Abstract
The lens in the vertebrate eye has been shown to be critical for proper differentiation of the surrounding ocular tissues including the cornea, iris and ciliary body. In mice, previous investigators have assayed the consequences of molecular ablation of the lens. However, in these studies, lens ablation was initiated (and completed) after the cornea, retina, iris and ciliary body had initiated their differentiation programs thereby precluding analysis of the early role of the lens in fate determination of these tissues. In the present study, we have ablated the lens precursor cells of the surface ectoderm by generation of transgenic mice that express an attenuated version of diphtheria toxin (Tox176) linked to a modified Pax6 promoter that is active in the lens ectodermal precursors. In these mice, lens precursor cells fail to express Sox2, Prox1 and αA-crystallin and die before the formation of a lens placode. The Tox176 mice also showed profound alterations in the corneal differentiation program. The corneal epithelium displayed histological features of the skin, and expressed markers of skin differentiation such as Keratin 1 and 10 instead of Keratin 12, a marker of corneal epithelial differentiation. In the Tox176 mice, in the absence of the lens, extensive folding of the retina was seen. However, differentiation of the major cell types in the retina including the ganglion, amacrine, bipolar and horizontal cells was not affected. Unexpectedly, ectopic placement of the retinal pigmented epithelium was seen between the folds of the retina. Initial specification of the presumptive ciliary body and iris at the anterior margins of the retina was not altered in the Tox176 mice but their subsequent differentiation was blocked. Lacrimal and Harderian glands, which are derived from the Pax6-expressing surface ectodermal precursors, also failed to differentiate. These results suggest that, in mice, specification of the retina, ciliary body and iris occurs at the very outset of eye development and independent of the lens. In addition, our results also suggest that the lens cells of the surface ectoderm may be critical for the proper differentiation of the corneal epithelium.
Keywords: lens, ablation, cornea, diphtheria toxin, iris, ciliary body, transgenic mice, retina, lacrimal glands, Harderian glands
INTRODUCTION
The vertebrate eye develops as a result of inductive interactions between tissues that are derived from the ectoderm, neural crest and mesoderm. During embryonic development, the neuroectoderm-derived optic vesicle induces the overlying ectoderm to upregulate the expression of transcription factors such as Pax6 and Sox2, leading to lens placode formation (reviewed in Chow and Lang, 2001). The lens placode invaginates to form the lens pit which subsequently, detaches from the overlying ectoderm to form the lens vesicle. Concurrently, the optic vesicle invaginates and forms the optic cup. The pre-lens ectoderm has been proposed to be a source of inductive signals critical for early development of the optic cup. Removal of the pre-lens ectoderm in chick embryos prevents optic vesicle invagination (Hyer et al., 2003). In mice ablation of the surface ectoderm by physical or enzymatic means at E9 (20–22 somite stage) resulted in induction of MITF, a retinal pigmented epithelial (RPE) marker, in the distal retina and conversion of the retina into an RPE (Hyer et al., 2003; Nguyen and Arnheiter, 2000). Conditional deletion of the Pax6 in the surface ectoderm results in the loss of lens placodes but does not affect optic cup formation and retinal differentiation (Ashery-Padan et al., 2000). In mice, the posterior/proximal part of the optic cup differentiates into the retinal pigmented epithelium (RPE) and the anterior/distal part differentiates to form the neural retina. The inductive signal for RPE differentiation has not been identified but in vitro studies in chicks have shown that periocular mesenchymal cells are both necessary and sufficient for induction of an RPE differentiation program in the neuroectoderm (Fuhrmann et al., 2000). In the cave fish Astyanax mexicanus, an inductive signal from the lens together with another signal from the RPE have been proposed to be essential for prevention of retinal apoptosis (Yamamoto and Jeffery, 2000). Collectively, these results suggest that interactions between the lens and surrounding ocular tissues are critical for proper development of the embryonic eye.
The lens, subsequent to detachment from the overlying ectoderm, initiates fiber differentiation. Posterior lens cells differentiate as lens fibers and express β- and γ-crystallins, while anterior cells remain cuboidal and retain expression of Pax6 and E-Cadherin (Govindarajan et al., 2005; Xu et al., 2002). An inductive signal from the neural retina is thought to be critical for initiation of lens fiber differentiation (Chow and Lang, 2001; Coulombre and Coulombre, 1963; Lovicu and McAvoy, 2005). After lens vesicle detachment, surface epithelial cells adjacent to the lens vesicle (defined by Sox2 expression) differentiate to form the corneal epithelium. In mice, onset of corneal epithelial differentiation is marked by Keratin 12 (K12) expression by embryonic day 15 (E15) (Kurpakus et al., 1994). The corneal stroma and endothelium are formed by neural crest and mesodermally-derived periocular mesenchymal cells (Gage et al., 2005). An inductive signal from the lens has been shown to be critical for the corneal endothelial precursors to develop junctional contacts and complete their transformation from mesenchyme to epithelium (Beebe and Coats, 2000; Zhang et al., 2007). Recent studies in chicks show that the lens also plays a critical role in shaping corneal innervation; Semaphorin 3A secreted by the lens mediates initial repulsion of trigeminal sensory axons from the cornea and is necessary for the proper formation of the nerve ring and positioning of the ventral plexus in the choroid fissure (Lwigale and Bronner-Fraser, 2007).
Ectodermal cells adjacent to the corneal epithelium differentiate to form the palpebral and bulbar conjunctival epithelia. Signals that delineate the boundary between corneal and conjunctival epithelium are not known. The two major ocular glands (the lacrimal glands and Harderian glands) are derived from palpebral and bulbar conjunctival epithelial precursors respectively (Govindarajan et al., 2000). Epithelial precursors of both these glandular rudiments retain expression of Pax6 (Govindarajan et al., 2000) and Pax6 has been shown to be critical for the development of these glands (Makarenkova et al., 2000).
The iris and ciliary body are derived from neuroectodermal precursors at the anterior margins of the retina and RPE. The ciliary epithelium is composed of two layers; the inner layer is derived from the neural retina and the outer layer from the anterior pigmented epithelium. An FGF signal from the neural retina and BMP signal from the periocular mesenchyme have been proposed to initiate ciliary body differentiation (Dias da Silva et al., 2007). The necessity of the pre-lens ectoderm for early specification of the iris and ciliary body has not been addressed in mice.
Lens ablation studies in mice have been performed by generating transgenic mice with lens-specific expression of diphtheria toxin A (DTA) or Tox176 (an attenuated version of DTA) (Breitman et al., 1989; Breitman et al., 1990; Key et al., 1992; Klein et al., 1992). The A subunit of the diphtheria toxin inactivates elongation factor-2 (EF-2) by ADP-ribosylation. Therefore, expression of the A subunit (DTA) is toxic to the cells where it is expressed. Previous lens ablation studies performed in mice relied on expression of DTA or Tox176 in the lens fibers. In these mice, the toxin is expressed in the lens after initiation of fiber differentiation. At this time during ocular development, the differentiation programs of the surrounding ocular tissues including cornea, iris, ciliary body, retina and the RPE have all been initiated (Zhang et al., 2007). These studies therefore, precluded the analysis of the early role of the lens in influencing cell fate specification of surrounding ocular tissues. In order to ablate the pre-lens ectodermal precursors, we targeted expression of Tox176 to the surface ectoderm using a modified Pax6 promoter. Lens placodes were not seen in the Pax6-Tox176 transgenic mice with concomitant loss of Sox2, Prox1 and αA-crystallin expression. The Tox176 transgenic mice however, showed normal optic vesicle invagination, optic cup formation and early differentiation of the neural retina. The retina underwent several foldings and the neuronal tissues between these folds transdifferentiated into RPE resulting in ectopic placement of the RPE in the anterior part of the eye. Early specification of the presumptive iris and ciliary body was unaltered but, subsequent differentiation of the iris and ciliary body was blocked. In the cornea, in place of a corneal epithelium, an epidermal layer that expressed skin epidermal differentiation markers such as Keratin 1 and 10 was seen and K12 expression was downregulated. The Pax6-Tox176 transgenic mice also lacked lacrimal and Harderian glands. These results suggest that, in mice, the lens is not essential for early specification of the retina, iris and ciliary body. However, the lens cells appear to be critical for proper differentiation of the corneal and conjunctival epithelium.
MATERIALS AND METHODS
Generation of Pax6-TOX176 transgenic mice
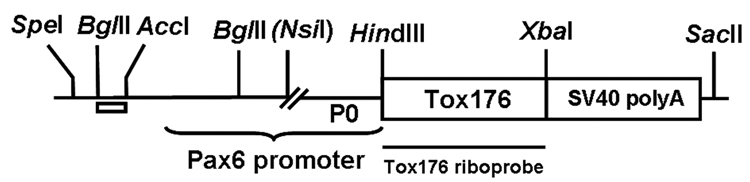
The Tox176 cDNA clone, encoding an attenuated version of diphtheria toxin (a gift from Ian Maxwell, University of Colorado Health Sciences Center, Denver), was digested with HindIII and XbaI and inserted between the Pax6 promoter/enhancer and the SV40 intron and polyadenylation sequences (Govindarajan et al., 2005). The Pax6 enhancer/promoter contains the lens enhancer (1.3 kb) and the P0 promoter (1 kb) of the Pax6 gene but does not include the pancreatic enhancer sequences present in the Pax6 promoters described previously (Kammandel et al., 1999; Williams et al., 1998). The injection fragment was generated by SpeI and SstII digestion and was microinjected into individual pronuclei of 1-cell stage FVB/N mouse embryos. Injected embryos were transferred into pseudopregnant ICR strain female mice. Animals were handled following the guidelines provided in US Public Health Service Policy on Humane Care and Use of Laboratory Animals. The embryos were allowed to develop to term and potential Pax6-Tox176 transgenic mice were identified by isolating genomic DNA from tail biopsies and screening by PCR, using primers specific for the Tox176 sequences: 5’- GAT GTT GTT GAT TCT TCT-3’ and 5’- ACG GTT CAG TGA GAC TTA-3’. The PCR cycle conditions are as follows: denaturation at 94°C for 45 seconds, annealing at 50°C for 45 seconds and extension at 72°C for 60 seconds for 35 cycles. A final extension step of 72°C for 10 minutes was also included.
Histological Analyses
Embryos were obtained by mating FVB/N females to heterozygous Tox176 transgenic males. Pregnant females were sacrificed at appropriate time points and transgenic offspring were identified by PCR. Heads of transgenic mice were removed, fixed in 10% formalin, dehydrated, embedded in paraffin, sectioned (5–7 µm) and used for histological analyses, in situ hybridizations and immunohistochemistry.
In situ hybridizations
To analyze expression of the Tox176 transgene, a [35S] UTP-labeled riboprobe specific to the Tox176 sequences of the transgene was generated. The Tox176 antisense riboprobe was synthesized using NcoI-digested Tox176 cDNA and T3 RNA polymerase (Promega). The BMP4 antisense probe was synthesized using EcoRI-digested mouse BMP4 cDNA and SP6 RNA polymerase. In situ hybridizations were performed using the same hybridization and washing conditions as described previously (Govindarajan et al., 2000). The hybridized slides were soaked in Kodak NTB-2 emulsion, dried and exposed for 6–9 days at 4°C. Following development and fixation, the slides were counterstained with Hematoxylin. Bright and dark-field images were captured separately using a Nikon Eclipse E600 microscope. Silver grains in the dark field images were pseudo-colored red using ADOBE Photoshop CS and overlaid on corresponding bright field images.
Immunohistochemistry
Immunohistochemistry on paraffin-embedded tissue sections was performed as follows. Slides containing ocular sections were first deparaffinized and rehydrated. Antigens were retrieved by microwave treatment in 10 mM Sodium Citrate buffer (pH 6.0). Following antigen retrieval, the tissue sections were blocked with 10% normal horse serum for 30 minutes, at room temperature. The slides were then incubated with anti-Pax6 (1:250; Covance, Berkeley, CA), anti-Sox2 (1:500; Chemicon International, Temecula, CA), anti-Prox1 (1;1000; Chemicon) (1:1000), anti-Keratin 1 (K1) (1:250; Covance), anti-Keratin 10 (K10) (1:500; Covance), anti-αA crystallin (1:10,000; Abcam, Cambridge, MA), anti-Keratin 12 (1:200; Cosmo Bio Company, Tokyo, Japan), anti-MITF (1:2000; Exalpha Biologicals Inc., Watertown, MA), anti-Otx1 (1;1000; Hybridoma Bank, IA), anti-Opticin (1:500; R & D systems, Minneapolis, MN), anti-α smooth muscle action (1:5000; Sigma, Saint Louis, Mo), anti- Brn3b (1;50; Santa Cruz Biotechnology, Santa Cruz, CA), anti-PKCα(1:400; Sigma), anti-NF160 (1:400; Sigma), anti-syntaxin (1:400; Sigma), TUJI (1:2000; Covance) antibody overnight at 4°C. Following brief washes in PBS, the slides were incubated with the appropriate biotinylated-secondary antibodies; anti-rabbit IgG (1:200; Vector Labs, Burlingame, CA) for Pax6, αA-crystallin, Sox2, PKCα, Prox1, K1, K10, K12, or TUJI, anti-mouse IgG (1:200; Vector Labs) for Otx1, MITF, NF160, α-smooth muscle actin, syntaxin and anti-goat IgG (1:200; Vector Labs) for Opticin and Brn3b for 30 minutes at 37°C. Antigen-antibody complexes were then detected using streptavidin-linked Alexa 594 (Invitrogen, Carlsbad, CA) at 1:1000 dilution. Sections were mounted using Prolong fade media containing DAPI (Invitrogen, Carlsbad, CA). Images were captured using a Nikon Eclipse E600 microscope.
Lacrimal glands
To visualize lacrimal glands, Pax6-Tox176 transgenic mice were mated to Le-Cre mice (generated by Ruth Ashery-Padan (Tel Aviv University, Tel Aviv, Israel)) (Ashery-Padan et al., 2000). The Le-Cre mice carry the Cre-IRES-GFP cassette driven by the Pax6 lens enhancer/promoter. These mice express GFP in the lacrimal epithelial cells. E17.5 embryos that carried Le-Cre and Pax6-Tox176 transgenes were identified by PCR using primers specific for Tox176 (5’- GAT GTT GTT GAT TCT TCT -3’ and 5’- ACG GTT CAG TGA GAC TTA -3’) and Le-Cre (5’- ATG CCC AAG AAG AAG AGG AAG GT-3’ and 5’- GAA ATC AGT GCG TTC GAA CGC TAG A -3’) transgenes. The PCR cycle conditions are as follows: denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds and extension at 72°C for 30 seconds for 30 cycles. A final extension step of 72°C for 10 minutes was also included. Skin from heads of E17.5 embryos was removed and lacrimal glands were photographed using Nikon AZ100 microscope.
RESULTS
In order to ablate the lens precursors in the murine eye, we generated transgenic mice with targeted expression of Tox176, an attenuated version of Diphtheria toxin A, under the control of a modified Pax6 promoter (Fig. 1). The Pax6 promoter is active in the surface ectoderm that includes the pre-placodal lens ectodermal precursors. The Pax6-Tox176 transgene was constructed by inserting the Tox176 cDNA between the Pax6 enhancer/promoter and SV40 intron and polyadenylation site. The transgene was microinjected and 5 transgenic founders were generated. Stable transgenic lines (GOV1-5) were established from these founders. Transgenic lines GOV1, 4 and 5 showed microphthalmia and GOV2 and 3 families did not show any ocular abnormalities. The GOV4 family carried multiple transgene insertions that segregated independently into GOV4A, 4B and 4C (data not shown). GOV4A mice were microphthalmic and GOV4B and C mice did not show any ocular abnormalities.
Figure 1. Schematic diagram of the Pax6-Tox176 transgene.
The Tox176 cDNA (~680 bp) was inserted between a modified Pax6 promoter (2.3 kb) and an intron and polyadenylation sequence derived from SV40 virus (850 bp). The lens/cornea enhancer is contained between the BglII/AccI sites (clear bar). Tox176 sequences were used to make riboprobes for detection of transgene expression.
Transgene expression
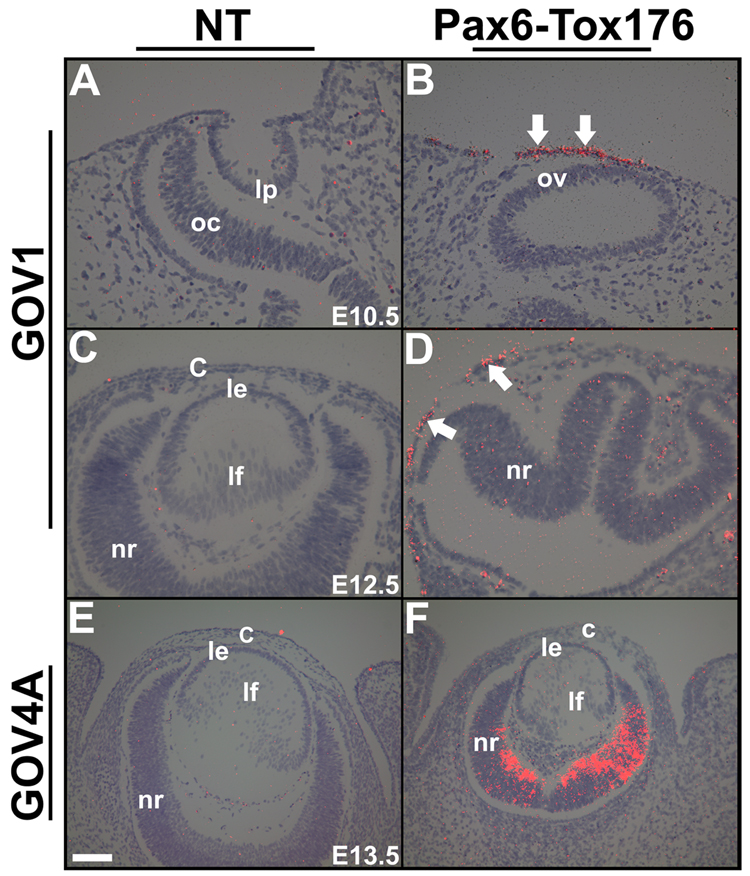
Transgene expression in the Pax6-Tox176 transgenic families was examined by situ hybridizations (Fig. 2). In the family GOV1, at E10.5, Tox176 transcripts were localized to ectodermal cells adjacent to the optic vesicle (Fig. 2B). At E12.5, transgene expression could be detected in a few cells of the surface ectoderm (Fig. 2D, arrows) and transgene expression was not seen elsewhere in the eye. A similar expression pattern was seen in the GOV5 family (data not shown). In the GOV4A family, transgene expression was seen in the differentiated ganglion cells and the undifferentiated neuroblasts of the retina, but not in the lens or in the cornea (Fig. 2F). No transgene expression was seen in the GOV2, 3, 4B and 4C families (data not shown). As transgene expression in the lens precursors was seen only in the GOV1 and 5 families, all the analyses were preformed on these two families. Since the alterations in ocular morphology were similar between GOV1 and 5 families, the results of the analyses of the GOV1 family are presented here.
Figure 2. Transgene expression.
In situ hybridizations were performed on ocular sections of Pax6-Tox176 transgenic mice using 35S-labeled Tox176 riboprobes. Dark-field images were overlaid on the respective bright-field images and silver grains were pseudocolored red. Ocular sections of nontransgenic (NT, A, C, E) and transgenic families GOV1 (B, D) and GOV4A (F) are shown. Transgene expression was detected in the surface ectoderm of the GOV1 (B, D, arrows) but not in the GOV4A (F) family. Abbreviations; c, cornea; le, lens epithelium; lf, lens fibers; nr, neural retina; oc, optic cup; ov, optic vesicle. The scale bar (E) represents 100 µm in A–D and 50 µm in E, F.
Tox176 transgenic mice show multiple ocular abnormalities
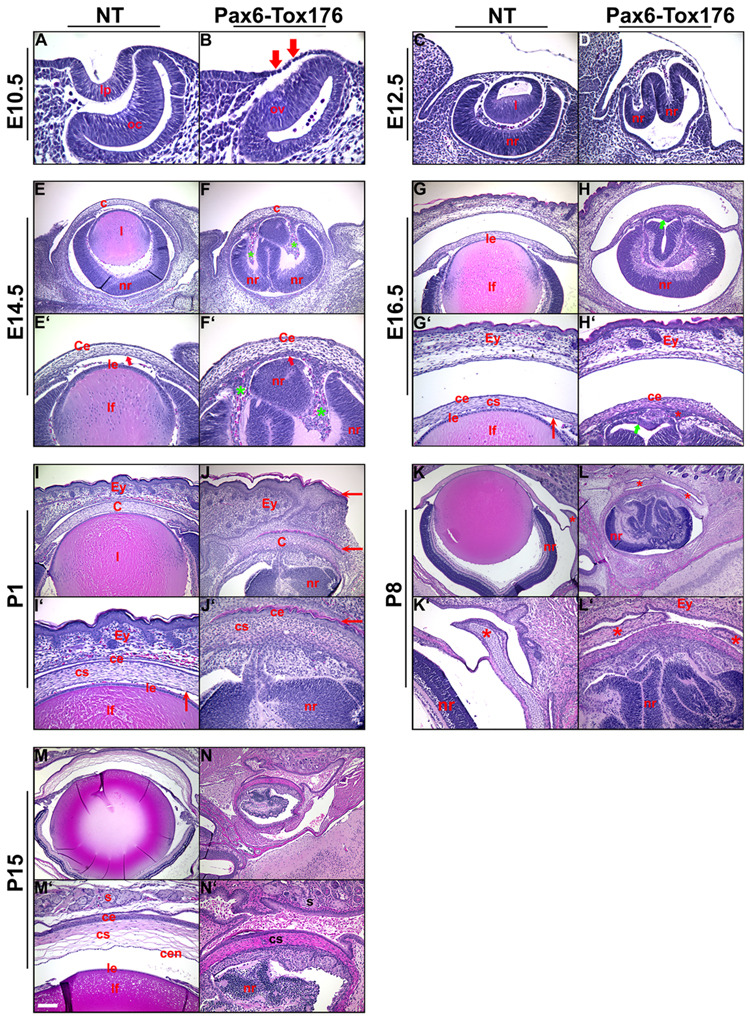
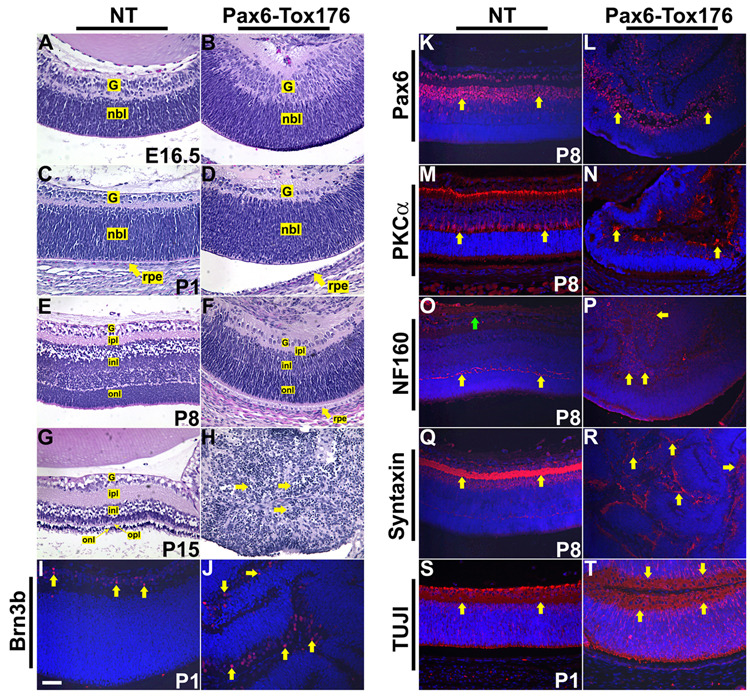
In order to assess the alterations in ocular development and morphology, sections of nontransgenic and Pax6-Tox176 transgenic mice were stained with hematoxylin and eosin (Fig. 3). Nontransgenic littermates were used as controls. At E10.5, the lens pit and optic cup were not seen in the Pax6-Tox176 mice (Fig. 3B). Nuclei of the Tox176 lens precursors were pyknotic suggestive of apoptosis (Fig. 3B, arrows). At 12.5, the lenses were absent in the Tox176 transgenic mice and the neural retina was folded (Fig. 3D). By E14.5, multiple abnormalities in the anterior segment were seen in the Tox176 transgenic mice. A distinctive corneal endothelium was not seen (Fig. 3F, F’, arrows) and the corneal stroma was disorganized. The periocular mesenchymal cells had migrated abnormally into the space between the retinal folds (Fig. 2F’, green asterisks). In addition, the Tox176 mice lacked anterior and vitreal chambers (Fig. 3F, F’). At E16.5, in between the folds of the neural retina, a monolayered epithelium histologically similar to the RPE was seen adjacent to the posterior part of the cornea (Fig. 3H, H’, arrow). Eyelid closure was not altered in the Tox176 transgenic mice (Fig. 3H, H’). At P1, the wild type corneal epithelium is multi-layered with a squamous morphology (Fig. 3I, I’). In contrast, the Tox176 corneal epithelium showed histological features that are more typical of the skin including a granular basal layer and a keratinized outer layer (Fig. 3J, J’, arrows). Other features of the skin including hair follicles were not seen (Fig. 3J, J’). Alterations in the ciliary body/iris and retinal differentiation are described later (Fig. 7 and Fig.8). At P8, the nictitating membrane could be seen in the wild type mice (Fig. 3K, K”, asterisk). In contrast, two nictitating membranes were seen in the Tox176 eyes (Fig. 3L’, asterisks). However, no cartilage growth was seen in these two membranes (Fig. 3L’). At P15, the Tox176 retina had degenerated and the eyes were microphthalmic (Fig. 3N, N’).
Figure 3. Pax6-Tox176 transgenic mice show altered ocular development.
Embryonic (E10.5, E12.5, E14.5, E16.5, A-H’) and postnatal (P1, P8, P15, I-N’) heads of nontransgenic (NT) and Tox176 transgenic mice were sectioned and stained with hematoxylin and eosin. E’–N’ are higher magnifications of E–N respectively. Loss of the lens placode in the Tox176 transgenic mice was apparent by E10.5 (B, arrows). Retinal folding and abnormal migration of the periocular mesenchymal cells were seen at E12.5 (D) and at E14.5 (F’, asterisks). By postnatal day 1 (P1), histological features of the skin such as a distinctive granular layer and stratum corneum were seen in the corneal epithelium (J, J’, arrows). Two nictitating membranes were seen in the Tox176 transgenic mice instead of one (compare L to K, L’ to K’, asterisks). By P15, the Tox176 transgenic retinas had degenerated (N, N’). Abbreviations; c, cornea; ce, corneal epithelium; cen, corneal endothelium; cs, corneal stroma; Ey, eyelids; l, lens; le, lens epithelium; lf, lens fibers; lp, lens pit; nr, neural retina; oc, optic cup; ov, optic vesicle; s, serous glands. The scale bar in panel M’ is 50 µm in C, D, E’–J’, 100 µm in E–J, K’–N’, 25 µm in A, B, 2.5 mm in K–N.
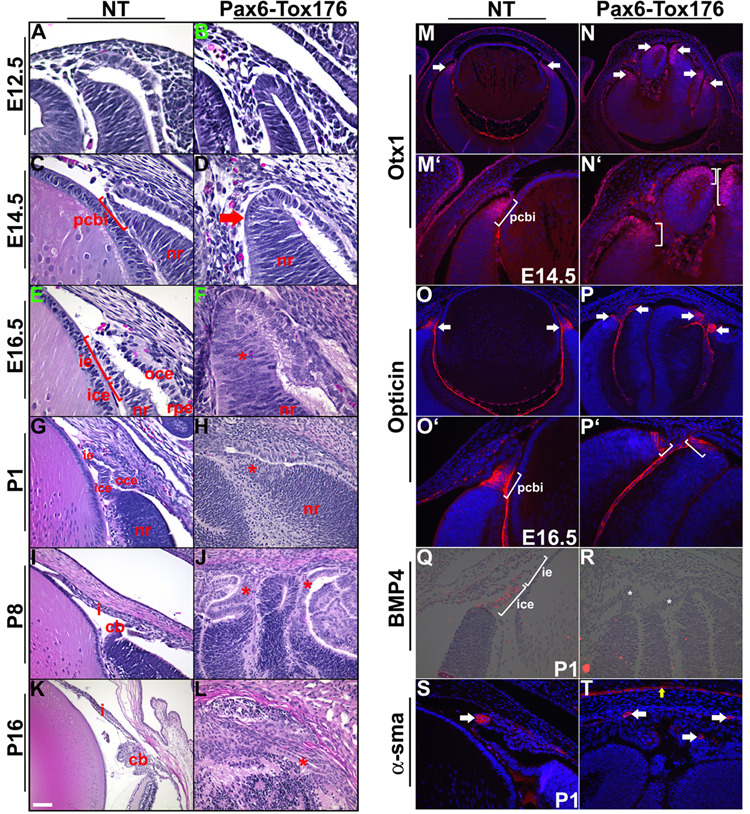
Figure 7. Ciliary body differentiation is altered in Tox176 transgenic mice.
A–L. Embryonic (E10.5, E12.5, E14.5, E16.5, A–F) and postnatal (P1, P8, P15, G–L) heads of nontransgenic (NT) and Tox176 transgenic mice were sectioned and stained with hematoxylin and eosin. The presumptive ciliary body and iris (pcbi), histologically distinct by E14.5, was not seen in the Tox176 mice (compare D to C and F to E). Though a rudimentary iris/ciliary body could be detected at P1, normal ciliary folds were not seen (compare H to G and J to I). By P16, tissues in the anterior segment had degenerated (L).
M–P’, S–T. Immunohistochemistry was performed on E14.5, E6.5 and P1 sections to detect expression of Otx1 (M–N’), Opticin (O–P’) and α-smooth muscle actin (α-sma) (S,T). M’–P’ are higher magnifications of M–P respectively. Antigen-antibody complexes are in red and nuclei are stained blue with DAPI. Multiple domains of Otx1 (N, arrows), Opticin (P, arrows) and α-sma (T, arrows) expression were seen in the Tox176 mice. α-sma expression was also seen in the corneal epithelial cells of the Tox176 mice (T, yellow arrow) similar to expression in skin epithelial cells. Q, R. In situ hybridizations were performed to assay for BMP4 expression in the ciliary and iridial epithelial cells on P1 sections. Abbreviations; cb, ciliary body; ice, inner ciliary epithelium; ie, iris epithelium; nr, neural retina; oce, outer ciliary epithelium. Scale bar in panel K is 25 µm in A–F, 50 µm in G–J, M’–P’, Q–T, 100 µm in K, L, M–P.
Figure 8. Retinal differentiation in Pax6-Tox176 transgenic mice.
A–H. Embryonic (E16.5, A, B) and postnatal day (P1, P8, P15, C–H) heads of nontransgenic (NT) and Tox176 transgenic mice were sectioned and stained with hematoxylin and eosin. Lamination of the different layers of the retina was seen in the Tox176 transgenic mice (B, D, F). By P15, retinal cells had degenerated (H, arrows). I–T. Immunohistochemistry was performed on P1 and P8 sections to assay for alterations in ganglion (Brn3b (I, J)), amacrine (Pax6 (K, L) and syntaxin (Q, R)), rod bipolar (PKCα(M, N)), horizontal (NF160 (O, P)) and neuronal (TUJI (S, T)) differentiation. Antigen-antibody complexes are in red and nuclei are stained blue with DAPI. Abbreviations; g, ganglion cell layer; inl, inner nuclear layer; ipl, inner plexiform layer; nbl, neuroblasts layer; onl, outer nuclear layer; rpe, retinal pigmented epithelium. Scale bar in panel I is 50 µm in A–T.
Ablation of lens precursors in Tox176 transgenic mice
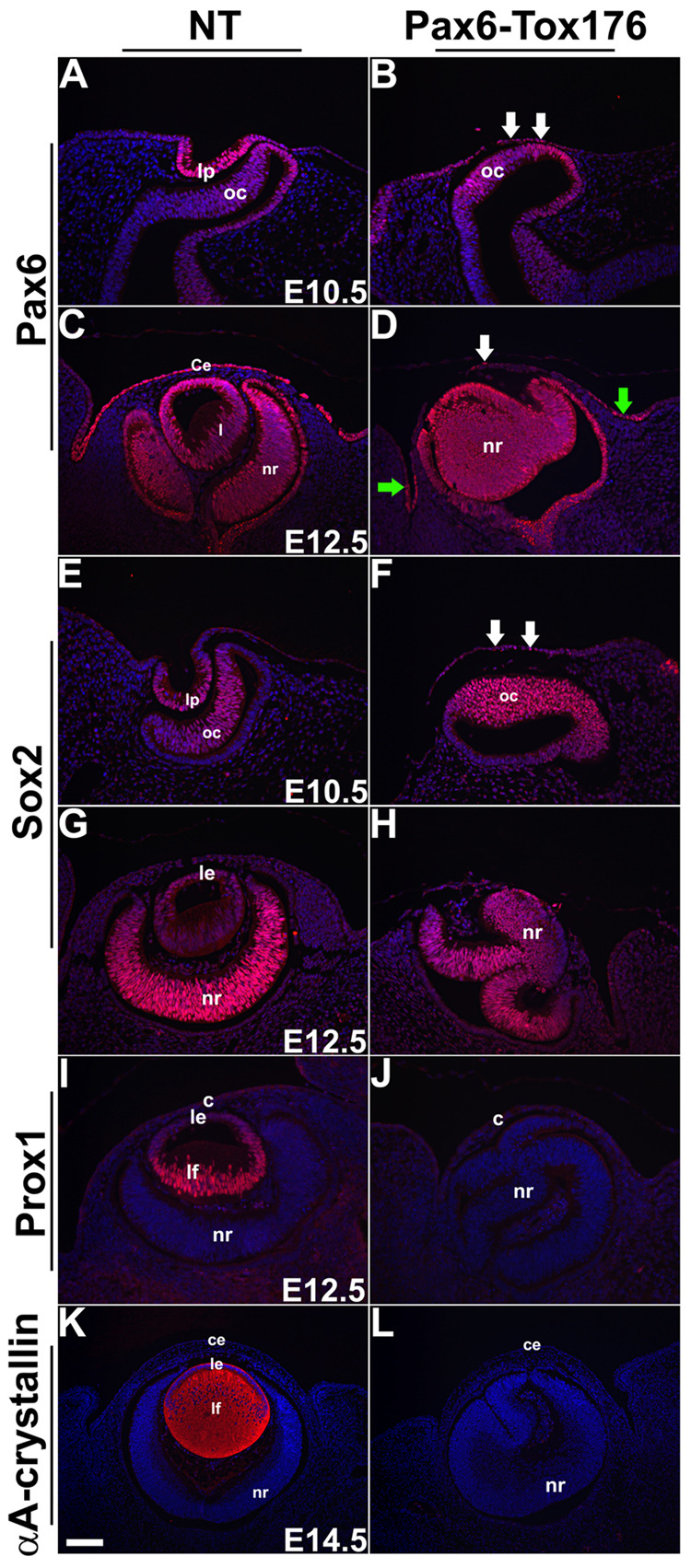
Loss of lenses in the Tox176 mice was assessed by immunohistochemistry. Pax6 expression is normally seen in the lens pit at E10.5 (Fig. 4A) and in the lens epithelial cells at E12.5 (Fig. 4C). In the Tox176 embryos, Pax6 expression in the surface ectodermal cells adjacent to the optic vesicle was reduced at E10.5 and at E12.5 (Fig. 4B, D). However, Pax6 expression in the neural retina was unaltered (Fig. 4B, D). At E12.5, Pax6 expression was seen in the few surviving ectodermal cells that would give raise to the conjunctival epithelium (Fig. 4D, arrows). Expression of Sox2, a transcription factor, is upregulated in the surface ectoderm in response to the signal from the optic vesicle (Furuta and Hogan, 1998). In the lens, Sox2 expression is seen in the lens pit at E10.5 (Fig. 4E) and in the lens epithelial cells at E12.5 (Fig. 4G). Sox2 expression in the Pax6-Tox176 ectoderm was reduced at E10.5 (Fig. 4F) and not seen at E12.5 (Fig. 4H). Sox2 expression in the Tox176 neural retinas was not altered (Fig. 4E–H). Prox1, a homeobox transcription factor, is expressed in the lens epithelial cells and is upregulated in the lens fibers during lens differentiation (Fig. 4I). Prox1 expression was not seen in the Tox176 eyes at E12.5 (Fig. 4J). αA-crystallin, normally localized to the lens epithelial and fiber cells (Fig. 4K), was not seen in Tox176 embryos (Fig. 4L). These results taken together suggested that lens induction and differentiation had been prevented in the Tox176 embryos.
Figure 4. Loss of lens ectodermal precursors in the Tox176 transgenic mice.
Immunohistochemistry was performed on E10.5, E12.5 and E14.5 sections to detect expression of Pax6 (A–D), Sox2 (E–H), Prox1 (I,J) and αA-crystallin (K, L). Antigen-antibody complexes are in red and nuclei are stained blue with DAPI. Pax6 (B, D) and Sox2 (F, H) expression were significantly reduced in the surface ectodermal cells of the Tox176 transgenic mice but their retinal expression (B, D, F, H) remained unaltered. Prox1 (J) and αA crystallin (L) expression were not seen in the Tox176 eyes. Abbreviations; c, cornea; ce, corneal epithelium; le, lens epithelium; lf, lens fibers; lp, lens pit; oc, optic cup; nr, neural retina. Scale bar in panel K is 50 µm in A–D, E–J and 100 µm in K and L.
Corneal differentiation is altered in Tox176 mice
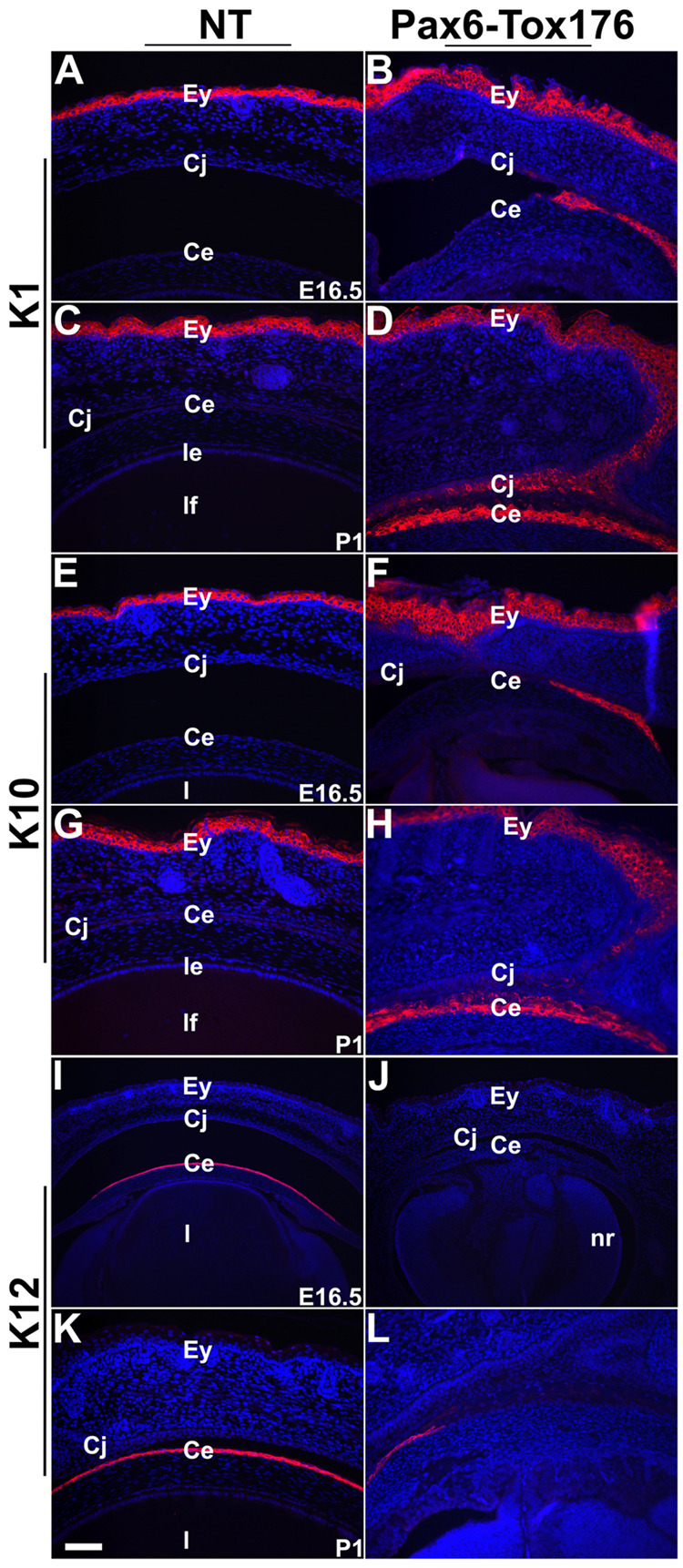
Histological analysis of Tox176 mice revealed changes in corneal epithelial morphology. Therefore, alterations in corneal epithelial differentiation were assessed by immunohistochemistry. Keratin 1 (K1) and Keratin 10 (K10) are normally expressed in the skin, but not in the corneal, epithelium (Fig. 5A, C, E and G). Keratin 12 (K12) is normally expressed in the corneal, but not in the skin, epithelium (Fig. 5I, K). In the Tox176 embryos, K1 and K10 expression in the skin epithelial cells was unaltered (Fig. 5B, D, F, H). However, K1 and K10 were upregulated in the Tox176 corneal epithelial cells at E16.5 (Fig. 5B, F) and P1 (Fig. 5D and H). In addition, K12 expression in the corneal epithelial cells was downregulated (Fig. 5J, L). These results suggested that the corneal differentiation was blocked in the Tox176 mice and instead, the skin epidermal differentiation program had been initiated. Expression of Pitx2 and Lmx1b, genes that are normally expressed in the corneal stromal precursors, were not altered in the Tox176 mice (data not shown).
Figure 5. Tox176 transgenic corneal epithelial cells express skin epidermal keratins.
Immunohistochemistry was performed on E16.5 and P1 sections to detect expression of Keratin 1 (K1) (A–D), Keratin 10 (K10) (E–H) and Keratin 12 (K12) (I–L). Antigen-antibody complexes are in red and nuclei are stained blue with DAPI. K1 (B, D) and K10 (F, H), markers of skin epidermal differentiation are upregulated and K12 (J, L), a marker of corneal epithelial differentiation is downregulated in the Tox176 corneas. Abbreviations; ce, corneal epithelium; cj, conjunctival epithelium; Ey, eyelid; l, lens. Scale bar in panel K is 50 µm in A–H and 100 µm in I–L.
Ectopic RPE differentiation in Tox176 mice
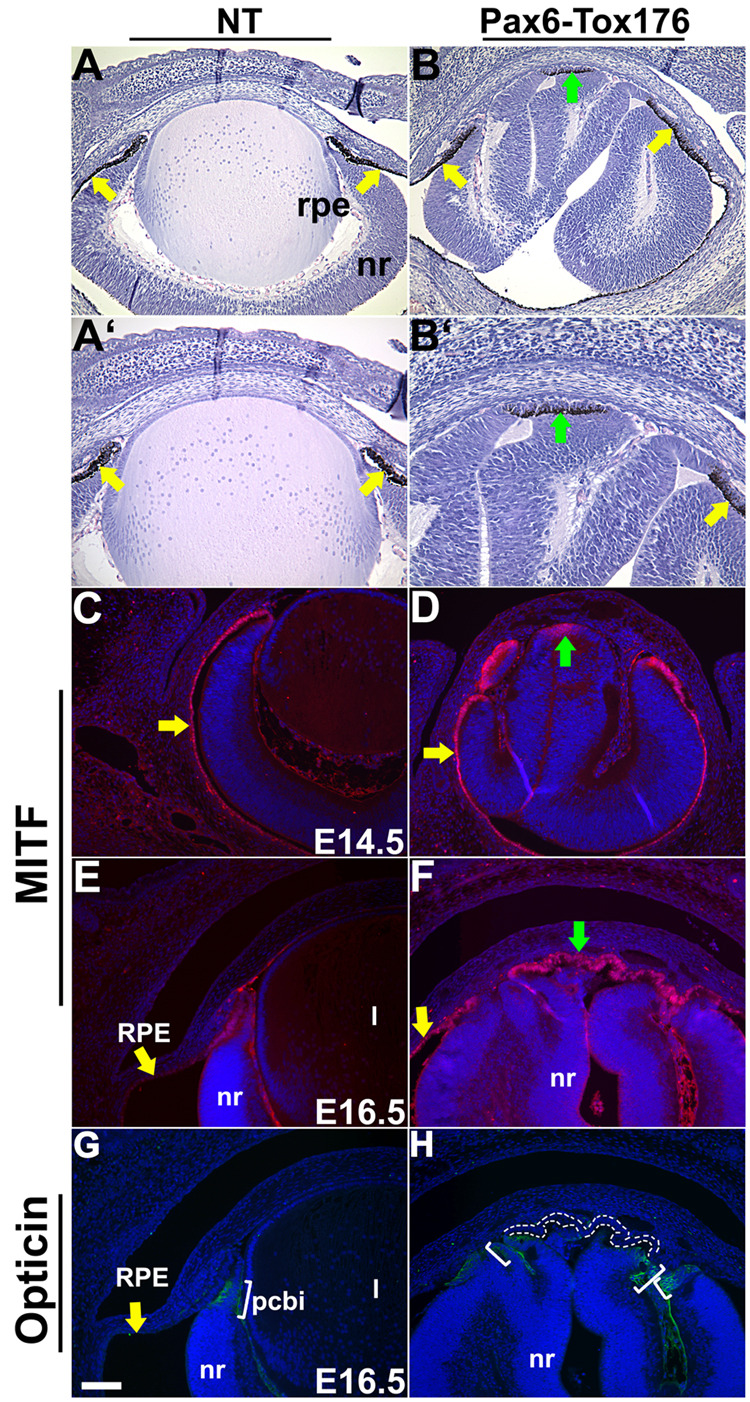
Histological analyses of the Tox176 embryos revealed a thin layer of epithelium between the retinal folds with an RPE-like morphology (Fig. 1H). In order to assess whether this tissue was indeed the RPE, Tox176 mice were mated to pigmented C57BL/6 mice and the ocular histology of pigmented offspring was analyzed (Fig. 6A–B’). The monolayered epithelium between the neural folds in the Tox176 mice was pigmented (Fig. 6B, B’, green arrow) similar to the endogenous RPE (Fig. 6A–B’, yellow arrows). In addition, microphthalmia transcription factor (MITF), a protein critical for RPE differentiation, was expressed in the ectopic RPE (Fig. 6F, green arrow) at E16.5. Interestingly, MITF expression was detectable in the neuroepithelium between the retinal folds by E14.5 (Fig. 6D, green arrows). These results taken together suggested that the neural-retinal precursors in between the folds of the Tox176 retinas had initiated the RPE differentiation program. In order to rule out the possibility that these neuro-epithelial precursors had initiated ciliary body differentiation, we analyzed the expression of Opticin, a glycoprotein and presumptive ciliary body marker, and MITF on adjacent sections. Opticin expression was not seen in the region between the folds of the Tox176 retinas (Fig. 6H, area circumscribed by dotted lines) but was seen in the ciliary body (Fig. 6G, H, brackets) (more details in Figure 7). These results support our conclusion that the anterior retinal epithelium in the Tox176 mice is not properly specified and differentiates as pigmented epithelium.
Figure 6. Ectopic placement of the retinal pigment epithelium in Tox176 transgenic mice.
E17.5 heads of pigmented nontransgenic (A, A’) and Tox176 (B, B’) transgenic mice were sectioned and stained with hematoxylin and eosin. A’ and B’ are higher magnifications of A and B respectively. Ectopic formation of the retinal pigment epithelium (RPE) (B, B’, green arrows) was seen in the Tox176 transgenic mice in addition to the normal RPE (A–B’, yellow arrows). Immunohistochemistry was performed on E14.5 (C, D) and E16.5 (E, F, G, H) sections to detect expression of MITF (C–F), a marker of RPE differentiation and Opticin (G, H), a marker of presumptive ciliary body differentiation. Antigen-antibody complexes are in red in C–F and green in G and H. Nuclei are stained blue with DAPI. Panels F and H show adjacent sections. MITF was upregulated in the cells between the neural folds adjacent to the corneal stroma (D, F, green arrow). Expanded domains of Opticin (H, brackets) expression were seen in the Tox176 mice but not in the region between the retinal folds (region circumscribed by dotted lines). Abbreviations; nr, neural retina. Scale bar in panel G is 100 m in A, B, E, F, G, H and 50 µm in A’, B’, C, D.
Iris and ciliary body specification in Tox176 mice
The effect of lens ablation on iris and ciliary body development was assessed by histology, immunohistochemistry and in situ hybridizations (Fig. 7). The presumptive ciliary body and iris (pcbi) is histologically distinct by E14.5 at the anterior margins of the retina and is considerably thinner than the neural retina (Fig. 7C). In Tox176 embryos, the pcbi is not apparent by E14.5 (Fig. 7D, arrow). At E16.5 and P1, the presumptive iris and ciliary folds (Fig. 7E, G) seen in the wild type mice were not seen in the Tox176 embryos (Fig. 7F, H). At P8, the anterior margins of the Tox176 retinas appear thin (Fig. 7H, J, asterisks) but ciliary folds were not seen. By P16, the Tox176 retina had degenerated and no further differentiation of the iris and ciliary epithelia was seen (Fig. 7L, asterisk).
Alterations in ciliary body and iris differentiation were also assessed by immunohistochemistry and in situ hybridizations (Fig. 7, M–T). Otx1, a transcription factor, and Opticin are normally expressed in the pcbi (Fig. 7M, M’, O, O’) at the anterior margins of the retina. In the Tox176 embryos, both Otx1 and Opticin expression were seen in the pcbi at E14.5 (Fig. 7N, N’, P, P’). Interestingly, multiple domains of Otx1 and Opticin expression were seen in the Tox176 embryos at the anterior margins of the retinal folds (Fig. 7N, P, arrows). BMP4 is normally expressed in the inner ciliary and iridial epithelium (Fig. 7Q) and has been shown to be necessary for the proper differentiation of these tissues (Zhao et al., 2002). BMP4 expression could not be detected in the Tox176 eyes at P1 (Fig. 7R). Expression of α-smooth muscle actin (α-sma), normally expressed in the iris sphincter muscles derived from the iridial epithelium was unaffected in the Tox176 eyes (Fig. 7T). In addition, multiple domains of α-sma expression were seen similar to Otx1 and Opticin (Fig. 7T, arrows). These results suggested that iris and ciliary body differentiation had been initiated, but not completed, in Tox176 mice.
Retinal differentiation in Tox176 mice
Alterations in retinal differentiation in the Tox176 mice were assessed by histological and immunohistochemical analyses (Fig. 8). At E16 and P1, the differentiating ganglion cell layer was histologically distinct in the Tox176 retinas (Fig. 8B, D) similar to nontransgenic retinas (Fig. 8A, C). At P8, the different layers of the retina including the ganglion cell layer, inner and outer nuclear layers and the inner plexiform layers were visible in the Tox176 eye (Fig. 8F). These results suggested that in the Tox176 mice, retinal lamination had not been altered. At P15, the retina had degenerated and cells with condensed/pyknotic nuclei were visible (Fig. 9H, arrows).
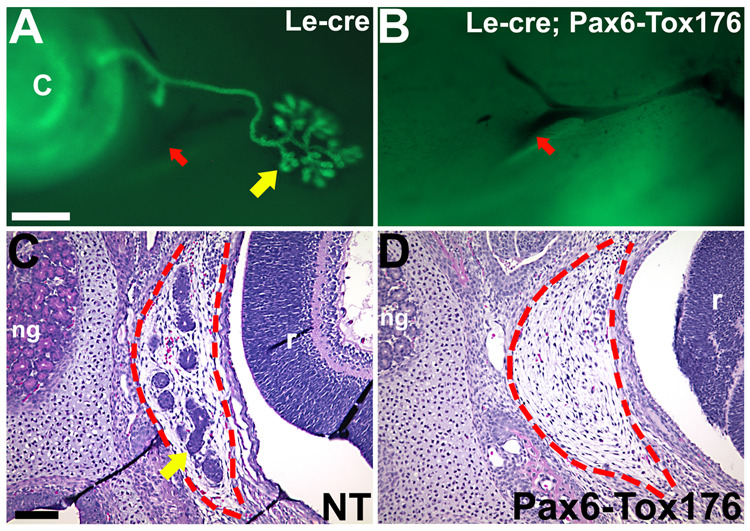
Figure 9. Lacrimal and Harderian gland development in Pax6-Tox176 transgenic mice.
Tox176 transgenic mice were crossed to Le-Cre mice that expressed GFP in the lacrimal epithelia (A, yellow). Tox176 transgenic lack lacrimal glands (B). Red arrows in A, B point to the facial artery. P2 heads were sectioned and stained with hematoxylin and eosin. Harderian gland is circumscribed by red dotted lines (C, D). Images shown in A, B and C, D were captured using different microscopes. Scale bar in A (300 µm) applies to panels A and B. Scale bar (100 µm) in C applies to panels C and D. Abbreviations; C, cornea; ng, nasal glands; r, retina.
Alterations in the differentiation of the major cell types in the retina were assayed by immunohistochemical analysis using Brn3b (ganglion) (Fig. 8I, J), Pax6 (amacrine) (Fig. 8K, L), protein kinase C-α(PKC-α) (rod bipolar) (Fig. 8M, N), Neurofilament 160 (NF-160) (horizontal and ganglion) (Fig. 8O, P), syntaxin (amacrine) (Fig. 8Q, R) and TUJI (neuron) (Fig. 8S, T) antibodies. Although these markers were expressed in the Tox176 retinas suggesting that differentiation of ganglion, amacrine, rod bipolar and horizontal cells had occurred, the patterns of expression were altered.
Tox176 mice lack lacrimal and Harderian glands
The lacrimal and Harderian glands are derived from the Pax6-expressing cells of the ectoderm (Govindarajan et al., 2000; Makarenkova et al., 2000). The lacrimal glands arise on the nasal side of the eye and are derived from the palpebral conjunctival epithelium (Fig. 9A). The Harderian glands arise from the temporal side of the eye from the bulbar conjunctival epithelium (Fig. 9C). Alterations in the lacrimal gland development were analyzed by mating the Tox176 mice to the Le-Cre transgenic line that expressed GFP in the lacrimal epithelium (Ashery-Padan et al., 2000) (Fig. 9A). Harderian gland development was assessed by histology (Fig. 9C). Both lacrimal and Harderian glands were absent in the Tox176 mice (Fig. 9B, D).
DISCUSSION
The studies presented here were performed to assess the notion that the lens functions as an ‘organizer’ of ocular development and is therefore required for normal cell fate determination of other ocular tissues including cornea, retina, iris and ciliary body. Inactivation of genes expressed in the presumptive lens ectoderm in mice has not yielded much information about this early role of the lens. Pax6 and Six3, genes expressed in the presumptive lens ectoderm, have been conditionally deleted in mice resulting in loss of the lens placode (Ashery-Padan et al., 2000; Liu et al., 2006). Inactivation of other genes expressed in the lens ectodermal precursors including FoxE3, Pitx3, Prox1 and Mab21l1 do not result in dominant inhibition of lens placode formation (Blixt et al., 2007; Blixt et al., 2000; Medina-Martinez et al., 2005; Semina et al., 2000; Wigle et al., 1999; Yamada et al., 2003). Alterations in corneal, conjunctival and ocular gland differentiation have not been reported in these mice.
In our studies, lens precursors were ablated by generation of transgenic mice that expressed diphtheria toxin (Tox176) in the Pax6-expressing cells of the ocular surface ectoderm including the lens precursors. Tox176 expression in these cells resulted in the loss of the lens placode and the lens. Corneal epithelial differentiation was blocked and the skin epidermal differentiation program was induced in its place. In the absence of the lens, the retina folded extensively. In addition, there was discontinuous and ectopic placement of the RPE in the anterior part of the eye. Major cell types in the retina including the ganglion, bipolar, horizontal and amacrine cells were detected suggesting that early neuronal differentiation in the retina can occur independent of the lens. In addition, the pcbi was specified correctly even in the absence of the lens. Multiple domains of expression of Otx1 and Opticin were seen at the anterior margins of the folded retina indicating the autonomous nature of iris and ciliary body differentiation. Other tissues derived from the Pax6-expressing cells of the surface ectoderm including the lacrimal and Harderian glands were absent in the Tox176 mice. Collectively, these results suggest that in mice, the lens is not necessary for the early development of the retina, iris and ciliary body. Pax6-expressing cells of the surface ectoderm are however, critical for the proper development of the corneal, lacrimal and Harderian epithelia.
Previous lens ablation studies performed in mice have yielded some insights into the early role of the lens in fate determination of surrounding ocular tissues (Breitman et al., 1989; Breitman et al., 1990; Kaur et al., 1989; Key et al., 1992; Klein et al., 1992; Zhang et al., 2007). The promoters used in these studies for directing toxin expression to the lens were active in differentiated lens fiber cells but inactive in the lens precursors. In the present study, we have utilized a modified version of the Pax6 promoter that includes the ectodermal enhancer. This promoter/enhancer is often active in cells of the pre-placodal lens ectoderm. In our transgenic mice, Tox176 transgene expression was specific to the ocular surface ectodermal cells and was not detected elsewhere in the eye. Histological analysis showed the loss of the lens placode in the Tox176 mice. Sox2 upregulation, a critical step preceding lens placode formation, was not seen in the Tox176 surface ectoderm. In addition, lens markers such as Prox1 and αA-crystallin were not seen in the Tox176 mice. Based on these results, we conclude that Tox176 expression resulted in the successful ablation of the pre-placodal lens ectoderm.
Even though differentiation programs of non-lenticular tissues are altered, it is important to note that leakage of the Tox176 protein from the dying lens cells is not likely to be the cause of these changes. Wild type diphtheria toxin is composed of A and B peptides. The B peptide normally binds to the cell surface and is needed for internalization of the A peptide. The Tox176 transgene encodes only the A peptide (Breitman et al., 1990; Saito et al., 2001). Therefore, tox176 is expected to be toxic only in those cells where it is actually synthesized.
There were multiple alterations to the corneal differentiation program in the Tox176 mice. Expression of corneal epithelial marker K12 was significantly reduced and instead, markers of skin epidermal differentiation K1 and K10 were upregulated. The switch from corneal epithelial to skin epidermal differentiation could be due to either of the following; 1) The Tox176 transgene is expressed in the corneal epithelial progenitors and loss of these cells by toxin ablation leads to loss of the corneal epithelium. Adjacent epithelial cells that do not express the transgene survive and replace the ablated corneal precursor cells. Due either to the absence of Pax6 expression or the absence of a lens, differentiation of this substitute epithelium leads to expression of K1 and K10 rather than K12 keratin. Corneal stromal precursors do migrate adjacent to the substitute ectoderm but in an altered pattern and a distinctive corneal endothelium was not seen. 2) Corneal epithelial differentiation is initiated normally but in the absence of the lens, not completed. In this case, absence of the lens would result in the lack of Pax6 promoter upregulation in the corneal epithelial precursors. This allows the cells to survive but blocks their ability to undergo normal differentiation. Since we do not see any evidence for a broader region of apoptosis, we prefer the second model. The reasons for the lack of lacrimal and Harderian glands could be similar to the reasons for the loss of the corneal epithelium i.e. either the glandular precursors in the conjunctival epithelium are ablated due to toxin expression resulting in the loss of the glands or the lack of a proper corneal epithelium results in the loss of Pax6 promoter upregulation in the conjunctival precursors which in turn, leads to abnormal differentiation of the ocular glands.
Eyelid closure and differentiation were not affected in the Tox176 mice. The palpebral conjunctival epithelium that lines the inner part of the eyelids and is normally derived from the Pax6-expressing cells differentiated as skin epidermis. However, this change did not alter eyelid morphogenesis. In addition, two nictitating membranes (third eyelid –a vestigial structure in mammals) were seen in the Tox176 mice. Though the reasons for this phenomenon are not clear, our results indicate that the forces that initiate and/or maintain eyelid morphogenesis are not dependant upon conjunctival and corneal differentiation and do not depend upon the maintenance of Pax6 expression in these regions of the eye.
The embryonic lens has been proposed to be the source of an instructive signal that induces retinal differentiation from bi-potential neuroectoderm (reviewed inChow and Lang, 2001). This proposal is based on the following observations; 1) when the chick optic cup was rotated 180° such that the RPE was closer to the pre-lens ectoderm, neuronal differentiation was induced in the RPE (Detwiler 1953, Dragomirov 1937 Lopashov & Stroeva 1964Mikami 1939). 2) Removal of the pre-lens ectoderm in chick embryos resulted in optic vesicles that initiated neural retinal differentiation but failed to invaginate (Hyer, 2003). However, our studies indicate that, in mice, ablation of the pre-placodal ectoderm (by induction of cell death) does not eliminate neuronal differentiation in the retina. The reasons for these differences in optic vesicle development between mice and chicks are not clear. It is possible that the inductive interactions that shape the mammalian and the avian eye are different. Alternatively, it is possible that not all of the pre-lens ectoderm had been successfully ablated in our Tox176 mice and that the presence of a few lens precursors that escape cell death are sufficient to initiate optic cup formation and retinal differentiation. Though this possibility cannot be ruled out, our analysis of lens differentiation markers would suggest that lens formation was completely blocked in the Tox176 mice. There is good evidence that lens induction requires physical contact between the lens competent surface ectoderm and cells of the optic vesicle (McAvoy, 1981; Wakely, 1977). Communication between these adjacent cell types may be reciprocal. In our mice, cell-cell contact would still occur. When the pre-lens ectoderm is ablated, this interaction may no longer be possible. By P15, the retina undergoes apoptosis. Since we did not detect Tox176 transgene expression in the retinal cells at any age (data not shown), these results suggest a role for the lens in prevention of postnatal retinal apoptosis, consistent with the results of studies in cave fish (Yamamoto and Jeffery, 2000).
In the absence of the lens, neural retina in the Tox176 mice folded over and the neural tissue between the folds differentiated into RPE. A similar phenotype has been reported in mice with conditional deletion of Pax6 in the surface ectoderm (Ashery-Padan et al., 2000). Though this phenomenon may reflect the spatial constraints of a small eye, the position of the ectopic RPE suggests another possible explanation. The ectopic RPE was found only where the neural tissue was in close proximity to the corneal stroma. Ectopic RPE was not seen in the posterior part of the retina. Therefore, it is possible that local signals from the periocular mesenchymal cells including corneal stromal cells upregulate MITF and induce the RPE differentiation program in neuroectodermal tissue. This hypothesis is consistent with optic vesicle-periocular mesenchymal co-culture studies performed in chick embryos (Fuhrmann et al. 2000). These ex-vivo experiments indicated that periocular mesenchymal cells are both necessary and sufficient for induction of RPE differentiation in optic vesicles. Removal of the periocular mesenchymal cells resulted in decreased expression of RPE markers MITF and Wnt13 (Fuhrmann et al., 2000). Optic vesicles that were co-cultured with periocular mesenchymal cells showed downregulation of the retina specific transcription factor Chx10. In this regard, the lens may function as a barrier that separates the retina from the posterior part of the cornea. In the Tox176 mice, the absence of the lens leads to the close apposition of neural retina and the periocular mesenchyme-derived corneal stroma which causes upregulation of MITF in the retina.
Our results suggest that in mice, even in the absence of the pre-placodal ectoderm, initial specification of the iris and ciliary body can occur. In fact, multiple pcbis were seen in the Tox176 mice. These results suggest that the specification of the pcbi is independent of the lens. Similar results have been reported in chick embryos (Dias da Silva et al., 2007). Therefore, the signals that initiate iris and ciliary epithelial differentiation are likely to come from within the retina and/or from the periocular mesenchymal cells. The ciliary/iris epithelial differentiation, though initiated, was not completed in the Tox176 mice. Similar results were obtained in our previous lens ablation studies (Zhang et al., 2007). In these studies, the lens was ablated after the initiation of iris and ciliary epithelial differentiation. In these mice, BMP4 expression was unaltered but ciliary body morphogenesis was blocked. In contrast, this present study shows that ablation of the pre-placodal lens ectoderm blocks BMP4 expression, ciliary fold formation and iris differentiation. These results taken together suggest that the lens may secrete a signal that is necessary for BMP4 expression in the ciliary epithelium and subsequent differentiation. An alternative possibility is that the block in ciliary/iris epithelial differentiation is simply a consequence of overgrowth of the retina. Our data do not allow us to distinguish between these possibilities.
Though ablation of the lens does not affect early specification of the retina, iris or ciliary body, morphogenesis and correct positioning of these tissues within the orbit become altered. It is likely that the absence of the lens, aqueous humor and vitreous humor collectively contribute to this phenomenon. Overall, our studies support the notion that the murine embryonic lens plays a critical role in shaping the differentiation programs of the cornea, iris and ciliary body. However, the mechanistic details of these interactions and the identities of the lens-derived signals require further investigation.
ACKNOWLEDGMENTS
The authors thank Dongcai Liang (Baylor College of Medicine, Houston, TX) for performing microinjections to generate the Pax6-Tox176 transgenic mice and Ruth Ashery-Padan (Tel Aviv University, Tel Aviv, Israel) and Peter Gruss (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for the Le-Cre mice. This research was supported by an NEI grant EY017610 (VG), revenue from Nebraska cigarette taxes and the Nebraska Tobacco Settlement Biomedical Research Development Fund (VG), Health Future Foundation (VG) and MRDDRC Core grant P30HD24064 (PAO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Blixt A, Landgren H, Johansson BR, Carlsson P. Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev Biol. 2007;302:218–229. doi: 10.1016/j.ydbio.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Breitman ML, Bryce DM, Giddens E, Clapoff S, Goring D, Tsui LC, Klintworth GK, Bernstein A. Analysis of lens cell fate and eye morphogenesis in transgenic mice ablated for cells of the lens lineage. Development. 1989;106:457–463. doi: 10.1242/dev.106.3.457. [DOI] [PubMed] [Google Scholar]
- Breitman ML, Rombola H, Maxwell IH, Klintworth GK, Bernstein A. Genetic ablation in transgenic mice with an attenuated diphtheria toxin A gene. Mol Cell Biol. 1990;10:474–479. doi: 10.1128/mcb.10.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens Development: Fiber Elongation And Lens Orientation. Science. 1963;142:1489–1490. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Dias da Silva MR, Tiffin N, Mima T, Mikawa T, Hyer J. FGF-mediated induction of ciliary body tissue in the chick eye. Dev Biol. 2007;304:272–285. doi: 10.1016/j.ydbio.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Harrison WR, Xiao N, Liang D, Overbeek PA. Intracorneal positioning of the lens in Pax6-GAL4/VP16 transgenic mice. Mol Vis. 2005;11:876–886. [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev Biol. 2003;259:351–363. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Kaur S, Key B, Stock J, McNeish JD, Akeson R, Potter SS. Targeted ablation of alpha-crystallin-synthesizing cells produces lens-deficient eyes in transgenic mice. Development. 1989;105:613–619. doi: 10.1242/dev.105.3.613. [DOI] [PubMed] [Google Scholar]
- Key B, Liu L, Potter SS, Kaur S, Akeson R. Lens structures exist transiently in development of transgenic mice carrying an alpha-crystallin-diphtheria toxin hybrid gene. Exp Eye Res. 1992;55:357–367. doi: 10.1016/0014-4835(92)90200-c. [DOI] [PubMed] [Google Scholar]
- Klein KL, Klintworth GK, Bernstein A, Breitman ML. Embryology and morphology of microphthalmia in transgenic mice expressing a gamma F-crystallin/diphtheria toxin A hybrid gene. Lab Invest. 1992;67:31–41. [PubMed] [Google Scholar]
- Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994;13:805–814. doi: 10.3109/02713689409025135. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. Embo J. 2006;25:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- McAvoy JW. The spatial relationship between presumptive lens and optic vesicle/cup during early eye morphogenesis in the rat. Exp Eye Res. 1981;33:447–458. doi: 10.1016/s0014-4835(81)80095-4. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum Mol Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Wakely J. Scanning electron microscope study of the extracellular matrix between presumptive lens and presumptive retina of the chick embryo. Anat Embryol (Berl) 1977;150:163–170. doi: 10.1007/BF00316648. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Williams SC, Altmann CR, Chow RL, Hemmati-Brivanlou A, Lang RA. A highly conserved lens transcriptional control element from the Pax-6 gene. Mech Dev. 1998;73:225–229. doi: 10.1016/s0925-4773(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Hasegawa T, Osumi N, Koseki H, Takahashi N. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130:1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Jeffery WR. Central role for the lens in cave fish eye degeneration. Science. 2000;289:631–633. doi: 10.1126/science.289.5479.631. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Overbeek PA, Govindarajan V. Perinatal ablation of the mouse lens causes multiple anterior chamber defects. Mol Vis. 2007;13:2289–2300. [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]