Abstract
Bacterial chromosomal mutations that confer antibiotic resistance often have deleterious effects that impose costs on reproductive fitness. This observation has led to the generalization that in the absence of the selection pressure exerted through treatment, the frequency of resistance will decrease. This model implies that the prudent use of antibiotics will eventually result in a decline in the prevalence of drug resistance. Recent work, however, suggests that some resistance-conferring mutations may not significantly impair fitness and that others may be accompanied by compensatory mutations that restore the organisms’ reproductive potential. Thus drug resistance, once introduced, may persist unless specific measures are implemented to target prevalent drug-resistant cases. Here we present ecological evidence to support the hypothesis that mutations at the 315 position of katG confer isoniazid resistance for Mycobacterium tuberculosis without diminishing virulence or transmissibility.
INTRODUCTION
RECENT GLOBAL SURVEYS reveal that drug-resistant tuberculosis exists in virtually every location examined.7 Antibiotic resistance in tuberculosis is ultimately a “man-made” phenomenon, and the burden of drug resistance is primarily due to inappropriate treatment of initially drug-sensitive disease. However, in places where recommended treatment strategies are employed, but where drug-resistant strains are nonetheless prevalent, the transmission of drug-resistant strains may cause the persistence or propagation of drug-resistant disease. While some have claimed that “the frequency of resistance will wane at a rate proportional to the fitness costs associated with resistance,”17 others have shown that these fitness costs may not be fixed.3 Understanding the relationship between antibiotic resistance and the transmissibility and virulence of tuberculosis is essential for predicting the future burden of drug-resistant disease. If resistance is associated with fitness deficits, the effect of this transmitted resistance may be negligible; however, if resistance does not incur a cost, transmission of resistant strains may undermine current control strategies.
Isoniazid (INH) is an agent with bactericidal activity against dividing bacilli and plays an essential role in short-course treatment regimens. INH is a prodrug that must be metabolized by mycobacterial catalase-peroxidase to exert its antibacterial activity. Most INH resistance in clinical isolates results from blocking prodrug activation through mutations in the gene katG that alter or eliminate mycobacterial catalase-peroxidase activity.25 Loss of catalase-peroxidase activity has been associated with loss of virulence in a mouse model.18 Although katG insertions, deletions, and frameshifts do occur occasionally and induce complete loss of the functional gene product and correspondingly high levels of INH resistance, the majority of the mutations identified in clinical isolates are single point mutations that result in intermediate levels of resistance.23 The INH resistance mutation that has been most commonly found in clinical isolates is a Ser315Thr point mutation in katG (Fig. 1). The mutant gene product manifests a reduced capacity for prodrug activation while retaining much of the catalase-peroxidase activity of the wild-type enzyme.24 These observations have led researchers to propose that these S315T katG mutations lead to clinically significant INH resistance without exacting a significant fitness cost. This hypothesis is consistent with both animal models of virulence and molecular epidemiological cluster studies.30
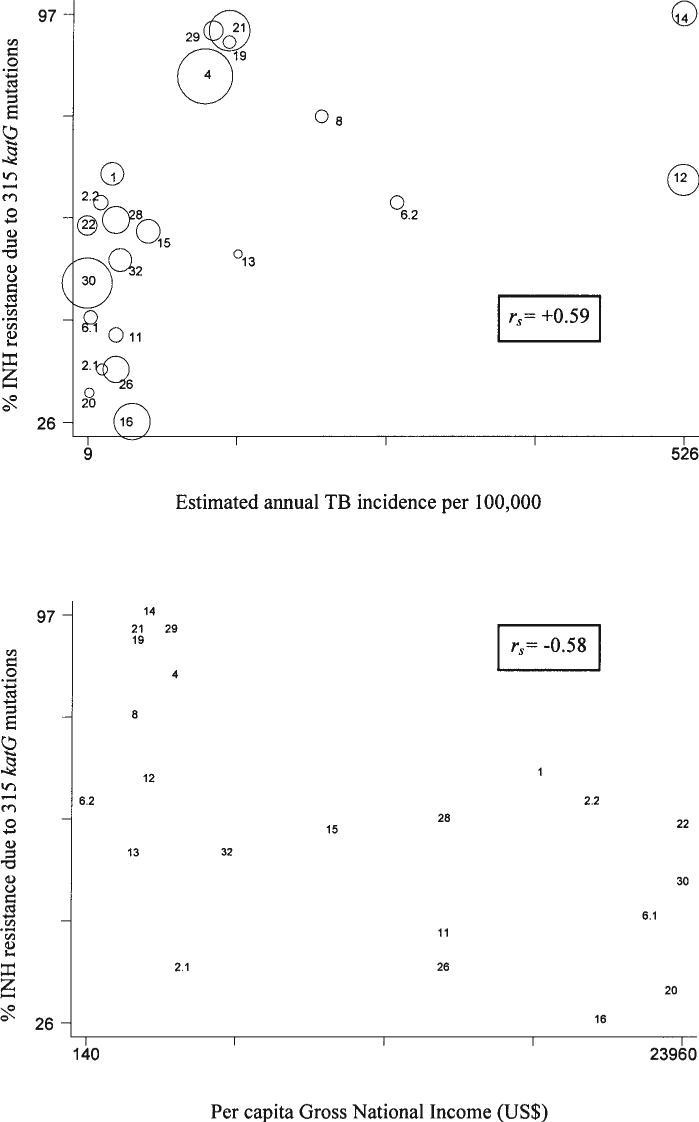
FIG. 1.
(Top) INH resistance due to 315 katG mutations and estimated incidence of tuberculosis by country. The areas of the circles are proportional to the sample size. Spearman correlation coefficients (rs) are given. (Bottom) INH resistance due to 315 katG mutations and estimated per capita Gross National Income by country. Data are provided in Table 1. Reference numbers, 1, Kuwait; 2.1, Lebanon; 2.2, UAE; 4, Lithuania; 6.1, Germany; 6.2, Sierra Leone; 8, Peru; 11, Spain; 12, South Africa; 13, Thailand; 14, South Africa; 15, South Korea; 16, Singapore; 19, Russian Federation; 20, Finland; 21, Russian Federation; 22, Netherlands; 26, Spain; 28, Spain; 29, Latvia; 30, Netherlands; 32, Mexico. Samples cited as 2.1 and 2.2 are from reference 2; samples cited as 6.1 and 6.2 are from reference 6.
We speculate that in geographic areas with a high incidence of tuberculosis infection, a relatively high proportion of INH resistance is due to transmitted disease. If this is indeed the case, the INH-resistant isolates most commonly observed should be those in which the mutations causing resistance have the least negative impact on the transmissibility of the strain. Conversely, in areas in which incidence of infection is low, there should be little transmission of resistant strains. In that case, a higher proportion of resistance may be due to mutations that have a deleterious effect on transmissibility but that have not been selected against, having not yet passed through the selective bottleneck of a transmission event. Assuming that tuberculosis notification rates are proxies for the incidence of infection, we conjectured that the proportion of INH-resistant strains that are due to S315T will be higher in those areas with higher case notification rates.
Because tuberculosis notification rates represent both recently acquired tuberculosis and the reactivation of remote infections, we consider these rates to be coarse indicators of actual transmission. We further postulate that the prevalence of S315T mutations among INH-resistant strains may be associated with socioeconomic indicators, which may serve as an additional proxy for high transmission rates. The inverse association between social and economic well being and the risk of tuberculosis transmission has long been recognized and has been confirmed in multiple recent epidemiologic studies.5,10 If certain drug-resistant mutants are indeed transmissible, they should be found in areas in which poverty favors both their emergence and their further spread.
METHODS
We reviewed the literature for all published surveys that attempted to quantify the amount of clinical INH resistance that was due to 315 point mutations in katG in different parts of the world. We conducted this search using the keywords “katG,” “mutation,” “resistance,” “isoniazid,” and “tuberculosis” and included studies that were written in English, provided data on origin of patients whose isolates were typed, and included a minimum of 10 strains per location.
We next evaluated the relationship between the proportion of INH resistance caused by these specific katG mutations and two indicators: (1) country-specific estimates of tuberculosis incidence, i.e., case notification rates and (2) a general measure of wealth, the World Bank Atlas estimate of per capita Gross National Income in 2002. For those countries for which there was more than one study of the proportion of INH resistance due to katG mutations, we tested the equality of the proportions using large-sample statistics.
Using the Shapiro-Wilk test for normality, we determined that neither incidence nor per capita income were distributed normally. Therefore, we analyzed these data using two different approaches. First, we used the nonparametric Spearman rank correlation coefficient (rs) to quantify these associations. To test for the presence of influential points, we recalculated rs after removing each observation individually and examined the distribution of the correlations for outliers; this distribution was normal, suggesting that there were no influential points. Next, using a Box-Cox transformation to normalize incidence and per capita income, we calculated Pearson correlation coefficients weighted by study sample size. Since some studies did not differentiate between S315T mutations and other mutations at the 315 locus in katG, we repeated these analyses including only those studies for which detailed sequence data were available.
RESULTS
Table 1 summarizes the available studies that report the proportion of INH-resistant strains that are due to mutations at codon 315 in katG. The table distinguishes studies that specifically identified these mutations from those which did not. Table 1 also indicates the sample size for each of the studies as well as the estimated tuberculosis incidence rate and gross per capita income from the country in which each study was performed. The prevalence of these mutants among INH-resistant strains increases as the estimated incidence of tuberculosis rises and as the estimated per capita gross income falls (Fig. 1). The crude Spearman correlation coefficients for these associations were 0.59 for incidence and −0.58 for income. Although we found that the two proportions of INH-resistant strains due to the 315 mutation from South Africa were statistically different (p 0.0001), removing each of these studies had no significant effect on the correlation coefficient. Higher coefficients were observed as the criteria for including the data selected for analysis became increasingly refined (Table 2).
Table 1.
Number of Drug-Resistant Isolates in Sample, Percentage of Resistance Due to katG Codon 315 Mutations, Estimated Incidence of Tuberculosis/100,000, and Estimated Per Capita Gross National Income by Location
| Location | Reference number | Number of drug-resistant isolates | % INH resistance due to 315 katG mutations | Estimated TB incidence per 100,000 (ref. 7) | World Bank estimated per capita GNI in US$, 2002 (ref. 27) |
|---|---|---|---|---|---|
| Finlanda | 20 | 13 | 31 | 11 | 23510 |
| Germanya | 6.1 | 25 | 44 | 12 | 22670 |
| Kuwait | 1 | 67 | 69 | 31 | 18270 |
| Latviaa | 29 | 51 | 94 | 118 | 3480 |
| Lebanon | 2.1c | 17 | 35 | 22 | 3990 |
| Lithuaniaa | 4 | 364 | 86 | 111 | 3660 |
| Mexico | 32 | 67 | 54 | 38 | 5910 |
| Netherlandsa | 22 | 51 | 60 | 9 | 23960 |
| Netherlands | 30 | 295 | 50 | ||
| Perua | 8 | 24 | 79 | 212 | 2050 |
| Russian Feda | 19 | 24 | 92 | 132 | 2140 |
| Russian Fed | 21 | 204 | 94 | ||
| Sierra Leonea | 6.2 | 25 | 64 | 278 | 140 |
| Singaporea | 16 | 160 | 26 | 48 | 20690 |
| South Africaa | 12 | 124 | 68 | 526 | 2600 |
| South Africaa | 14 | 79 | 97 | ||
| South Koreaa | 15 | 71 | 59 | 62 | 9930 |
| Spaina | 26 | 95 | 32 | 34 | 14430 |
| Spaina | 11 | 29 | 41 | ||
| Spaina | 28 | 94 | 61 | ||
| Thailand | 13 | 11 | 55 | 140 | 1990 |
| United Arab Emirates | 2.2 | 28 | 64 | 21 | 20340b |
Table 2.
Correlation Coefficients for the Association between Prevalence of katG Codon 315 Mutants and Measures of TB Incidence and Income
| Test | Correlation with incidence | Correlation with income |
|---|---|---|
| Spearman | 0.59 | −0.58 |
| Spearmana | 0.72 | −0.78 |
| Pearson (weighted by sample size) | 0.61 | −0.78 |
| Pearsona (weighted by sample size) | 0.58 | −0.83 |
Including only studies which included information on SNPs.
DISCUSSION
This analysis shows a strong correlation between the proportion of INH resistance-conferring mutations due to S315T measured in clinical isolates and several different indicators of tuberculosis transmission intensity, supporting the hypothesis that mutations at the 315 position of katG confer INH resistance for Mycobacterium tuberculosis without diminishing virulence or transmissibility. Our study has several important limitations. First, we used data from published reports in which clinical INH-resistant isolates were characterized by resistance-conferring mutations. Because methods of detecting mutations at the 315 locus varied, not all 315 mutations were confirmed to be S315T substitutions. Other mutations observed at this site have led to Ser-Asn, Ser-Ile, Ser-Arg, and Ser-Leu substitutions, although these have been observed less frequently than S315T.4,6,12,15,22,26,28 Because these mutations are relatively rare, they may indeed be less fit than their Ser-Thr counterpart. If this is the case, their inclusion would most likely diminish any association detected between 315 mutations and transmissibility.
In addition, the strategies by which isolates were selected differed significantly between studies reviewed and thus our conclusions may be based on nonrepresentative “convenience” samples. Although many of the studies included isolates from specific local regions within countries, we used country-level estimates of tuberculosis incidence published by the World Health Organization to ensure uniformity of data collection techniques. These country-level estimates may not be an accurate measure of the relevant local disease burden. The observed relationship between the 315 mutations and estimates of per capita income is also subject to the ecologic fallacy because we cannot attribute national averages to the individuals included in these surveys. Finally, although mutations that lead to drug resistance may alter the reproductive fitness of M. tuberculosis strains, there may be other genetic polymorphisms that are associated with the differential fitness of strains. For example, several recent studies have suggested that members of the Beijing family of strains display marked differences in pathogenicity and in the rate of intracellular growth compared to other clinical isolates.31 Although an ideal study of the fitness of drug-resistant mutants would use strains that were isogenic in all other respects, such a study cannot be conducted in the human populations in which tuberculosis transmission takes place.
Despite these limitations, we have shown that the correlation between the proportion of the resistant isolates that are due to 315 mutations and tuberculosis transmission is robust in several sensitivity analyses. The observational data derived from field studies support the hypothesis that the S315T mutation does not impair the fitness of M. tuberculosis isolates circulating in human populations. Further testing with studies designed to evaluate the relative likelihood of specific resistant strains spreading within communities is needed. Molecular epidemio-logical studies, in which strains are characterized by DNA fingerprint pattern and classified as clustered (i.e., part of a recent transmission chain) or unique (i.e., most likely the result of the reactivation of a latent infection), will further our understanding of the impact of specific mutations on fitness. In the Netherlands, van Soolingen et al. found that strains with mutations in katG resulting in amino acid substitutions at the 315 position were more likely to be clustered than those with other INH resistance-conferring mutations and equally likely to be clustered as the susceptible strains.30 Similar cluster analyses conducted in high-incidence countries would be invaluable for discerning the impact of the 315 mutant phenotype on the emergence of drug resistance in locations where the threat of uncontrolled epidemics of drug-resistant tuberculosis is most severe. Additionally, prospective studies among the contacts of those with active tuberculosis could be conducted to compare the distributions of drug-resistance mutations among resistant cases that do not result in secondary cases and those that do.
Given the prolonged course of tuberculosis epidemics, tracking of trends in drug resistance over the course of several decades is necessary to understand the long-term effects of policies aiming to control the emergence of drug resistance. We predict that, in the absence of policies directed at providing effective treatment for those with drug-resistant disease, highly fit resistant strains may have a major impact on the future course of tuberculosis epidemics. Mathematical models that incorporate differential levels of reproductive fitness will be essential for estimating the future burden of drug-resistant disease and identifying effective strategies for its control.
ACKNOWLEDGMENTS
This work was supported by NIAID grant T32 AI07433 (T.C.), the Bill & Melinda Gates Foundation (M.B.), and NIAID grants K08 AI01930-01 and R01 AI046669-02 (M.M.). All authors contributed equally to the conception, research, and writing of this paper. None of the authors has a conflict of interest in the presentation of this work. Funding sources are specified above.
REFERENCES
- 1.Abal AT, Ahmad S, Mokaddas E. Variations in the occurrence of the S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Kuwait. Microb. Drug Resist. 2002;8:99–105. doi: 10.1089/107662902760190644. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad S, Fares E, Araj GF, Chugh TD, Mustafa AS. Prevalence of S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Dubai and Beirut. Int. J. Tuberc. Lung Dis. 2002;6:920–926. [PubMed] [Google Scholar]
- 3.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 4.Bakonyte D, Baranauskaite A, Cicenaite J, Sosnovskaja A, Stakenas P. Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis clinical isolates in Lithuania. Antimicrob. Agents Chemother. 2003;47:2009–2011. doi: 10.1128/AAC.47.6.2009-2011.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr RG, Diez-Roux AV, Knirsch CA, Pablos-Mendez A. Neighborhood poverty and the resurgence of tuberculosis in New York City, 1984−1992. Am. J. Public Health. 2001;91:1487–1493. doi: 10.2105/ajph.91.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobner P, Rusch-Gerdes S, Bretzel G, Feldmann K, Rifai M, Loscher T, Rinder H. Usefulness of Mycobacterium tuberculosis genomic mutations in the genes katG and inhA for the prediction of isoniazid resistance. Int. J. Tuberc. Lung Dis. 1997;1:365–369. [PubMed] [Google Scholar]
- 7.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement: Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. J. Am. Med. Assn. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Escalante P, Ramaswamy S, Sanabria H, Soini H, Pan X, Valiente-Castillo O, Musser JM. Genotypic characterization of drug-resistant Mycobacterium tuberculosis isolates from Peru. Tuber. Lung Dis. 1998;79:111–118. doi: 10.1054/tuld.1998.0013. [DOI] [PubMed] [Google Scholar]
- 9.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, Hoffner S, Rieder HL, Binkin N, Dye C, Williams R, Raviglione MC. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N. Engl. J. Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 10.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N. Engl. J. Med. 1995;333:229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez N, Torres MJ, Aznar J, Palomares JC. Molecular analysis of rifampin and isoniazid resistance of Mycobacterium tuberculosis clinical isolates in Seville, Spain. Tuber. Lung Dis. 1999;79:187–190. doi: 10.1054/tuld.1998.0195. [DOI] [PubMed] [Google Scholar]
- 12.Haas WH, Schilke K, Brand J, Amthor B, Weyer K, Fourie PB, Bretzel G, Sticht-Groh V, Bremer HJ. Molecular analysis of katG gene mutations in strains of Mycobacterium tuberculosis complex from Africa. Antimicrob. Agents Chemother. 1997;41:1601–1603. doi: 10.1128/aac.41.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imwidthaya P, Mieskes K, Rienthong S. Evaluation of katG codon 315 mutations among isoniazid sensitive and resistant Mycobacterium tuberculosis isolates from Thailand. J. Med. Assoc. Thai. 2001;84:864–869. [PubMed] [Google Scholar]
- 14.Kiepiela P, Bishop KS, Smith AN, Roux L, York DF. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber. Lung Dis. 2000;80:47–56. doi: 10.1054/tuld.1999.0231. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Park YJ, Kim WI, Lee SH, Ludgerus Chang C, Kang SJ, Kang CS. Molecular analysis of isoniazid resistance in Mycobacterium tuberculosis isolates recovered from South Korea. Diagn. Microbiol. Infect. Dis. 2003;47:497–502. doi: 10.1016/s0732-8893(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee AS, Lim IH, Tang LL, Telenti A, Wong SY. Contribution of kasA analysis to detection of isoniazid-resistant Mycobacterium tuberculosis in Singapore. Antimicrob. Agents Chemother. 1999;43:2087–2089. doi: 10.1128/aac.43.8.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin BR. Models for the spread of resistant pathogens. Neth. J. Med. 2002;60(7 Suppl):58–64. discussion 64−66. [PubMed] [Google Scholar]
- 18.Li Z, Kelley C, Collins F, Rouse D, Morris S. Expression of katG in Mycobacterium tuberculosis is associated with its growth and persistence in mice and guinea pigs. J. Infect. Dis. 1998;177:1030–1035. doi: 10.1086/515254. [DOI] [PubMed] [Google Scholar]
- 19.Marttila HJ, Soini H, Eerola E, Vyshnevskaya E, Vyshnevskiy BI, Otten TF, Vasilyef AV, Viljanen MK. A Ser315Thr substitution in KatG is predominant in genetically heterogeneous multidrug-resistant Mycobacterium tuberculosis isolates originating from the St. Petersburg area in Russia. Antimicrob. Agents Chemother. 1998;42:2443–2445. doi: 10.1128/aac.42.9.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marttila HJ, Soini H, Huovinen P, Viljanen MK. katG mutations in isoniazid-resistant Mycobacterium tuberculosis isolates recovered from Finnish patients. Antimicrob. Agents Chemother. 1996;40:2187–2189. doi: 10.1128/aac.40.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokrousov I, Narvskaya O, Otten T, Limeschenko E, Steklova L, Vyshnevskiy B. High prevalence of KatG Ser315Thr substitution among isoniazid-resistant Mycobacterium tuberculosis clinical isolates from northwestern Russia, 1996 to 2001. Antimicrob. Agents Chemother. 2002;46:1417–1424. doi: 10.1128/AAC.46.5.1417-1424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musser JM, Kapur V, Williams DL, Kreiswirth BN, van Soolingen D, van Embden JD. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J. Infect. Dis. 1996;173:196–202. doi: 10.1093/infdis/173.1.196. [DOI] [PubMed] [Google Scholar]
- 23.Pym AS, Saint-Joanis B, Cole ST. Effect of katG mutations on the virulence of Mycobacterium tuberculosis and the implication for transmission in humans. Infect. Immun. 2002;70:4955–4960. doi: 10.1128/IAI.70.9.4955-4960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouse DA, DeVito JA, Li Z, Byer H, Morris SL. Site-directed mutagenesis of the katG gene of Mycobacterium tuberculosis: effects on catalase-peroxidase activities and isoniazid resistance. Mol. Microbiol. 1996;22:583–592. doi: 10.1046/j.1365-2958.1996.00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Slayden RA, Barry CE., 3rd The genetics and biochemistry of isoniazid resistance in Mycobacterium tuberculosis. Microbes Infect. 2000;2:659–669. doi: 10.1016/s1286-4579(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 26.Telenti A, Honore N, Bernasconi C, March J, Ortega A, Heym B, Takiff HE, Cole ST. Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J. Clin. Microbiol. 1997;35:719–723. doi: 10.1128/jcm.35.3.719-723.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The World Bank Group http://www.worldbank.org/dat/countrydata/countrydata.html.
- 28.Torres MJ, Criado A, Gonzalez N, Palomares JC, Aznar J. Rifampin and isoniazid resistance associated mutations in Mycobacterium tuberculosis clinical isolates in Seville, Spain. Int. J. Tuberc. Lung Dis. 2002;6:160–163. [PubMed] [Google Scholar]
- 29.Tracevska T, Jansone I, Broka L, Marga O, Baumanis V. Mutations in the rpoB and katG genes leading to drug resistance in Mycobacterium tuberculosis in Latvia. J. Clin. Microbiol. 2002;40:3789–3792. doi: 10.1128/JCM.40.10.3789-3792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Soolingen D, de Haas PE, van Doorn HR, Kuijper E, Rinder H, Borgdorff MW. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in the Netherlands. J. Infect. Dis. 2000;182:1788–1790. doi: 10.1086/317598. [DOI] [PubMed] [Google Scholar]
- 31.van Helden P, Warren R, Victor T, van der Spuy G, Richardson M, van Helden H. Strain families of Mycobacterium tuberculosis. Trends Microbiol. 2002;10:167–168. doi: 10.1016/s0966-842x(02)02317-x. [DOI] [PubMed] [Google Scholar]
- 32.Viader-Salvado JM, Luna-Aguirre CM, Reyes-Ruiz JM, Valdez-Leal R, del Bosque-Moncayo Mde L, Tijerina-Menchaca R, Guerrero-Olazaran M. Frequency of mutations in rpoB and codons 315 and 463 of katG in rifampin- and/or isoniazid-resistant Mycobacterium tuberculosis isolates from northeast Mexico. Microb. Drug Resist. 2003;9:33–38. doi: 10.1089/107662903764736328. [DOI] [PubMed] [Google Scholar]