Abstract
Painful degenerative disc disease is a major health problem and for successful tissue regeneration, MSCs must endure and thrive in a harsh disc microenvironment that includes matrix acidity as a critical factor. MSCs were isolated from bone marrow of Sprague-Dawley rats from two different age groups (<1 month, n=6 and 4–5 months, n=6) and cultured under four different pH conditions representative of the healthy, mildly or severely degenerated intervertebral disc (pH 7.4, pH 7.1, pH 6.8, pH 6.5) for 5 days. Acidity caused an inhibition of aggrecan, collagen-1 and TIMP-3 expression, as well as a decrease in proliferation and viability and was associated with a change in cell morphology. Ageing had generally minor effects but young MSCs maintained greater mRNA expression levels. As acidic pH levels are typical of increasingly degenerated discs, our findings demonstrate the importance of early interventions and predifferentiation when planning to use MSCs for reparative treatments.
Keywords: intervertebral disc, mesenchymal stem cells, pH, regeneration, gene expression, proliferation, viability, ageing
Introduction
Tissue engineering approaches for intervertebral disc repair and regeneration require a sufficient number of functional cells, and progenitor cells such as mesenchymal stem cells (MSCs) have attracted special attention. Progenitor cells may hold more promise than native cells isolated from the respective damaged tissue, since degenerative conditions diminishes functionality and total numbers of native cells available. Regenerative strategies for degenerative disc disease face multiple challenges, most of which evolve from the disc’s uncommon and harsh microenvironment. The disc microenvironment is characterized by high mechanical loads [1], reduced oxygen supply [2] and reduced nutrition [3;4] as well as high [5]. Perhaps the most challenging microenvironment condition of the intervertebral disc is its matrix acidity, which increases with degeneration [6]. The pH conditions in the disc are more pronounced than in cartilage [6;7], with the pH levels in a healthy disc ranging between 7.2 and 7.0 [8] and around 0.5 pH units lower than that of surrounding fluids [5]. Matrix acidity can drop drastically during disc degeneration with pH levels of 6.5 most representative of severely degenerated discs, although values of pH 5.7 have been recorded [6;9] (Table 1).
Table 1.
Extracellular pH in the Intervertebral Disc.
Acidification during degenerative processes can be explained by a loss of nutrient transportation routes (e.g. due to smoking [10]) as well as by the presence of cytokines which may enhance lactic acid production [11;12]. In general, two main factors are responsible for the acidic conditions in the normal intervertebral disc: The disc is avascular and therefore energy metabolism is mostly based on anaerobic glycolysis, which means that disc cells produce ATP by transforming glucose into lactic acid [13].. In addition, the intervertebral disc is characterized by a high negative charged fixed density and therefore also by high concentrations of free cations [14] such as H+ because of high proteoglycan concentrations.
Matrix acidity is known to play an important role in the functionality and viability of disc cells [4; 14; 15] and was previously determined to be a crucial factor in MSC behavior [16]. Values of pH drop substantially in advanced degeneration making knowledge of pH effects on MSCs critical, however, the behavior of MSCs to varying pH environments remains largely uninvestigated and is the purpose of this study. The hypothesis of this study is that gene expression, proliferation and viability will be maintained at neutral pH but that function and viability of MSCs will be significantly reduced at pH levels below a certain threshold. The clinical relevance is to determine how different acidity levels affect MSC behavior in order to establish if a pH range exists when there is optimal likelihood of successful regenerative applications.
Methods
Cell isolation and expansion
Femurs from 12 Sprague-Dawley rats were bilaterally excised within 1 hour after death and the ends of the bones were removed. Six animals were 4–5 months old and another six animals was 3–4 weeks old. The bone marrow was detached from the femur by a short centrifugation step (1500 rcf, 30 seconds). Bone marrow aspirates of both femurs of one rat were pooled and resuspended in culture medium consisting of DMEM with 10% FCS, Penicillin (100 units/ml), Streptoymcin (100 μg/ml) and Amphotericin (2.5 μg/ml). Freshly isolated cells of each rat were incubatred at 37°C, 5% CO2 and 21% O2 and after 24 hours, culture medium was changed to remove non-adherent cells, therefore identifying MSCs by the Colony Forming Unit-Fibroblast assay (CFU-F assay) [17] and expanded thereafter. All reagents were purchased from Invitrogen (Carlsbad, CA).
Cell culture under IVD-typical pH conditions
MSCs in passage 1 were trypsinized, resuspended in culture medium consisting of DMEM supplemented with 10% FCS, Penicillin (50 units/ml), Streptoymcin (50 μg/ml) and Amphotericin (1.25 μg/ml) and aliquoted into 25cm2 cell culture flasks (gene expression analysis) or 24 well plates (all other analysis methods). Medium with 4 different pH values were prepared by adding an appropriate amount of 1M HCl, to the supplemented DMEM, resulting in pH values of 7.4 (= body fluid), 7.1 (= healthy IVD), 6.8 (= mildly degenerated IVD) and 6.5 (= severely degenerated IVD). The culture medium was kept in the incubator for 18 hours to allow pH equilibrium (CO2-dependent) to be reached. Normal medium was replaced by the appropriate pH adjusted medium one day later, with each rat MSCs being cultured under 4 different pH conditions. On day 3, medium was replaced by fresh, equilibrated pH medium and on day 5, cells were harvested and analyzed.
Gene expression analysis (real-time RT PCR)
MSCs were lysed and RNA was isolated by use of the GenElute mammalian total RNA Kit (Sigma, St. Louis, MO) and reverse transcribed into cDNA with MultiScribe reverse transcriptase using the TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). For each sample, duplicate analysis of the mRNA levels were performed using real-time RT-PCR on the GeneAmp 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) and the TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Gene expression of matrix proteins (aggrecan, collagen-1, collagen-2), matrix degrading enzymes (MMP-2, ADAMTS4), anti-catabolic genes (TIMP-3), apoptosis markers (p53, caspase-3) and a housekeeping gene (18S-RNA) was measured as previously described [18;19] (for primer sequences see Table 2). Duplicate Ct values for each sample were analyzed and the relative amount of mRNA was computed according to the comparative Ct Method. For each rat, gene expression was calculated relative to 18S-RNA and gene expression at pH 7.1, pH 6.8 and pH 6.5 was calculated relative to gene expression at pH 7.4.
Table 2.
Primer/Probe sequences for real-time RT-PCR.
| Gene | Primer/Probe | Sequence (5′ → 3′) |
|---|---|---|
| Aggrecan | Forward | ggactgggaagagcctcga |
| Reverse | cgtccgcttctgtagcctgt | |
| ProbeFAM/TAMRA | tcacttgcacagaccccaacacctaca | |
|
| ||
| Caspase-3 | Forward | aattcaagggacgggtcatg |
| Reverse | gcttgtgcgcgtacagtttc | |
| ProbeFAM/TAMRA | ttcatccagtcactttgcgccatg | |
|
| ||
| Collagen-1 | Forward | gcccagaagaatatgtatcaccaga |
| Reverse | ggccaacaggtccccttg | |
| ProbeFAM/TAMRA | cttgggtccctcgactcctatgacttctg | |
|
| ||
| Collagen-2 | Forward | gcacatctggtttggagagacc |
| Reverse | tagcggtgttgggagcca | |
| ProbeFAM/TAMRA | cggcttccacttcagctacggcg | |
|
| ||
| MMP2 | Forward | Applied Biosystems Assays-on-Demand |
| Reverse | ||
| ProbeFAM/TAMRA | ||
|
| ||
| P53 | Forward | tgcgtgtggagtatttggatg |
| Reverse | tggtacagtcagagccaacaag | |
| ProbeFAM/TAMRA | aaacacttttcgacatagtgtggtggtgcc | |
|
| ||
| TIMP3 | Forward | cctttggcactctggtctacact |
| Reverse | ctttcagaggcttccgtgtga | |
| ProbeFAM/TAMRA | aagcaaatgaagatgtaccgaggattca | |
|
| ||
| 18S | Forward | Applied Biosystems Assays-on-Demand |
| Reverse | ||
| ProbeVIC/TAMRA | ||
Measurement of proliferation (MTT Assay)
A fresh, sterile solution of MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide from Sigma, St. Louis, MO] with a concentration of 0.5 mg/ml in DMEM was prepared, 500 μl was added to each well and incubated for 4 hours at 37°C. MTT medium was discarded and 200 μl sterile DMSO (Sigma, St. Louis, MO) was added to lyse cells (10 min, 37°C, under shaking). Two aliquots were measured in duplicate at 565 nm and absorbance at pH 7.1, pH 6.8 and pH 6.5 was calculated relative to the absorbance at pH 7.4.
Analysis of DNA content (Picrogreen Assay)
DNA content of samples was analyzed using the Picogreen dsDNA quantitation Kit (Invitrogen, Carlsbad, CA). Briefly, MSCs were trypsinized, centrifugated and the cell pellet was digested with 400 μl of sterile papain solution at 60°C over night [NaOAc (100 mM), EDTA (10 mM), L-Cysteine (10mM), papain (300μg/ml) (Sigma, St. Louis, MO)]. Samples (50 μl, in duplicate) were incubated with Picogreen dsDNA quantitation Kit in a 96 well plate and quantified photometrically (extinction 480 nm, emission 520 nm) relative to DNA standards.
Analysis of cell viability (Fluoresceindiacetate/Propidiumiodide Staining)
A fresh, sterile staining solution with a concentration of 20.8 μg Fluoresceindiacetate (in Acetone) and 16.7 μg Propidiumiodide (in Ringer) (Sigma, St. Louis, MO) per 1 ml Ringer was prepared, 500 μl were added to each well and incubated in the dark at 37°C for 20 min. After incubation, cells were washed with sterile PBS (3x) and an inverted fluorescence microscope was used to detect red and green fluorescence, determining the ratio of viable to dead/necrotic cells under each pH conditions.
Statistical Analysis
A two way ANOVA was performed to evaluate effects of pH level (repeated measures) and aging (young vs mature). Post-hoc comparisons were performed using Dunnet’s test to evaluate effects of pH using pH 7.4 as control. When significant interactions between pH and age were found, Fisher’s PLSD was performed to evaluate the pH levels where young and mature donors had significant differences. All analyses were performed using StatView (Version 5.0.1., SAS Institute Inc., Cary, NC, USA) with a significance level of p < 0.05.
Results
Gene expression (real-time RT-PCR)
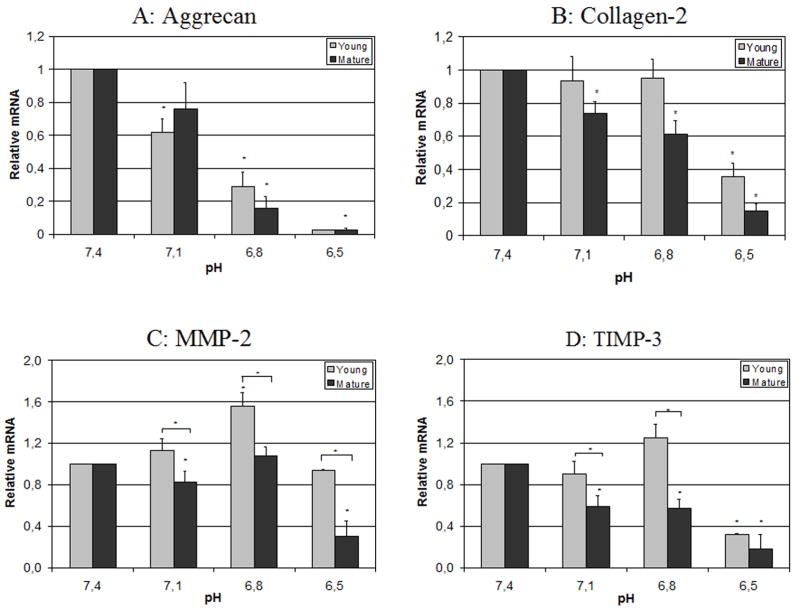
The pH conditions strongly influenced gene expression (Figure 1), with a significant inhibition of mRNA levels for aggrecan, collagen-1 and TIMP-3 expression under acidic conditions relative to pH 7.4, with severe effects already at pH 6.8 for aggrecan and at pH 6.5 for collagen-1 and TIMP-3. Effects for collagen-1 were less pronounced than for aggrecan. Only minor or no changes in the expression of MMP-2 and caspase-3 were detected until pH levels were reduced all the way to 6.5. The mRNA expression of collagen-2 and p53 were under the detection level and could not be analyzed. Some age effects were noted with pH having a larger effect on the down-regulation of TIMP-3 for mature MSCs at pH 7.1 and 6.8. The young MSCs up-regulated MMP-2 expression at pH 7.1 and 6.8 while mature MSCs showed hardly any alterations of MMP-2 mRNA levels at the same pH levels. In summary, acidity caused a drop in relative biosynthesis rate (aggrecan, collagen-1, TIMP-3) in our cell culture experiments, particularly for mature cells with evidence of a decreased biosynthesis rate and altered phenotype with age.
Figure 1.
Aggrecan (A), collagen-1 (B), TIMP-3 (C) and MMP-2 (D) mRNA expression under different pH levels, relative to pH 7.4 (real-time RT-PCR). Statistically significant differences (p<0.05) are marked with *.
Cell proliferation (MTT assay)
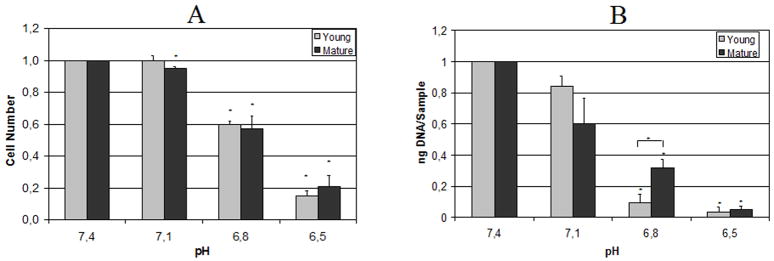
Cell proliferation was also strongly influenced by pH (Figure 2A), with proliferation being highest for pH 7.4 and 7.1 and decreasing at more acidic pH conditions with no effects of rat age. We found a significant inhibition of proliferation for all pH levels compared to 7.4.
Figure 2.
A) Cell proliferation under different pH levels, relative to pH 7.4 (MTT assay).
B) DNA content under different pH levels, relative to pH 7.4 (Picogreen assay). Statistically significant differences (p<0.05) are marked with *.
DNA content (Picogreen assay)
Measurement of DNA content revealed a dose-dependent decrease that was statistically significant from pH 7.4 for pH 6.8 and 6.5 (Figure 2B) and showed high similarities to data obtained via MTT assay (as shown in Figure 2A). There was a significant effect of animal age at pH 6.8 and 7.1, but this effect was of small magnitude and results were generally consistent for both age groups.
Cell viability (fluoresceindiacetate/propidiumiodide)
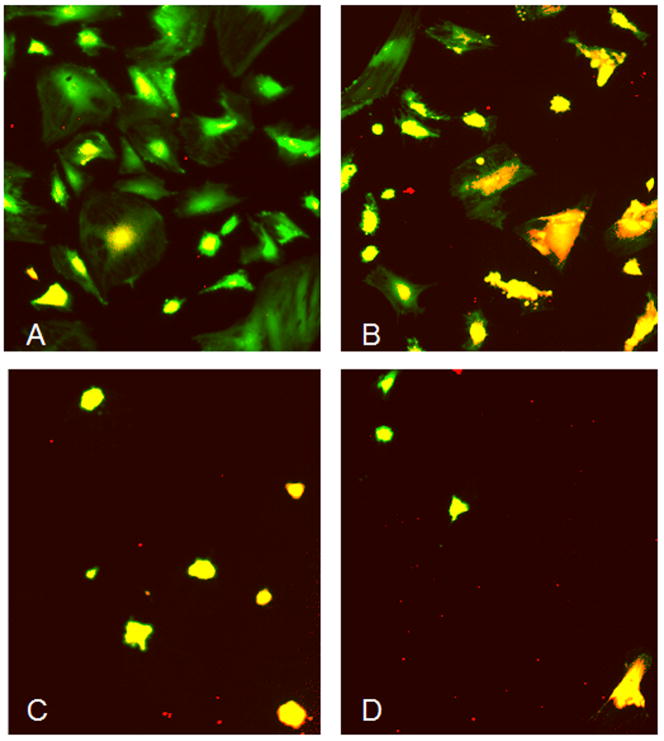
Cell viability decreased with acidity, showing a decreased number of living cells (green) and an increased number of dead cells (red) from pH 7.4 to pH 6.5 (Figure 3), with the most severe effects occurring below pH 6.8. Morphological changes were also noted as MSCs showed a loss in cytoskeleton size under acidic pH conditions. The total number of cells also decreased from pH 7.4 to pH 6.5, therefore supporting the data obtained from MTT and Picogreen assay. No differences were observed between cells from young and mature animals.
Figure 3.
Cell viability and morphology under different pH levels, where green fluorescence indicates viable cells (fluoresceindiacetate) and red indicates dead/necrotic cells (propidiumiodide). Pictures represent pH levels of 7.4 (A), 7.1 (B), 6.8 (C) and 6.5 (D).
Discussion
The intervertebral disc is characterized by an acidic pH, especially during the process of disc degeneration. Matrix acidity may negatively influence disc cell functionality and may also limit the success of MSC based tissue regeneration for the intervertebral disc. To date, this is the first study to directly investigate the responses of MSCs to pH levels found in the disc, and we used a cell culture system to address several clinically important questions: Is there a pH level where biosynthesis rates of MSCs are greatly diminished? Does pH cause phenotypic changes? At what pH level do MSCs stop proliferating and lose viability? Does donor age affect the response of MSCs to pH?
This study revealed a drop in biosynthesis rate, as indicated by the down-regulation of aggrecan, collagen-1 and TIMP-3 mRNA expression, that occurred with increasing acidity, as would be expected with advanced disc degeneration. It was noteworthy that functionality and viability were generally maintained at pH 7.1 (representing a fairly healthy disc) while significant loss of cellularity and decreased biosynthesis rates were found at pH 6.5 (representing a severely degenerated disc). This decrease in mRNA expression was gradual with decreasing pH particularly for MSCs from mature donor animals (especially when considering the logarithmic nature of the pH scale). While MSCs from young and mature donors reacted similarly, anabolic and catabolic mRNA expression was maintained at (or even increased) in young MSCs with pH values as low as 6.8 suggesting more of a threshold response compared to mature MSCs.
Gene expression and morphology results further indicated pH levels affected phenotype as well as biosynthesis rates. The decrease in anabolicand anti-catabolic mRNA measurements as well as the general lack of change (or increase) in MMP-2 expression at all but the lowest pH level suggested a shift to a more catabolic response. In a study by Bischoff et al., it was shown that a drop in pH can stimulate IL-8 production (via p38 and NFκB pathways) [20] and certain cytokines are known to influence matrix protein expression and production [21], which may be a potential mechanism for findings in this study, although more investigation is necessary to determine underlying pathways for our observed changes.
A drop in pH revealed negative effects on proliferation and viability of MSCs as indicated by MTT assay, Picogreen assay and viability staining. For other cell types such as fibroblasts, pH was already shown to control proliferation as well as expression of extracellular matrix proteins [22]. In addition to showing an increase in apoptotic/necrotic cells under more acidic conditions, viability staining also indicated a change in cell morphology from cells with a large cytoskeleton to roundish small cells. The change in cytomorphology and the shift in mRNA expression further support an altered phenotype with acidity prior to, or concomitant with, loss of cellularity. Cytomorphological alteration may have arisen due to (1) changes in cytoskeleton, (2) changes in cell adhesion molecules [23] or (3) survival and therefore selection of a certain cell type from the heterogeneous MSC pool. Further characterization of the remaining cells will be necessary to develop appropriate interventions.
Even though viability staining revealed a pH dependency, it was difficult to make conclusions about apoptotic processes. Measurement of caspase-3 expression did not show significant effects of pH condition, which might be due to the fact that expression of caspase-3 is an early apoptosis marker that may have reached a plateau or steady state after 5 days of culture. When culturing fibroblasts at acidic pH levels (pH 6.7) for shorter time periods (1–3 days), an increase in the expression of certain apoptosis markers such as FLAME-1 or TRAIL APO2-L could be observed by proteomics [24]. Expression of p53 was also evaluated in this study but was not detectable (even though the primers/probe were shown to be efficient on RNA isolated from disc tissue) which suggests additional cells in pellet and/or 3-D culture would be important for future studies.
Matrix acidity seems to be more detrimental to MSCs than to disc cells when compared to the literature. Bovine nucleus pulposus cells showed a decrease in synthesis of sulphated glycosaminoglycans below pH 6.8 (compared to pH 7.2), measured by 35S-sulphate incorporation [14;15]; incorporation of 3H-Proline was shown to be less sensitive to extracellular pH [15]. In a similar culture system, Bibby et al. found a decrease in cell viability when decreasing the pH to 6.7 and a more obvious decrease for pH 6.2, especially when culturing cells under nutrient deficit conditions [3]. We hypothesize that differentiated cells (disc cells) are better adapted to the harsh pH conditions than undifferentiated stem cells such as our nascent MSCs. Intervertebral disc cells express acid sensing ion channel proteins (ASIC), which was shown to be likely associated to their ability to survive under acidic conditions [25] and this may be responsible for observed differences. One way for successful disc regeneration may be the use of predifferentiated MSCs, using growth factors such as TGF-beta [26], or to subject MSCs to a more gradual acclimation to altered pH as would occur in vivo. This hypothesis is supported by a recent study from Guehring et al., showing that notochordal cells are also more sensitive to changes in pH than disc cells [27], which could be due to the gradual increase in acidity that occurs with disc cells throughout aging and degeneration.
The choice for 2D culture allowed high proliferation rates and high cell yields while keeping passage number low, yet had some limitations, particularly with regard to defining cell phenotype. For example collagen-2 mRNA was hardly detectable, possibly due to the use of a 2D system [28], especially as primers/probe were successfully used before on disc tissue [18;19]. More precise evaluation of cell phenotype would require a 3D culture system. This study determined that donor age may be an important factor for MSC regeneration; future studies with an aged group would provide additional insight beyond the effects of maturation as explored in the current study, yet our relatively young age groups were expected to represent cells with the most promise for adaptation to media pH changes.
Results indicated that MSC functionality, phenotype, and viability were minimally restricted at pH 7.1, representing a fairly healthy disc, and severely compromised at pH 6.5, representing a severely degenerated disc. The MSC response to pH levels was gradual although there was some evidence that cells from young donors might exhibit a response with maintained biosynthesis rates until a lower pH threshold. We conclude that MSC therapies for degenerative disc regeneration offer the greatest promise when applied at early stages of degeneration with pH levels of 6.8 or above. Findings also suggested a likelihood of failure for MSC based regeneration in severely degenerated discs using nascent MSCs. However, pH challenges are less detrimental to disc cells than to MSCs, and we speculate that treatment of moderately and severely degenerated IVDs may be possible using MSCs after predifferentiation and/or more gradual acclamation of cells to matrix acidity.
Acknowledgments
Supported by a grant from the National Institutes of Health (R01 AR051146) and AO Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–62. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30–5. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 3.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 4.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30:858–64. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 6.Kitano T, Zerwekh JE, Usui Y, Edwards ML, Flicker PL, Mooney V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993:372–7. [PubMed] [Google Scholar]
- 7.Grodzinsky AJ. Electromechanical and physicochemical properties of connective tissue. Crit Rev Biomed Eng. 1983;9:133–99. [PubMed] [Google Scholar]
- 8.Ichimura K, Tsuji H, Matsui H, Makiyama N. Cell culture of the intervertebral disc of rats: factors influencing culture, proteoglycan, collagen, and deoxyribonucleic acid synthesis. J Spinal Disord. 1991;4:428–36. doi: 10.1097/00002517-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–6. doi: 10.1007/BF02146615. [DOI] [PubMed] [Google Scholar]
- 10.Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–9. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- 11.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–7. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 14.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341–9. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079–82. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. 2008;33:1843–9. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–7. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff DS, Zhu JH, Makhijani NS, Yamaguchi DT. Acidic pH stimulates the production of the angiogenic CXC chemokine, CXCL8 (interleukin-8), in human adult mesenchymal stem cells via the extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and NF-kappaB pathways. J Cell Biochem. 2008;104:1378–92. doi: 10.1002/jcb.21714. [DOI] [PubMed] [Google Scholar]
- 21.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borsi L, Allemanni G, Gaggero B, Zardi L. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer. 1996;66:632–5. doi: 10.1002/(SICI)1097-0215(19960529)66:5<632::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Serrano CV, Jr, Fraticelli A, Paniccia R, Teti A, Noble B, Corda S, Faraggiana T, Ziegelstein RC, Zweier JL, Capogrossi MC. pH dependence of neutrophil-endothelial cell adhesion and adhesion molecule expression. Am J Physiol. 1996;271:C962–70. doi: 10.1152/ajpcell.1996.271.3.C962. [DOI] [PubMed] [Google Scholar]
- 24.Bumke MA, Neri D, Elia G. Modulation of gene expression by extracellular pH variations in human fibroblasts: a transcriptomic and proteomic study. Proteomics. 2003;3:675–88. doi: 10.1002/pmic.200300395. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama Y, Cheng CC, Danielson KG, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Expression of Acid-Sensing Ion Channel 3 (ASIC3) in Nucleus Pulposus Cells of the Intervertebral Disc is Regulated by p75NTR and ERK Signaling. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070805. [DOI] [PubMed] [Google Scholar]
- 26.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 27.Guehring T, Sumner M, Wilde G, Urban JP. Notochordal Cells Diappearance In The Human Intervertebral Disc - The Role Of Nutritional Demands. Annual Meeting of the International Society for the Study of the Lumbar Spine; Hong Kong. 2007. [Google Scholar]
- 28.Marlovits S, Hombauer M, Truppe M, Vecsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004;86:286–95. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]