Abstract
The nicotinic partial agonist varenicline (VCL) is a recently approved medication for the treatment of tobacco dependence, yet very little preclinical research on this drug has been published. The present experiment examined the nicotinic partial agonist properties of VCL and its parent compound, cytisine (CYT), in a nicotine discrimination assay. Rats were trained to discriminate nicotine (0.4 mg/kg, s.c.) from saline using a two-lever discrimination procedure, followed by generalization and antagonism tests with VCL and CYT. Antagonism was examined across a range of nicotine doses. In generalization tests, VCL produced a maximum of 63% responding on the nicotine-appropriate lever, indicating partial generalization. In antagonism tests, VCL decreased the % responding on the nicotine-appropriate lever at 0.2 and 0.4 mg/kg nicotine, indicating antagonism of nicotine's discriminative stimulus effects. No dose of VCL produced significant effects on response rate. The two highest doses of CYT weakly substituted for nicotine, producing a maximum of 23% nicotine-appropriate responding. CYT produced a weak antagonism of the discrimination of moderate nicotine doses, but not of the training dose. These results demonstrate that VCL and CYT partially generalize to and partially antagonize nicotine's discriminative stimulus effects, consistent with a partial agonist mechanism of action.
Although current medications for smoking cessation (e.g., nicotine replacement therapy, bupropion, nortriptyline) are helpful, the majority of smokers who use them fail to quit (Fiore et al. 2008). Thus, discovery and development of more effective medications is needed. To this end, numerous biological mechanisms in the pathophysiology of nicotine dependence have been examined as potential targets for medication development, such as pharmacodynamic processes in glutamatergic, GABAergic, opioidergic, or dopaminergic systems (Lerman et al., 2007; Wonnacott et al., 2005), and the pharmacokinetic process of nicotine distribution to brain (LeSage et al., 2006). Nicotinic acetylcholine receptors (nAChRs) in the brain, the primary site of action for nicotine in dependence, obviously comprise a critical medication target. The α4β2-containing subtypes of nAChRs are particularly relevant targets for medication development for several reasons. These nAChRs form high-affinity nicotine binding sites in the brain (Gotti and Clementi, 2004), and are distributed throughout the CNS including in dopaminergic areas that have been shown to be important in the reinforcing and rewarding effects of nicotine (Corrigall et al., 1994; Corrigall et al., 1992; Laviolette and van der Kooy, 2004; Wonnacott et al., 2005). The discriminative stimulus and reinforcing effects of nicotine are blocked by the administration of nicotinic antagonists that act at β2-containing receptors (Corrigall et al., 1994; Stolerman et al., 1997; Watkins et al., 1999). Nicotine does not produce reinforcing effects in β2 knock-out mice (Picciotto et al., 1998), whereas α4 knock-in mice display heightened sensitivity to nicotine-induced reward and locomotor sensitization, as well as increased tolerance to the hypothermic effects of nicotine (Tapper et al., 2004).
Given evidence such as this, recent attention has been paid to development of α4β2*-directed partial agonists for the treatment of tobacco dependence (Rollema et al., 2007b). A partial agonist binds to and activates a receptor (e.g., α4β2* nicotinic receptors), but has only partial efficacy at the receptor compared to a full agonist (e.g., nicotine). In addition, a partial agonist can act as a competitive antagonist by competing with the full agonist for receptor occupancy. In the case of α4β2* nicotinic receptors, the former action would result in effects similar to, but of lesser magnitude than those of nicotine, while the latter action would prevent nicotine from producing its maximal effect.
For example, the recently-approved medication varenicline (Chantix/Champix, Pfizer) is a partial agonist at α4β2* nicotinic receptors (Coe et al., 2005a; Coe et al., 2005b; Rollema et al., 2007a), although the compound does have affinity for other receptor subtypes (Mihalak et al., 2006). Consistent with its partial agonist mechanism, varenicline has been shown to produce increases in dopamine release and turnover in the nucleus accumbens that are significantly lower (40-60%) than those produced by nicotine, and varenicline pretreatment attenuates nicotine-induced increases in dopamine release and turnover to a level near the maximal response produced by varenicline alone (Coe et al., 2005a; Rollema et al., 2007a). In addition, varenicline has been shown to exhibit partial-to-full generalization to nicotine in drug discrimination assays (Rollema et al., 2007a; Smith et al., 2007), maintain self-administration behavior under a progressive-ratio schedule at lower breaking points compared to nicotine (Rollema et al., 2007a), and suppress nicotine self-administration (NSA) under a fixed-ratio (FR) schedule at doses that did not suppress food-maintained behavior in rats (Rollema et al., 2007a).
Cytisine is another α4β2* partial agonist, but with lower affinity compared to varenicline (Coe et al., 2005a; Coe et al., 2005b). Like varenicline, cytisine has been shown to partially substitute for the discriminative stimulus effects of nicotine (Brioni et al., 1994; Chandler and Stolerman, 1997; Craft and Howard, 1988; Pratt et al., 1983; Reavill et al., 1990; Smith et al., 2007; Stolerman et al., 1984). However, it has not yet been clearly shown to antagonize such effects (Reavill et al., 1990).
Clinically there have been issues with both compounds. Varenicline showed good efficacy in early clinical trials where it was generally well-tolerated (Gonzales et al., 2006; Jorenby et al., 2006; Nides et al., 2006; Oncken et al., 2006), but there have been recent reports of psychiatric side effects (Freedman, 2007; Kohen and Kremen, 2007; Kristensen et al., 2008); cytisine has been used since the 1960s as an aid for smoking cessation in eastern and central European countries (Etter et al., 2008), but there is only a small amount of data on its efficacy, which may be limited by its affinity for other nAChR subtypes and limited blood-brain barrier penetration (Rollema et al. 2007b). However, from a preclinical perspective both varenicline and cytisine are useful tools to examine mechanisms by which a medication may influence tobacco use and cessation, since both animal and human preclinical studies can be done.
In this study we have used drug discrimination methods to assess the effects of varenicline and cytisine in animals trained to discriminate nicotine from saline. Varenicline-induced suppression of NSA is the only evidence published to date of its ability to attenuate the behavioral effects of nicotine in preclinical models. The extent to which this effect is attributable to antagonism of nicotine binding at nicotinic receptors is unclear. Suppression of NSA could also be achieved by virtue of varenicline's agonist properties, since nicotinic receptor agonists also decrease NSA (Corrigall and Coen, 1989; Green et al., 2000; LeSage et al., 2003; LeSage et al., 2002; Stairs et al., 2007). The drug discrimination assay provides a tool to directly assess both the agonist and antagonist functions of varenicline, as agonism and antagonism are exhibited by distinct effects in this assay. However, antagonism tests were not conducted in the existing studies examining varenicline's effects in nicotine discrimination assays (Rollema et al., 2007a; Smith et al., 2007). Thus, the purpose of the present study was to examine the ability of varenicline to both generalize to and antagonize nicotine's discriminative stimulus effects in order to clarify its partial agonist effects at the behavioral level. For comparison, cytisine was also examined in this assay.
1. Methods
1.1. Subjects
Sixteen experimentally-naïve male Long Evans rats weighing 300-400 g were maintained with limited access to food (18 g/day rat chow) and unlimited access to water. Each rat was individually housed in a temperature- and humidity-controlled colony room under a 12h light/dark cycle (lights on at 7:00 am). Animal husbandry and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation, in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
1.2. Apparatus
Experimental sessions occurred in sixteen identical operant-conditioning chambers (Med Associates Inc., St. Albans, VT). The front panel contained two response levers, a stimulus light over each response lever, and an aperture between the levers for delivery of 45-mg food pellets (PJAI-0045, Research Diets, New Brunswick, NJ). A house light was located on the back panel near the chamber ceiling to provide ambient illumination. Each chamber was enclosed in a sound-attenuating box equipped with an exhaust fan that provided masking noise.
1.3. Discrimination Training
Methods similar to those reported by Rosecrans and colleagues were used (Philibin et al., 2005; Rosecrans, 1989). Rats were initially trained to lever press for food pellets during daily 15-min sessions. During this phase, each response on either lever produced a single 45-mg food pellet. Once responding was stable (50-60 total responses/session), lever pressing was reinforced under a variable interval (VI) 3 sec schedule. Under this schedule, the first response on the lever to occur after an average period of 3 sec produced a food pellet. The VI schedule was then gradually increased across several sessions until the terminal VI-15 sec schedule was established and stable performance was maintained (∼40-60 reinforcers per session). At this point, discrimination training began. Rats were trained to respond on one lever (right lever for half of the rats, left lever for the other rats) following a s.c. injection of 0.4 mg/kg nicotine and on the opposite lever following saline. Rats were injected, placed in the operant chamber, and the session was started. Each session began with a 5 min timeout period, during which the house light and cue lights were off and lever presses had no programmed consequence. The timeout was immediately followed by onset of the houselight and cue lights to signal the beginning of the discrimination training or testing period. Training occurred in double alternating sessions (i.e., Nic-Nic-Sal-Sal) and learning of the discrimination was assessed twice weekly (Tuesdays and Fridays) during 2-min extinction test sessions followed by a 15-min training session until criterion levels of performance were achieved during the extinction test sessions (>80% responding on the injection-appropriate lever). Sessions were conducted Monday through Friday. After criterion levels of performance were obtained (mean 73 sessions, range 62-81), the protocol for test sessions was changed such that a) rats received an i.p. injection of saline 25 min prior to s.c. injection of the nicotine training dose or saline and b) the 2-min extinction test session was followed by a 15 min session in which responding was reinforced on either lever according to the VI 15-sec schedule. This additional session maintained the daily level of reinforcement, avoiding any motivational effects that may have occurred when reducing reinforcement of lever pressing from five to three days per week if only extinction sessions were run on test days. Data from these non-differential reinforcement sessions were not analyzed. Discrimination was considered stable when a) discrimination criteria were met during two consecutive saline and nicotine test sessions, b) >95% injection-appropriate responding was exhibited on six consecutive training sessions, and c) response rates (total responses per session) were stable (no trend across the four consecutive test sessions and six consecutive training sessions). At this point, generalization and antagonism testing began.
1.4. Generalization and Antagonism Tests
Test sessions occurred twice weekly (Tuesdays and Fridays) as described above, subject to stable discrimination performance on intervening training days (discrimination criteria were met and response rate was within baseline range for 2 consecutive training sessions). During these test sessions, VCL (0.3, 1.0, and 3.0 mg/kg), CYT (0.1, 0.3, 1.0, and 3.0 mg/kg), or saline was administered i.p. at a volume of 1 ml/kg 25-min prior to administration of saline (generalization tests) or a range of nicotine doses (0.0, 0.05, 0.1, 0.2, or 0.4 mg/kg, antagonism tests). Nicotine generalization dose-effect functions were determined prior to testing each compound and at the end of the protocol to examine the stability of nicotine discrimination over the course of the experiment. For all rats, generalization tests were completed prior to antagonism tests, and assessment of VCL was completed prior to CYT. These tests involved administration of saline 25-min prior to a range of nicotine doses (0.0, 0.05, 0.1, 0.2, and 0.4 mg/kg). Antagonism tests with VCL were restricted to the 1.0 and 3.0 mg/kg doses, while those with CYT were restricted to the 0.3 and 1.0 mg/kg doses. The antagonism tests with 3.0 mg/kg CYT were not conducted because this dose alone significantly reduced response rates during generalization tests. The dose range and pretreatment time for VCL and CYT were based on those shown to be effective in blocking nicotine-induced elevations in dopamine in the nucleus accumbens and substituting for nicotine in drug discrimination assays (Coe et al. 2005a; Smith et al. 2007; Rollema et al. 2007a; Reavill et al. 1990).
1.5. Drugs
(-)-Nicotine hydrogen tartrate salt and cytisine (Sigma, St. Louis, MO) were dissolved in saline, and the pH adjusted to 7.4. 6,7,8,9-Tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine (varenicline) was provided by Research Triangle Institute (Research Triangle Park, NC) and synthesized as the dihydrochloride salt using reported methods (Coe and Brooks, 2002; Brooks et al., 2004), and was also dissolved in saline. All doses were administered in a volume of 1 ml/kg. Nicotine was administered s.c., while cytisine and varenicline were administered i.p.. Nicotine doses are expressed as that of the base, while VCL and CYT doses are expressed as that of the salt.
1.6. Data Analysis
Only data from the 2-min extinction test sessions were analyzed. The percentage of responding on the nicotine-appropriate lever (%NLR) and overall response rate (response/second) served as the primary dependent measures. Generalization functions were analyzed by repeated-measures ANOVA followed by Dunnett's post-hoc tests comparing each dependent measure at a given nicotine, VCL or CYT dose to saline. Antagonist functions were analyzed by multivariate ANOVA for repeated-measures with Bonferroni post-hoc tests comparing each dependent measure at a given nicotine dose following VCL or CYT pretreatment to that following saline pretreatment. Full generalization was defined as %NLR greater than or equal to 80%, while partial generalization was defined as %NLR greater than or equal to 20% but less than 80%. Four rats failed to meet training criteria in a timely fashion and were excluded from the study. One rat's performance became unstable following VCL testing and failed to return to criterion performance in a timely fashion to continue with CYT testing. Thus, the final sample size was 12 for VCL assessment and 11 for CYT assessment.
2. Results
2.1. Effects of varenicline
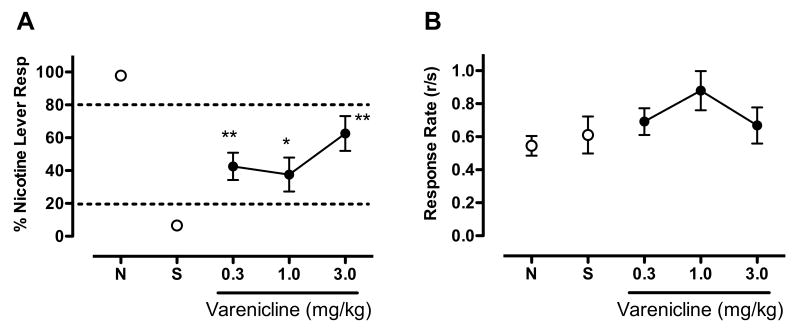
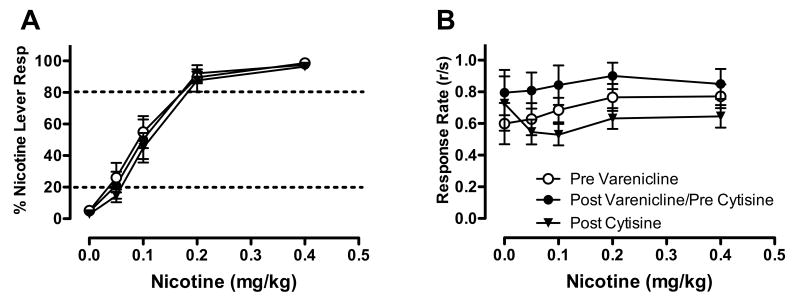
Figure 1 shows the effects of VCL substitution on %NLR (panel A) and overall response rate (panel B). VCL showed partial generalization to nicotine (F(3,11)=10.34, p<0.001), with the 0.3, 1.0 and 3.0 mg/kg VCL doses producing 43, 38, and 63 %NLR, respectively (t(11)=3.53, p<0.01; t(11)=3.04, p<0.05; and t(11)=5.50, p<0.001; respectively). VCL had no significant effect on response rate.
Figure 1.
Effects of VCL on percent responding on the nicotine-appropriate lever (panel A) and overall response rate (total responses/sec, panel B). Each point represents the mean (±SEM) of 12 subjects. Points derived from sessions prior to which the nicotine training dose or saline were administered are indicated by N and S, respectively. Significantly different from saline, *p<0.05, **p<0.01
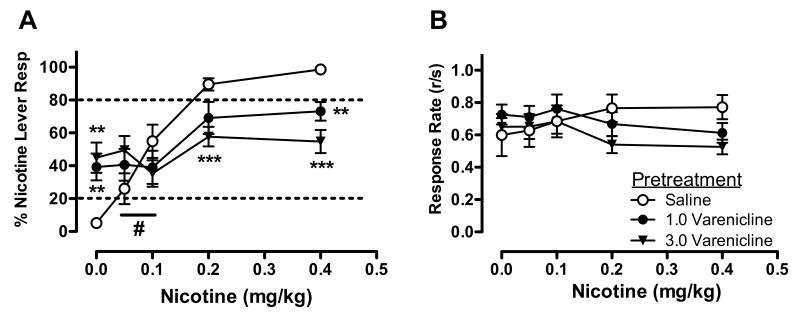
Figure 2 shows the effects of varenicline pretreatment on the nicotine discrimination dose-effect function (panel A) and overall response rate dose-effect function (panel B). Statistical analysis indicated no significant main effect of VCL, but a significant main effect of nicotine (F(4,8)=42.11, p<0.001) and nicotine × VCL interaction (F(8,4)=27.15, p<0.01). Simple effects tests indicated that VCL dose-dependently attenuated nicotine discrimination. The 1.0 mg/kg VCL dose reduced the mean %NLR from 98.63% to 73.13% at the nicotine training dose (t(11)=4.40, p<0.01), while the 3.0 mg/kg VCL dose reduced %NLR to 54.73% at the training dose (t(11)=6.38,p<0.001) and from 89.56% to 57.72% at the 0.2 mg/kg nicotine dose (t(11)=4.72, p<0.01). Also evident was a significant increase in mean %NLR produced by both of these VCL doses (t(11)=-4.40, p<0.01 and t(11)=-4.30, p<0.01 for the 1.0 and 3.0 mg/kg doses, respectively) when administered prior to saline (0 mg/kg nicotine), replicating the effects of these doses during generalization testing. Although VCL tended to increase %NLR at the 0.05 mg/kg nicotine dose, these simple effects were not statistically significant. Nonetheless, the direction of effect of 3.0 mg/kg VCL was reversed between the two lowest nicotine doses, indicated by a significant simple interaction between nicotine and VCL at this segment of the nicotine dose-response curve (F(1,11)=4.9, p<0.05). Moreover, while %NLR at 0.2 and 0.4 mg/kg nicotine after 1.0 mg/kg VCL was significantly higher than that induced by 1.0 mg/kg VCL alone (q(11)=3.26, p<0.01, q(11)=3.70, p<0.01, respectively), no dose of nicotine elicited higher levels of %NLR after administration of 3.0 mg/kg VCL compared to that dose of VCL alone. Statistical analysis indicated no significant main effect of VCL or nicotine, or a VCL × nicotine interaction, on response rate.
Figure 2.
Effects of VCL pretreatment on percent responding on the nicotine-appropriate lever (panel A) and overall response rate (panel B) produced by a range of nicotine doses. Each point represents the mean (±SEM) of 12 subjects. The legend in panel B applies to both panels. Significantly different from saline, **p<0.01, ***P<0.001. #Significant interaction between 3.0 mg/kg VCL and nicotine (0.05 versus 0.1 mg/kg), p<0.05.
2.2. Effects of cytisine
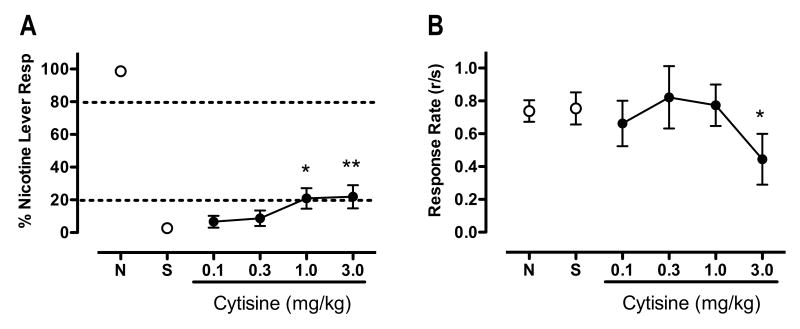
Figure 3 shows the effects of CYT substitution on %NLR (panel A) and overall response rate (panel B). CYT showed marginal partial generalization to nicotine (F(4,10)=3.90, p<0.01), with the 1.0 and 3.0 mg/kg doses producing only 21, and 23 %NLR, respectively (t(10)=2.93 and 3.09, p<0.05, respectively). In addition, CYT significantly attenuated response rate (F(5,10)=3.41, p<0.01), with the 3.0 mg/kg dose reducing response rate by 41% compared to saline (t(10)=3.0, p<0.05).
Figure 3.
Effects of CYT on percent responding on the nicotine-appropriate lever and overall response rate. Each point represents the mean (±SEM) of 10 subjects. For further details refer to Figure 1. Significantly different from saline, *p<0.05, **p<0.01
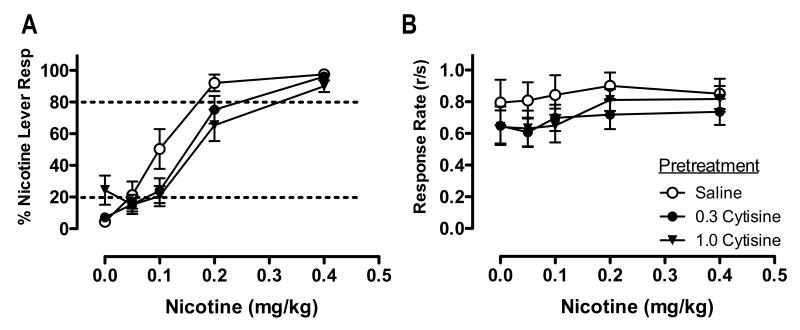
Figure 4 shows the effects of CYT pretreatment on the nicotine discrimination dose-effect function (panel A) and overall response rate dose-effect function (panel B). Statistical analysis indicated a significant main effect of CYT (overall, F(2,8)=6.85, p<0.05; saline vs 0.3 mg/kg CYT, t(10)=3.53, p<0.01; saline vs 1.0 CYT, t(10)=2.56, p<0.05) and nicotine (F(4,6)=98.28, p<0.001), but no significant nicotine × CYT interaction. Although the mean %NLR following 0.3 and 1.0 mg/kg CYT pretreatment appeared lower compared to saline pretreatment at the 0.1 and 0.2 mg/kg nicotine dose, these simple effects were not statistically significant. Similarly, although a higher mean %NLR was observed following 1.0 mg/kg CYT administered prior to saline, this effect was not statistically significant. No statistically significant main effect of CYT or nicotine, or a CYT × nicotine interaction, on response rate was observed.
Figure 4.
Effects of CYT pretreatment on percent responding on the nicotine-appropriate lever and overall response rate produced by a range of nicotine doses. Each point represents the mean (±SEM) of 10 subjects. For further details, refer to Figure 2.
Figure 5 shows the nicotine discrimination (panel A) and overall response rate (panel B) dose-response curves obtained prior to varenicline testing (from Figure 2), as well as prior to (from Figure 4) and following cytisine testing. No statistically significant changes in %NLR were observed across nicotine generalization dose-effect determinations, indicating the stability of nicotine discrimination over the course of the experiment. However, a significant main effect of time (F(2,8)=11.3, p<0.01) on response rate was observed, such that rates were somewhat higher overall during nicotine generalization testing prior to cytisine assessment compared to that prior to varenicline or following cytisine assessment.
Figure 5.
Nicotine discrimination (panel A) and overall response rate (panel B) dose-effect functions obtained prior to VCL assessment, as well as prior to and following CYT assessment. Each point is the mean (±SEM) of either 12 (pre VCL) or 10 subjects (pre and post CYT).
3. Discussion
The main findings of the present study are that 1) VCL partially generalized to the nicotine discriminative stimulus, with a maximum of 63% nicotine lever responding at the highest VCL dose and without significant effect on response rate, 2) CYT showed marginal generalization to nicotine, with a maximum of 22% nicotine lever responding and significant suppression of response rate at the highest CYT dose, 3) VCL produced a dose dependent antagonism of nicotine discrimination, while cytisine produced only a marginal overall antagonism. The present study is the first to demonstrate VCL antagonism of nicotine discrimination.
The generalization between VCL and nicotine observed in the present study is similar to prior studies, though varying degrees of generalization have been reported. Smith et al. (2007) found that VCL partially generalized to nicotine within the same dose range and to a similar maximal effect (60% NLR) as found in the present study. In contrast, Rollema et al. (2007a) reported that VCL fully generalized to nicotine at a dose of 1 mg/kg. While these findings together clearly show that VCL exhibits agonist-like effects in a nicotine discrimination assay, the full generalization reported by Rollema et al. is not entirely consistent with a partial agonist mechanism of action. Although VCL doses above those used in the present study may have produced full generalization, Smith et al. found that a higher dose (5.0 mg/kg) produced no greater generalization than lower doses. The difference in degree of generalization between studies may be related to the training schedule used (i.e., VI in the present study and Smith et al. versus FR in Rollema et al.), the pretreatment interval (i.e., 30 min in the present study and Smith et al. 2007 versus 5 min in Rollema et al. 2007a), features of the test sessions (i.e., extinction in the present study versus nondifferential reinforcement in the other studies), or a combination of these factors.
The addition of antagonist tests in the present study helps to clarify VCL's partial agonist properties in the nicotine discrimination assay. In contrast to the rightward shift typically produced by strict nAChR antagonists, VCL tended to produce a flattening (i.e., “clockwise rotation”) of the nicotine dose-response curve. This was particularly evident at the 3.0 mg/kg VCL dose, where no dose of nicotine produced any greater generalization than that VCL dose alone, and the direction of the effect of this VCL dose reversed between the two lowest doses of nicotine. Together with the agonist test data discussed above, these findings appear to provide a clear demonstration of VCL's partial agonist mechanism of action at a behavioral level. Although we cannot exclude the possibility that the decrease in nicotine discrimination could also be the result of the combination of VCL and nicotine producing a distinct discriminative stimulus, this seem unlikely in light of the known neuropharmacological profile of VCL (see below).
Although the present finding that cytisine partially generalized to nicotine is in agreement with several other studies, the magnitude of generalization was much lower by comparison. For example, most studies have shown that cytisine at doses similar to those used in the present study exhibits a peak generalization of between 40-60% NLR (Brioni et al., 1994; Craft and Howard, 1988; Reavill et al., 1990; Smith et al., 2007), in contrast to only just over 20% NLR in the present study. This discrepancy may be related to features of the present study that differ from prior studies, including strain or sex of the rats, training schedule, and parameters of the test sessions.
Although CYT produced an overall attenuation of nicotine discrimination in the present study, it was relatively weak and only apparent at nicotine doses below the training dose. This marginal antagonism is consistent with a prior report which, to our knowledge, is the only other study that has examined the ability of cytisine to antagonize the discriminative stimulus effects of nicotine. Specifically, Reavill et al. (1990) reported that 2.4 mg/kg CYT decreased %NLR in rats trained to discriminate 0.1 mg/kg nicotine from saline. However, a higher dose (3.9 mg/kg) failed to attenuate %NLR, and both doses produced significant suppression of response rates. Taken together with the present study, these data suggest that cytisine exhibits, at best, relatively weak antagonism of nicotine's discriminative stimulus effects.
The present findings show that the nicotine discrimination assay is sufficiently sensitive to distinguish between α4β2* nicotinic partial agonists. For example, VCL and CYT generalized to different extents to the nicotine stimulus, with varenicline showing greater generalization than cytisine. Similarly, VCL produced greater antagonism of nicotine discrimination than CYT. Also, the inability of CYT to attenuate nicotine discrimination at the training dose compared to lower nicotine doses suggests that the CYT's effects are surmountable, whereas the VCL data show a flattening of the dose-effect curve toward a level of generalization comparable to that induced by the VCL alone. However, a limitation of the present study was the lack of testing nicotine doses greater than the training dose. Consequently, it is unknown whether VCLs antagonist effects on nicotine discrimination are surmountable with higher nicotine doses.
Several lines of evidence suggest that the neuropharmacological mechanism mediating differences in the behavioral effects of VCL and CYT in the present study are due to differences in action at α4β2* nicotinic receptors. First, the greater efficacy of VCL in the present study is consistent with prior studies showing that, compared to CYT, VCL produces greater current in Xenopus oocytes expressing human α4β2* nicotinic receptors and greater attenuation of nicotine-induced dopamine turnover in nucleus accumbens (Coe et al., 2005a). Second, the present findings are also consistent with the greater affinity of VCL for α4β2* nicotinic receptors, and consequently its ability to compete with nicotine for receptor occupancy (Coe et al., 2005a; Smith et al., 2007). Third, in contrast to nicotine and VCL, CYT lacks efficacy at the subpopulation of α4β2* nicotinic receptors with high acetylcholine sensitivity, which is the receptor subtype that is upregulated during chronic nicotine exposure (Moroni et al. 2006; Isabel Bermudez, personal communication). Finally, the present study is consistent with several others suggesting that central α4β2* nicotinic receptors play a key role in the discriminative stimulus effects of nicotine, in contrast to other central nicotinic receptors (e.g., α3β4*, α7*, (Brioni et al., 1996; Gommans et al., 2000; Smith et al., 2007; Stolerman et al., 2004). However, differences between VCL and CYT in their affinity and efficacy at α4β2* nicotinic receptors may not entirely account for differences in their behavioral effects, as CYT also shows relatively poor brain penetration (Rollema et al., 2007b).
There is little to distinguish VCL and CYT in their actions at other nAChR targets. They are both full agonists at α7* receptors and have similar low efficacy at β2-containing receptors and high efficacy at α3β4* and α7* receptors (Mihalek et al. 2006; Luetje et al. 1991; Slater et al. 2003; Houlihan et al. 2001). Both VCL and CYT have also shown partial agonist activity at α6-containing receptors (Milalak et al 2006; Evans et al. 2003). Given the limited data available for comparing the neuropharmacological actions of VCL and CYT, it remains unclear whether differences in their actions at receptor targets beyond α4β2* contribute to the behavioral effects observed in the present study.
Taken together with the present study, the extant preclinical data on the comparative efficacy of VCL and CYT appear to have good predictive validity, as clinical trials show VCL also has a higher odds ratio for smoking cessation compared to CYT (Cahill et al., 2007). Thus, as a whole, research on nicotinic partial agonists for nicotine dependence represents an important advance in translational research in medication development, where the predictive validity of preclinical models has not been well established (Lerman et al. 2007).
Acknowledgments
This work was supported by startup funds from the University of Minnesota, Department of Psychiatry and the Minneapolis Medical Research Foundation (W.A. Corrigall), and NIDA grant DA020136 (M.G. LeSage). Varenicline was synthesized on NIDA grant DA12002 (F.I. Carroll). The authors thank Danielle Burroughs for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brioni JD, Kim DJ, O'Neill AB. Nicotine cue: lack of effect of the alpha 7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol. 1996;301:1–5. doi: 10.1016/0014-2999(96)00010-6. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, O'Neill AB, Williams JE, Decker MW. Clozapine attenuates the discriminative stimulus properties of (-)-nicotine. Brain Res. 1994;643:1–9. doi: 10.1016/0006-8993(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2007:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl) 1997;129:257–264. doi: 10.1007/s002130050188. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005a;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Coe JW, Vetelino MG, Bashore CG, Wirtz MC, Brooks PR, Arnold EP, Lebel LA, Fox CB, Sands SB, Davis TI, Schulz DW, Rollema H, Tingley FD, 3rd, O'Neill BT. In pursuit of alpha4beta2 nicotinic receptor partial agonists for smoking cessation: carbon analogs of (-)-cytisine. Bioorg Med Chem Lett. 2005b;15:2974–2979. doi: 10.1016/j.bmcl.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Craft RM, Howard JL. Cue properties of oral and transdermal nicotine in the rat. Psychopharmacology (Berl) 1988;96:281–284. doi: 10.1007/BF00216050. [DOI] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend. 2008;92:3–8. doi: 10.1016/j.drugalcdep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Evans NM, Bose S, Benedetti G, Zwart R, Pearson KH, McPhie GI, Craig PJ, Benton JP, Volsen SG, Sher E, Broad LM. Expression and functional characterization of a human chimeric nicotinic receptor with alpha6beta4 properties. Euro J Pharmacol. 2003;466:31–39. doi: 10.1016/s0014-2999(03)01540-1. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Freedman R. Exacerbation of schizophrenia by varenicline. Am J Psychiatry. 2007;164:1269. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2000;152:289–294. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Slater Y, Guerra DL, Peng JH, Kuo YP, Lukas RJ, Cassels BK, Bermudez I. Activity of cytisine and its bominated isosteres on recombinant human alpha7, alpha4beta2 and alpha4beta4 nicotinic acetylcholine receptors. J Neurochem. 2001;78:1029–1043. doi: 10.1046/j.1471-4159.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kohen I, Kremen N. Varenicline-induced manic episode in a patient with bipolar disorder. Am J Psychiatry. 2007;164:1269–1270. doi: 10.1176/appi.ajp.2007.07010173. [DOI] [PubMed] [Google Scholar]
- Kristensen PL, Pedersen-Bjergaard U, Thorsteinsson B. Varenicline may trigger severe hypoglycaemia in Type 1 diabetes. Diabet Med. 2008 doi: 10.1111/j.1464-5491.2008.02419.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003 doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neuroscience. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Vann RE, Varvel SA, Covington HE, 3rd, Rosecrans JA, James JR, Robinson SE. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Reavill C, Walther B, Stolerman IP, Testa B. Behavioural and pharmacokinetic studies on nicotine, cytisine and lobeline. Neuropharmacology. 1990;29:619–624. doi: 10.1016/0028-3908(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Nicotine as a discriminative stimulus: a neurobehavioral approach to studying central cholinergic mechanisms. J Subst Abuse. 1989;1:287–300. [PubMed] [Google Scholar]
- Slater YE, Houlihan LM, Maskell PD, Exley R, Bermudez I, Lukas RJ, Valdivia AC, Cassels BK. Halogenated cytisine derivatives as agonists at human neuronal nicotinic acetylcholine receptor subtypes. Neuropharmacology. 2003;44:503–515. doi: 10.1016/s0028-3908(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Wei X, Boustany C, Hojahmat M, Cassis LA, Crooks PA, Dwoskin LP, Bardo MT. Effects of nornicotine enantiomers on intravenous S(-)-nicotine self-administration and cardiovascular function in rats. Psychopharmacology (Berl) 2007;190:145–155. doi: 10.1007/s00213-006-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]