Abstract
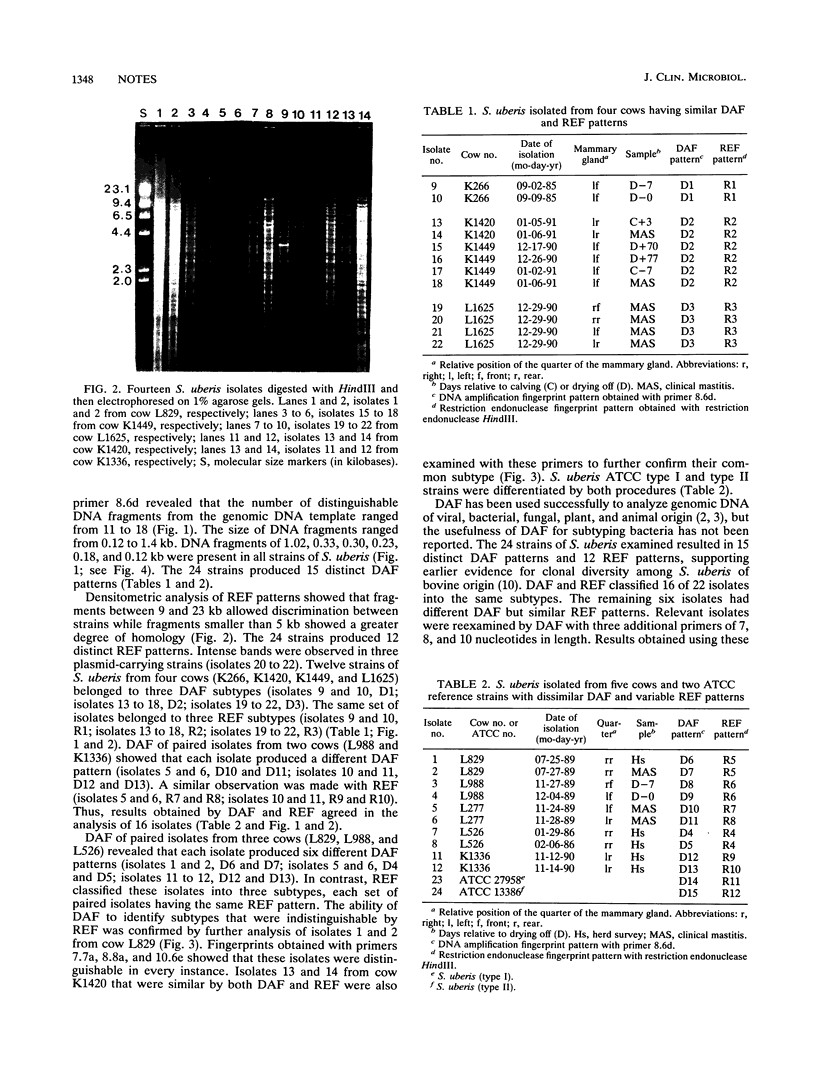
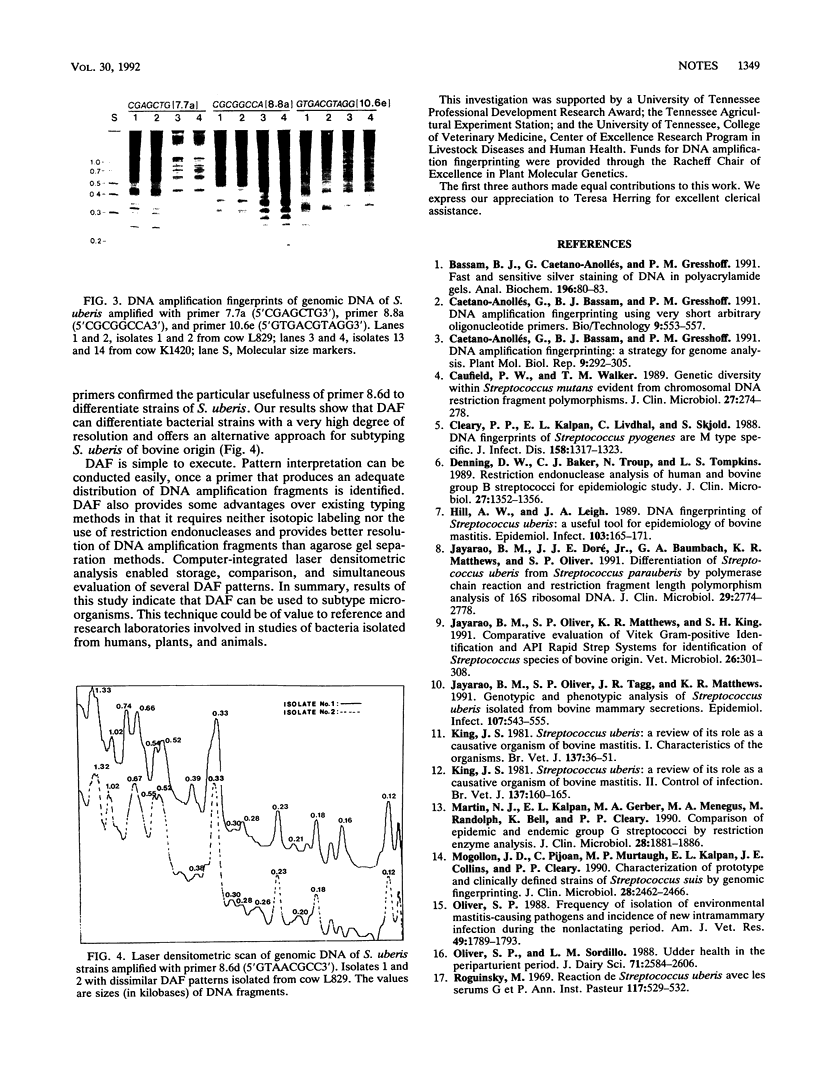
Total DNA of Streptococcus uberis from cows with mastitis was analyzed by DNA amplification fingerprinting (DAF) and compared with restriction endonuclease fingerprinting (REF). DAF grouped 22 strains into 15 distinct patterns, while REF grouped them into 12 patterns. These results suggest that DAF is a useful technique for subtyping strains of S. uberis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassam B. J., Caetano-Anollés G., Gresshoff P. M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991 Jul;196(1):80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Bassam B. J., Gresshoff P. M. DNA amplification fingerprinting using very short arbitrary oligonucleotide primers. Biotechnology (N Y) 1991 Jun;9(6):553–557. doi: 10.1038/nbt0691-553. [DOI] [PubMed] [Google Scholar]
- Caufield P. W., Walker T. M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989 Feb;27(2):274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Kaplan E. L., Livdahl C., Skjold S. DNA fingerprints of Streptococcus pyogenes are M type specific. J Infect Dis. 1988 Dec;158(6):1317–1323. doi: 10.1093/infdis/158.6.1317. [DOI] [PubMed] [Google Scholar]
- Denning D. W., Baker C. J., Troup N. J., Tompkins L. S. Restriction endonuclease analysis of human and bovine group B streptococci for epidemiologic study. J Clin Microbiol. 1989 Jun;27(6):1352–1356. doi: 10.1128/jcm.27.6.1352-1356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. W., Leigh J. A. DNA fingerprinting of Streptococcus uberis: a useful tool for epidemiology of bovine mastitis. Epidemiol Infect. 1989 Aug;103(1):165–171. doi: 10.1017/s0950268800030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Doré J. J., Jr, Baumbach G. A., Matthews K. R., Oliver S. P. Differentiation of Streptococcus uberis from Streptococcus parauberis by polymerase chain reaction and restriction fragment length polymorphism analysis of 16S ribosomal DNA. J Clin Microbiol. 1991 Dec;29(12):2774–2778. doi: 10.1128/jcm.29.12.2774-2778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Oliver S. P., Matthews K. R., King S. H. Comparative evaluation of Vitek gram-positive identification system and API Rapid Strep system for identification of Streptococcus species of bovine origin. Vet Microbiol. 1991 Feb 1;26(3):301–308. doi: 10.1016/0378-1135(91)90023-9. [DOI] [PubMed] [Google Scholar]
- Jayarao B. M., Oliver S. P., Tagg J. R., Matthews K. R. Genotypic and phenotypic analysis of Streptococcus uberis isolated from bovine mammary secretions. Epidemiol Infect. 1991 Dec;107(3):543–555. doi: 10.1017/s0950268800049244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. S. Streptococcus uberis: a review of its role as a causative organism of bovine mastitis. I. Characteristics of the organism. Br Vet J. 1981 Jan;137(1):36–52. doi: 10.1016/s0007-1935(17)31786-4. [DOI] [PubMed] [Google Scholar]
- King J. S. Streptococcus uberis: a review of its role as a causative organism of bovine mastitis. II. Control of infection. Br Vet J. 1981 Mar-Apr;137(2):160–165. doi: 10.1016/s0007-1935(17)31733-5. [DOI] [PubMed] [Google Scholar]
- Martin N. J., Kaplan E. L., Gerber M. A., Menegus M. A., Randolph M., Bell K., Cleary P. P. Comparison of epidemic and endemic group G streptococci by restriction enzyme analysis. J Clin Microbiol. 1990 Sep;28(9):1881–1886. doi: 10.1128/jcm.28.9.1881-1886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogollon J. D., Pijoan C., Murtaugh M. P., Kaplan E. L., Collins J. E., Cleary P. P. Characterization of prototype and clinically defined strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1990 Nov;28(11):2462–2466. doi: 10.1128/jcm.28.11.2462-2466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. P. Frequency of isolation of environmental mastitis-causing pathogens and incidence of new intramammary infection during the nonlactating period. Am J Vet Res. 1988 Nov;49(11):1789–1793. [PubMed] [Google Scholar]
- Oliver S. P., Sordillo L. M. Udder health in the periparturient period. J Dairy Sci. 1988 Sep;71(9):2584–2606. doi: 10.3168/jds.S0022-0302(88)79847-1. [DOI] [PubMed] [Google Scholar]
- Roguinsky M. Réactions de Streptococcus uberis avec les sérums G et P. Ann Inst Pasteur (Paris) 1969 Oct;117(4):529–532. [PubMed] [Google Scholar]
- Skjold S. A., Quie P. G., Fries L. A., Barnham M., Cleary P. P. DNA fingerprinting of Streptococcus zooepidemicus (Lancefield group C) as an aid to epidemiological study. J Infect Dis. 1987 Jun;155(6):1145–1150. doi: 10.1093/infdis/155.6.1145. [DOI] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]