonstrated that S. pneumoniae that had acquired resistant penicillin-binding protein alleles had a reduced ability to compete for upper respiratory tract colonization with susceptible ancestors [19]. The animal studies support our speculation that the reduction in pneumococcal resistance rates during the warm months results from the reduced fitness of antibiotic-resistant strains. The reduction in antibiotic resistance during the warm months was seen mainly among Jewish children, in whom a remarkable reduction (36%) in antibiotic use (and thus in antibiotic pressure) was observed during the warm months.
In the presence of multidrug resistance, even a large reduction in the use of mainly a single drug may not be successful in reducing overall resistance rates. In our study, the “synchronized” pattern in which all oral antibiotics were reduced during the same season among the Jewish children might have contributed to the reduction in single-drug and multidrug resistance in the population.
Regardless of the mechanism involved, the finding that antibiotic resistance rapidly decreases each year among AOM-causing S. pneumoniae isolates when antibiotic prescription rates decrease suggests that interventions to reduce antibiotic use may reduce resistance in the community faster than previously thought. A recent study from France [7] suggests that this might be the case; an intervention to reduce prescriptions or change the dose and duration of antibiotic treatment in the community led to reduced antibiotic use and was associated with a significant reduction in PNSP carriage in the intervention groups.
To what extent should antibiotic use be reduced so as to affect resistance among S. pneumoniae in the community? A model designed by Austin et al. [6] predicts that the decline will be slow. In the present study, the decrease in antibiotic prescription rates during the summer was marked, especially in Jewish children, with approximately twice as many prescriptions during peak months as during trough months. It is unclear whether a more moderate reduction in prescription rates would have achieved a similar reduction in antibiotic resistance.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 27−30 September 2006 (abstract G-345).
References
- 1.Dagan R. Treatment of acute otitis media: challenges in the era of antibiotic resistance. Vaccine. 2000;19(Suppl 1):S9–16. doi: 10.1016/s0264-410x(00)00272-3. [DOI] [PubMed] [Google Scholar]
- 2.Low DE. Changing trends in antimicrobial-resistant pneumococci: it's not all bad news. Clin Infect Dis. 2005;41(Suppl 4):S228–33. doi: 10.1086/430782. [DOI] [PubMed] [Google Scholar]
- 3.Felmingham D, Reinert RR, Hirakata Y, Rodloff A. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and comparative in vitro activity of the ketolide, telithromycin. J Antimicrob Chemother. 2002;50(Suppl S1):25–37. doi: 10.1093/jac/dkf808. [DOI] [PubMed] [Google Scholar]
- 4.Wise R, Hart T, Cars O, et al. Antimicrobial resistance is a major threat to public health. BMJ. 1998;317:609–10. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruinsma N, Kristinsson KG, Bronzwaer S, et al. Trends of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 2004;54:1045–50. doi: 10.1093/jac/dkh458. [DOI] [PubMed] [Google Scholar]
- 6.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–6. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillemot D, Varon E, Bernede C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930–8. doi: 10.1086/432721. [DOI] [PubMed] [Google Scholar]
- 8.Barkai G, Greenberg D, Givon-Lavi N, Dreifuss E, Vardy D, Dagan R. Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005;11:829–37. doi: 10.3201/eid1106.050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennessy TW, Petersen KM, Bruden D, et al. Changes in antibiotic-prescribing practices and carriage of penicillin-resistant Streptococcus pneumoniae: a controlled intervention trial in rural Alaska. Clin Infect Dis. 2002;34:1543–50. doi: 10.1086/340534. [DOI] [PubMed] [Google Scholar]
- 10.Belongia EA, Sullivan BJ, Chyou PH, Madagame E, Reed KD, Schwartz B. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575–83. doi: 10.1542/peds.108.3.575. [DOI] [PubMed] [Google Scholar]
- 11.Granizo JJ, Aguilar L, Casal J, Garcia-Rey C, Dal-Re R, Baquero F. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and beta-lactam consumption in Spain (1979−1997). J Antimicrob Chemother. 2000;46:767–73. doi: 10.1093/jac/46.5.767. [DOI] [PubMed] [Google Scholar]
- 12.Berg HF, Tjhie JH, Scheffer GJ, et al. Emergence and persistence of macrolide resistance in oropharyngeal flora and elimination of nasal carriage of Staphylococcus aureus after therapy with slow-release clarithromycin: a randomized, double-blind, placebo-controlled study. Antimicrob Agents Chemother. 2004;48:4183–8. doi: 10.1128/AAC.48.11.4183-4188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias R, Canica M. Emergence of invasive erythromycin-resistant Streptococcus pneumoniae strains in Portugal: contribution and phylogenetic relatedness of serotype 14. J Antimicrob Chemother. 2004;54:1035–9. doi: 10.1093/jac/dkh469. [DOI] [PubMed] [Google Scholar]
- 14.Drlica K. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother. 2003;52:11–7. doi: 10.1093/jac/dkg269. [DOI] [PubMed] [Google Scholar]
- 15.Klugman KP, Lonks JR. Hidden epidemic of macrolide-resistant pneumococci. Emerg Infect Dis. 2005;11:802–7. doi: 10.3201/eid1106.050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorkman J, Andersson DI. The cost of antibiotic resistance from a bacterial perspective. Drug Resist Updat. 2000;3:237–45. doi: 10.1054/drup.2000.0147. [DOI] [PubMed] [Google Scholar]
- 17.Andersson DI. Persistence of antibiotic resistant bacteria. Curr Opin Microbiol. 2003;6:452–6. doi: 10.1016/j.mib.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Lipsitch M. The rise and fall of antimicrobial resistance. Trends Microbiol. 2001;9:438–44. doi: 10.1016/s0966-842x(01)02130-8. [DOI] [PubMed] [Google Scholar]
- 19.Trzcinski K, Thompson CM, Gilbey AM, Dowson CG, Lipsitch M. Incremental increase in fitness cost with increased β-lactam resistance in pneumococci evaluated by competition in an infant rat nasal colonization model. J Infect Dis. 2006;193:1296–303. doi: 10.1086/501367. [DOI] [PubMed] [Google Scholar]
- 20.Rozen DE, McGee L, Levin BR, Klugman KP. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2007;51:412–6. doi: 10.1128/AAC.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistical abstracts of Israel, 1998. No. 49. Central Bureau of Statistics. Hemed Press; Jerusalem: 1998. [Google Scholar]
- 22.Levy A, Fraser D, Vardi H, Dagan R. Hospitalizations for infectious diseases in Jewish and Bedouin children in southern Israel. Eur J Epidemiol. 1998;14:179–86. doi: 10.1023/a:1007439908351. [DOI] [PubMed] [Google Scholar]
- 23.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 24.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 25.Samore MH, Lipsitch M, Alder S, et al. Mechanisms by which antibiotics promote dissemination of resistant pneumococci in human populations. Am J Epidemiol. 2006;163:160–70. doi: 10.1093/aje/kwj021. [DOI] [PubMed] [Google Scholar]
- 26.Vanderkooi OG, Low DE, Green K, Powis JE, McGeer A. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005;40:1288–97. doi: 10.1086/429242. [DOI] [PubMed] [Google Scholar]
- 27.Pantosti A, Moro ML. Antibiotic use: the crystal ball for predicting antibiotic resistance. Clin Infect Dis. 2005;40:1298–300. doi: 10.1086/429248. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Rey C, Aguilar L, Baquero F, Casal J, Dal-Re R. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J Clin Microbiol. 2002;40:159–64. doi: 10.1128/JCM.40.1.159-164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dagan R, Barkai G, Leibovitz E, Dreifuss E, Greenberg D. Will reduction of antibiotic use reduce antibiotic resistance? The pneumococcus paradigm. Pediatr Infect Dis J. 2006;25:981–6. doi: 10.1097/01.inf.0000239266.20642.26. [DOI] [PubMed] [Google Scholar]
- 30.McCormick AW, Whitney CG, Farley MM, et al. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med. 2003;9:424–30. doi: 10.1038/nm839. [DOI] [PubMed] [Google Scholar]
- 31.Levin BR, Lipsitch M, Perrot V, et al. The population genetics of antibiotic resistance. Clin Infect Dis. 1997;24(Suppl 1):S9–16. doi: 10.1093/clinids/24.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 32.Rieux V, Carbon C, Azoulay-Dupuis E. Complex relationship between acquisition of β-lactam resistance and loss of virulence in Streptococcus pneumoniae. J Infect Dis. 2001;184:66–72. doi: 10.1086/320992. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CN, Briles DE, Benjamin WH, Jr, Hollingshead SK, Waites KB. Relative fitness of fluoroquinolone-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005;11:814–20. doi: 10.3201/eid1106.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]