Abstract
This paper employs substrates that are patterned with shapes having well-defined geometric cues to characterize the influence of curvature on the polarization of highly metastatic B16F10 rat melanoma cells. Substrates were patterned using microcontact printing to define adhesive islands of defined shape and size on a background that otherwise prevents cell adhesion. Cells adherent to these surfaces responded to local curvature at the perimeter of the adhesive islands; convex features promoted the assembly of lamellipodia and concave features promoted the assembly of stress filaments. Cells adherent to rectangular shapes displayed a polarized cytoskeleton that increased with the aspect ratio of the shapes. Shapes that combined local geometric cues, by way of concave or convex edges, with aspect ratio were used to understand the additive effects of shape on polarization. The dependence of cell polarity on shape was determined in the presence of small molecules that alter actomyosin contractility and revealed a stronger dependence on contractility for shapes having straight edges, in contrast to those having curved edges. This study demonstrates that the cytoskeleton modulates cell polarity in response to multiple geometric cues in the extracellular environment.
Keywords: Micropatterning, Lamellipodia, Stress Fibers, Geometric Cues
INTRODUCTION
The development of surface chemistries that either support or prevent cell adhesion, in combination with methods that can pattern substrates with these chemistries, has enabled detailed studies of how cell shape influences many aspects of cell behavior. Early studies using these techniques demonstrated that size alone could have a strong influence on the growth or apoptosis of individual cells (Chen et al. 1997; Singhvi et al. 1994). More recently, geometric cues presented by the micropatterned surfaces have been used to induce directional motility, and polarity, across a population of individual cells (Jiang et al. 2005; Thery et al. 2006b). We used patterned substrates to independently vary the geometry of adherent cells at cellular and sub-cellular length scales to understand the roles of global and local geometric cues, respectively, in controlling motility structures in the cell. This paper shows that both local and global shape cues independently modulate cytoskeletal distribution and cell polarity.
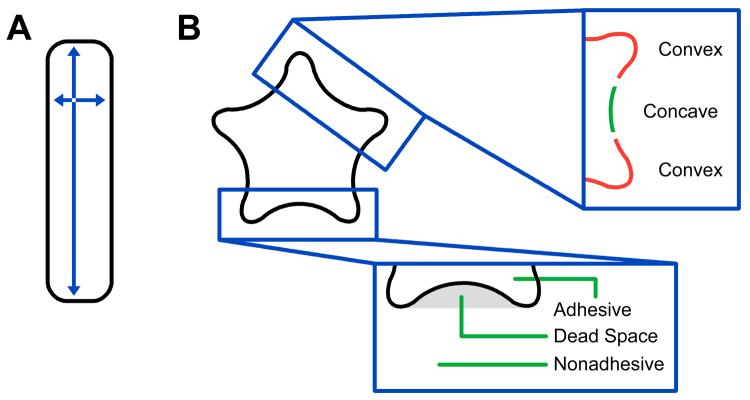
The relationship between the shape of individual cells and their cytoskeletal structure was first studied by Ingber and Whitesides, using microcontact printing to generate adhesive islands surrounded by a non-adhesive background (Chen et al. 2003; Mrksich et al. 1997). Individual NIH 3T3 fibroblasts and bovine endothelial cells spread to fill the adhesive islands and extended lamellipodia into nonadhesive regions after stimulation with platelet-derived growth factor. The direction of these lamellipodial protrusions was dictated by geometric features of the adhesive islands (Brock et al. 2003; Parker et al. 2002). Cells adherent to circular islands formed lamellipodia at all regions of the perimeter. Cells on square or triangular adhesive islands preferentially extended lamellipodia at the corners, demonstrating a qualitative preference for forming lamellipodia at acute rather than obtuse corners. A second cue for ordering cytoskeletal structures was identified by Bornens and coworkers, using shapes on patterned surfaces that required cells to span non-adhesive regions of the substrate—we refer to non-adhesive regions that underlie the cell as ‘dead space’ in this paper (Thery et al. 2006a). Cells generated strong contractile stress fibers along cell edges that spanned dead space on the substrate. These stress fibers straightened over time, demonstrating an increased contractile force that resisted membrane tension. When stress fiber contractility was blocked with Y27632, an inhibitor of ROCK (Thery et al. 2006a), cells lost their ability to generate straight edges over nonadhesive regions. This study, along with work by Ingber (Brock et al. 2003; Parker et al. 2002), demonstrates that local geometric features in the extracellular environment can control the spatial distribution of specific force-generating motility structures.
The correlation between cell polarity and cell shape was first reported by Whitesides and coworkers (Jiang et al. 2005), who used a switchable substrate to release cells from micropatterned surfaces. This method was based on an electrical potential that could switch non-adhesive regions of the substrate to adhesive and therefore allowed initially patterned cells to migrate while the direction and speed could be measured. Cells released from rectangular islands with a high aspect ratio migrated along the long axis of the adhesive island, while cells released from adhesive islands with a low aspect ratio migrated without a directional preference. This study established that aspect ratio, as a global property of shape, plays a dominant role in polarizing both cytoskeletal architecture and cell motility. A second study by Bornens and coworkers extended these findings in reporting that local geometric features in the extracellular environment could also polarize cells in the absence of a high aspect ratio (Thery et al. 2006b). Adhesive islands that presented cells with both convex curvature and asymmetrical dead space generated polarity, as did a series of islands which generated a square shape in cells but varied in the patterning of nonadhesive dead space. Adhesive islands with dead space along all four sides of the square cell perimeter did not induce a polarized distribution of the Golgi structure in a large cell population. When dead space was only present on three sides of a square, however, the position of the Golgi structure shifted towards the remaining adhesive edge, demonstrating cell polarity. Together, these studies by Ingber, Whitesides, Bornens, and co-workers identify cell geometries that modulate cellular behavior with either local or global shape cues in the extracellular adhesive environment.
In the present paper, we address the role of shape cues in modulating the cytoskeleton and polarity of patterned, metastatic cells, using substrates patterned with a range of shapes that have both local and global geometric cues (Figure 1). We use large cell populations to determine the statistical significance of local or global shape cues on cell polarity, allowing greater sensitivity in understanding the effect of various shape cues. We find that both local and global shape cues can independently modulate cell polarity, and that cell populations polarize in response to global but not local shape cues through actomyosin contractility.
MATERIALS AND METHODS
Microcontact Printing
Patterns were designed using AutoCAD software (Autodesk, Inc., San Rafael, CA), with the constraint that each shape had an area of 1000 μm2. The file was exported to L-Edit CAD (Version 11, Tanner EDA, Monrovia, CA), where the features were enlarged 25 times and incorporated into a stepper mask file. A transparency mask was generated using a 5080 dpi printer, then placed in a wafer stepper to reduce the features 5 times onto a 3-inch chrome mask (Telic, Santa Monica, CA). The resist was developed in AZ 327 MIF Developer (Clariant, Somerville, NJ) for 10 seconds. The exposed metal was removed in chrome etchant (1020AC, Transene, Danvers, MA) for 45 seconds and then the remaining resist was removed with acetone. The pattern on the 3-inch mask was transferred to a 5-inch chrome mask (Telic Santa Monica, CA) using contact alignment and UV flood exposure (Fusion Flood Exposure System, Rockville, MD). The 5-inch chrome mask was used in the wafer stepper to reduce features by 5 times onto a second 3-inch mask and achieve the final desired size of raised features.
The resist master mold was prepared by spin-coating hexamethyldisilazane (HMDS) adhesion promoter (Sigma-Aldrich, St. Louis, MO), then AZ4620 photoresist (Clariant, Somerville, NJ), onto a 3-inch Pyrex glass flat (Esco, Oak Ridge, NJ). The glass flat was soft baked for 7.5 minutes at 90°C to achieve a 15 micron-thick layer. Contact optical lithography was carried out using the fabricated optical mask in a contact mask aligner (Karl Suss MJB3, SUSS MicroTec, Garching, Germany), after which the photoresist was developed for 2.5 minutes in pure AZ-Developer (Clariant, Somerville, NJ). The master mold was hard-baked for 10 minutes at 90°C, followed by an oxygen plasma clean for 30 seconds at 300 W (PlasmaTherm Oxygen Reactive Ion Etcher). Finally, the resist master was exposed to chlorotrimethylsilane (Fisher Scientific, Hanover Park, IL) in vapor-phase for 75 seconds to facilitate removal of polydimethylsiloxane (PDMS). PDMS (Dow Corning, Midland, MI) was cast on the resist mold and cured for 15 minutes at 90°C. Stamps were cut out of the 5 mm thick PDMS layer.
The PDMS stamp was inked with octadecanethiol (10 mM in ethanol: Sigma-Aldrich, St. Louis, MO), dried under a stream of nitrogen and brought into contact with a gold-coated slide (prepared by electron beam evaporation of a 50 Å titanium adhesion layer followed by a 200 Å gold film onto a glass coverslip). The stamp was removed after one minute to generate an array of self-assembled monolayers (SAM) with the appropriate shapes. Slides were then incubated in a solution of tri(ethylene glycol)-terminated alkanethiol (3 mM in ethanol) for 14 hours to modify remaining regions of the slide with a surface chemistry that prevents protein adsorption and cell adhesion (LeDuc et al. 2002). Slides were washed in ethanol, dried with nitrogen, then incubated with 25 μg/ml fibronectin (Sigma-Aldrich, St. Louis, MO) for 1 hour. Slides were washed with PBS before cells were added.
Cells and Toxins
Early passage B16F10 cells (ATCC, Manassas, Va) were maintained in DMEM (Gibco, Gaithersburg, MD) supplemented with 10% FBS (Gibco, Gaithersburg, MD) and penicillin/streptomycin (Gibco, Gaithersburg, MD). For a survey of many different shapes (Figure 3), cells were allowed to spread onto a patterned surface for six hours before fixation. In all other experiments, cells were allowed to spread on the coverslips for three hours before fixation. In experiments using toxins, cells were allowed to spread in drug-free media for two hours, placed into media with 50 μM blebbistatin (Sigma-Aldrich, St. Louis, MO) or 10 μM nocodazole (Sigma-Aldrich, St. Louis, MO) for one hour, then fixed. Experiments were repeated multiple times, with collection of similar sizes of cell populations.
Immunostaining
Primary antibodies included rabbit anti-fibronectin H-300 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-giantin (AbCam, Cambridge, MA), and mouse anti-cortactin 4F11 (Upstate Biotechnology, Charlottesville, VA). Secondary antibodies were from Jackson ImmunoResearch (West Grove, PA), and included anti-rabbit CY5 and anti-mouse Texas Red. Cells were also stained with AlexaFluor 488-phalloidin and Hoescht 33258, both from Molecular Probes (Eugene, OR).
Cells on coverslips were stained using two separate methods. Slides stained for cortactin and the Golgi apparatus were prepared with a methanol fixation. Cells were fixed in -20°C methanol for 8 minutes, fixed in 4% paraformaldehyde (Ted Pella, Redding, CA) at 4°C for 20 minutes, and finally permeabilized in 0.5% TritonX-100 (Sigma-Aldrich, St. Louis, MO) for 15 minutes. Cells which were stained for phalloidin or fibronectin were prepared by simultaneous fixation and permeabilization in 4% paraformaldehyde and 0.5% Triton X-100 for 20 minutes at room temperature. All slides were stained for primary and then secondary antibodies at 37°C for 40 minutes.
Imaging and Analysis
All single cells that filled an adhesive island were imaged with a Hamamatsu back-thinned EM-CCD camera on a Olympus IX81 spinning disk confocal microscope (Center Valley, PA) using a 100x oil-immersion objective. Images for each cell population were taken with identical exposure times and on the same day to allow accurate comparison of fluorescence intensities between images. Images were exported from Slidebook (Intelligent Imaging Innovations, Inc, Denver, CO) into ImageJ (NIH, Bethesda, MD) for analysis. To construct a heat map, images of cortactin immunostained cells were digitally aligned and averaged to generate a ‘average cell’, here termed a cortactin map. For each image, the number of cells used to generate the composite cortactin map is indicated at the lower right corner.
Images of the nucleus and the Golgi were converted into binary images, and ImageJ was used to calculate the centroids of both nuclei and the Golgi apparatus. The position of the nucleus, relative to the Golgi, was plotted from coordinates of the centroids to determine the direction of cell polarity. Cells were classified as polarized if the angle of Golgi-to-nucleus was either between −15° to 15° or between 165° to 210° relative to the long axis of the patterned shape. Angles for the cell population were plotted as a polar histogram, using Origin 7.0 (OriginLab, Northampton, MA). Statistical analysis was completed using SPSS 16.0 (SPSS Inc., Chicago, IL). Error bars for each cell population, indicating the distribution of angles, were calculated for a 95% confidence interval (CI). Cell populations were compared using Analysis of Variance (ANOVA) and Tamhane's post-hoc ANOVA test (Cramer and Howitt 2004).
RESULTS
Approach
We used B16F10 cells to evaluate the roles of global and local geometric cues in establishing polarity in adherent cells. These cells derive from a metastatic mouse melanoma line that is highly motile and characterized by a non-polarized random walk on unpatterned substrates (Madeja et al. 2001). B16F10 cells do not maintain a persistent spatial segregation of lamellipodia and stress fibers during motility, but may instead exhibit multiple lamellipodia along the perimeter of the cell (Ballestrem et al. 1998). In this study, we identified lamellipodial structures by immunostaining for cortactin, a protein that colocalizes with Arp2/3 (Cosen-Binker and Kapus 2006; Weaver et al. 2001). The direction of cell polarity was calculated by determining the position of the Golgi apparatus centroid, relative to the centroid of the nucleus, with the former representing the front of the cell (Jiang et al. 2005; Magdalena et al. 2003; Thery et al. 2006b). We used cortactin and Golgi-to-nucleus localization as local indicators of actin architecture and global indicators of cell polarity, respectively, to examine the influence of cell shape on cell behavior.
Aspect Ratio Influences a Continuum of Polarity in a Cell Population
We first characterized the dependence of cytoskeletal polarization on the aspect ratio of rectangular shapes. For cells adherent to square shapes (Figure 2 A-E), stress fibers assembled along straight edges of the shape and, as indicated by cortactin staining, lamellipodia assembled at both the corners and edges of the adhesive island. To quantitatively assess lamellipodial distribution across a large population, we averaged cortactin stains from 71 cells to generate a heat map. This integrated image confirmed that lamellipodia were present in all parts of patterned cell, with a modest preference for corners of the shape. We also measured the position of the cell nucleus relative to the Golgi apparatus, and found that 12 ± 5% (CI = 95%) of cells were aligned within 15° of the horizontal axis (−15° to 15°, or 165° to 195°). This score was consistent with a random distribution of angles from the Golgi-to-nucleus localization, confirming that the cell population did not assume a global axis of polarity.
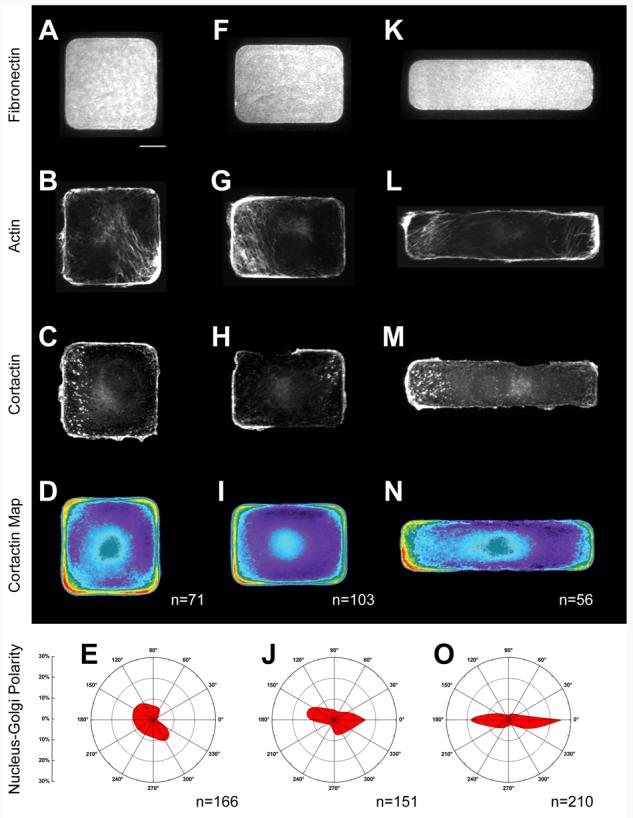
We next characterized cells that were patterned onto rectangles having a moderate aspect ratio of 3:2 (Figure 2 F-J). While aspect ratio has been identified as a global shape cue that induces polarity, patterns having similarly moderate aspect ratios did not induce directed migration of cells (Jiang et al. 2005). As with the square shapes, cells on these rectangular islands displayed stress fibers along all edges of the rectangle. However, lamellipodia were more prevalent along the short edges of the rectangular pattern and were strongest at the corners of the pattern. This trend was clearly observed in an analysis of a population of cells, where the heat map demonstrated stronger cortactin localization around the corners and the short edge of the rectangle relative to the long edge. Global polarity, measured by the distribution of the nucleus relative to the Golgi, indicated that 30 ± 7.5% (CI = 95%) of cells were polarized along the long axis of the rectangle.
We next tested whether the degree of polarization observed in the cell population was directly correlated to the magnitude of the global cue. When allowed to spread onto rectangular patterns with a high aspect ratio of 4:1 (Figure 2 K-O), cells demonstrated clear spatial segregation of cytoskeletal structures. Strong stress fibers were restricted to the long edges of the rectangle, while lamellipodia were observed at the short ends of the rectangle. While most cells had lamellipodia on one short end of the rectangle, several cells had lamellipodia on both short ends of the rectangle. The simultaneous presence of two lamellipodia indicated that cell motility structures responded directly to local extracellular cues, which were identical on both ends of the rectangles, and were not constrained by the global polarity of the cell, which would favor a single ‘front edge’. A heat map generated from a population of cells again showed the clear trend for the localization of lamellipodia at the short ends of the rectangle, with exclusion of lamellipodia from the long sides of the rectangle. By again measuring the position of the nucleus relative to the Golgi structure, we found that 50 ± 6.5% (CI = 95%) of the cells were polarized along the long axis of the rectangle.
These findings suggest that polarity within a population of cells increases with the aspect ratio of the cell shape. To assess the significance of this trend, we used an ANOVA multiple comparisons test to compare the polarization of cells on different patterns. Populations that are statistically different have a p-value < 0.005, while populations that are similar have a p-value > 0.005. In this case, ANOVA demonstrated that differences in population polarity between cells on squares, short rectangles, and long rectangles were significantly different (p-value < 0.001). This result was confirmed by Tamhane's post-hoc ANOVA test (Cramer and Howitt 2004), a more sensitive statistical analysis that does not assume that populations have similar distributions, again demonstrating that increased aspect ratio can generate significant increases in polarity in a population of cells.
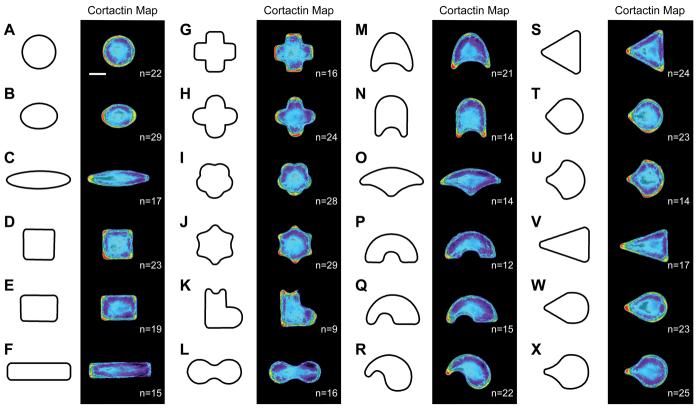
As part of our early studies, we investigated the localization of cortactin in cells adherent to a broad range of shapes and summarize these findings in Figure 3. These data are consistent with the trend that polarity increases with aspect ratio of the shapes, and further suggests a strong influence of curvature on the localization of cytoskeletal structures (Figure 3 A-F, and discussed below). While earlier studies identified aspect ratio as a global cue for polarity (Jiang et al. 2005), the improved sensitivity and larger cell population used in this work show that polarization of actin-based motility structures is graded and proportional to the strength of global shape cues.
Local Shape Cues Direct Cell Motility Structures
We next describe experiments that show the positions of lamellipodia are controlled not only by the global shape of the cell, but also by local shape cues. Previous studies have identified local shape cues that lead to an organization of cytoskeletal structures, including the assembly of focal adhesions and lamellipodia at sharp corners and of stress fibers across dead space (Chen et al. 2003; Singhvi et al. 1994; Thery et al. 2006a). The heat maps in Figure 3, taken from several shapes of patterned cells, demonstrate that lamellipodial formation is strongly dependent on local curvature. Cells formed lamellipodia at convex curves; conversely, lamellipodia were largely absent from concave curves (Figure 3 H-L). A survey of these data clearly reveals a correlation between the locations of lamellipodia and curvature of the pattern. Cells assembled more pronounced and localized lamellipodia at regions that presented acute convex curves (Figure 3 J) as compared to regions that were broadly curved (Figure 3 M, N). These findings suggest that actin-based structures are primarily influenced by the presence of local shape cues.
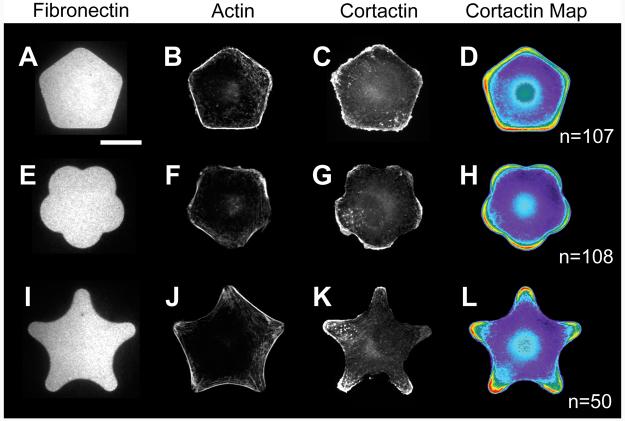
We used a subset of these patterns to more rigorously examine the role of curvature as a dominant local shape cue that promotes the assembly of specific actin-based structures. We compared cytoskeletal structure in cells adherent to three patterns, each with pentagonal symmetry but with differences in curvature of the edges and the sharpness of the corners. On pentagon shapes with straight edges (Figure 4 A-D), individual cells formed stress fibers along the edges, with lamellipodia along the perimeter at both corners and edges. On these patterns, the straight edges and corners did not tightly control cell motility structures, serving instead as a middle ground that allowed both lamellipodia and stress fiber assembly.
When the edges of the pentagon were made into convex curves, approximating the shape of a flower (Figure 4 E-H), the distribution of cytoskeletal structures changed distinctly. Cells on these adhesive islands formed large lamellipodia at the convex regions; this is consistent with lamellipodia on non-patterned surfaces, which take on broad, convex shapes. Lamellipodia on the patterned flower were typically separated by the concave corners between each petal, while stress fibers spanned the nonadhesive dead space between the convex curves. A few lamellipodia spread across adjacent convex curves, but most cells generated distinct lamellipodia at the convex curves of the modified pentagon. We generated heat maps of cells immunostained for cortactin and found that this lamellipodia-associated protein was brightest at the apex of each convex curve.
We next characterized shapes where the edges of the pentagon were altered to be concave; cells which spread on these adhesive islands generated a five pointed star (Figure 4 I-L). The cytoskeletal structures observed in the flower pattern were reversed on this star pattern, with strong stress fibers along the concave edges of the shape and lamellipodia restricted to short convex corners at the points of the star. This geometry of stress fibers is also seen with cells on non-patterned surfaces, where stress fibers at the cell perimeter take on a straight to concave shape (Wang and Suo 2005). Patterned cells extended beyond the underlying pattern of matrix protein at the concave regions to generate dead space. This behavior is consistent with previous findings that actin contractility leads to a time-dependent straightening of the cell edge across concave regions, stretching the cell across non-adhesive regions of the substrate and into dead space (Thery et al. 2006a). Together, these three shapes clearly illustrate the extent to which cytoskeletal structures can be spatially organized within the cell using local shape cues based on curvature. Lamellipodia preferentially localize at regions with high convexity, while stress fibers are prominent across nonadhesive dead space.
Local and Global Cues Reinforce Cell Polarity and Cell Motility Structures
The examples reported above illustrate the strong influence that local shape cues have on motility structures in adherent cells. We next examined the additive roles of aspect ratio and local curvature in promoting a polarized cytoskeleton in patterned cells. These experiments used a set of shapes that shared an aspect ratio of 2:5 but varied in the type and arrangement of local curvature. For a population of cells that spread onto a rectangle with no local curvature, 22 ± 9.5% (CI = 95%) of the cells showed a polarization in response to the shape, confirming polarization in response to moderate aspect ratio (Figure 5 A-E). For a rectangular shape that had convex curvature at the long ends—and which therefore provided both local and global shape cues to the patterned cells—the population of cells demonstrated higher lamellipodia localization at convex corners of the shape (Figure 5 F-J). This distribution of cytoskeletal structures was consistent with previous findings, and was associated with a small increase in polarity to 33 ± 12.5% (CI = 95%). A rectangular shape that had a concave curvature along the long edges also showed a greater localization of lamellipodia to the short ends of the shape (Figure 5 K-O). For a population of these cells, 48 ± 12.5% (CI = 95%) were polarized along the long axis of the shape. The role of local curvature in modulating the fraction of cells that are polarized on these shapes is on the edge of being statistically significant. The distinctions between these populations imply that variations in local geometric cues have more subtle effects on cell polarity than variations in aspect ratio, a global shape cue.
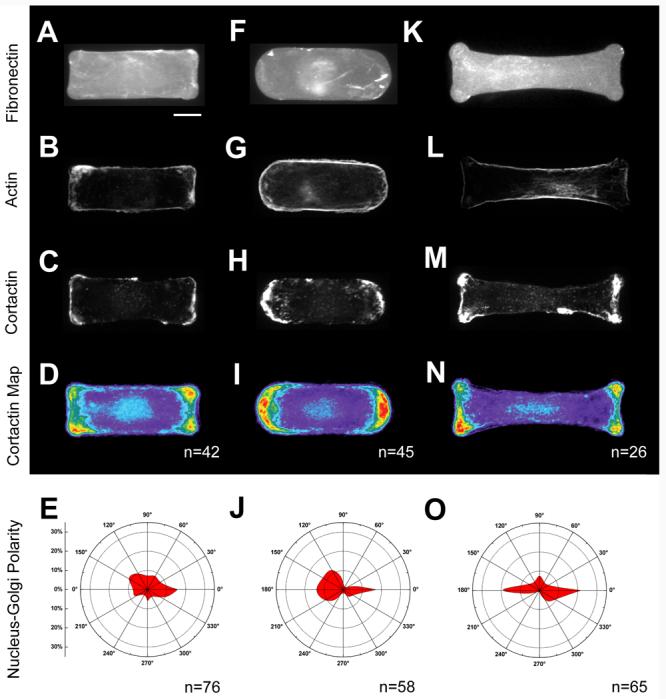
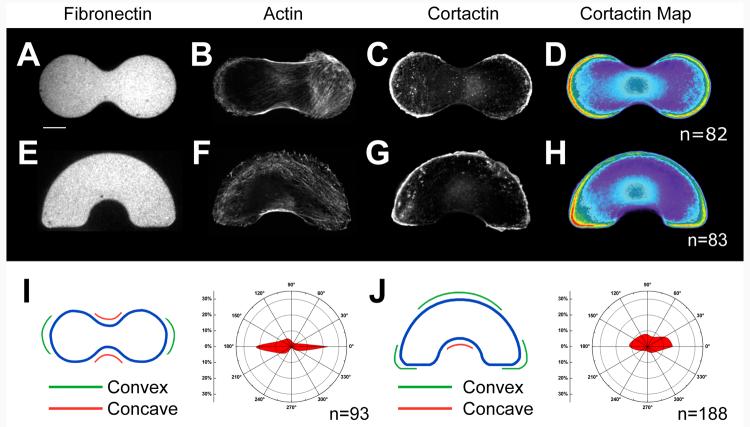
We next characterized cells adherent to a dumbbell shape with an aspect ratio of 1:2.5 that combined both convex curvature along the long ends of the shape and concave curvature at the short ends of the shape (Figure 6 A-D, I). For this pattern, we expected local curvature and global aspect ratio to cooperate in enforcing global polarity. Indeed, cells formed lamellipodia at the convex curves and assembled stress fibers that spanned the concave curves along the length of the cells. The segregation of protrusive and contractile elements in this polar shape was consistent with the local curvature. We found that 54 ± 10.5% (CI = 95%) of the cells had their Golgi structure polarized along the long axis of the cell. Though we recognize that the narrowing of the pattern near the center of the cell may have contributed to the displacement of the Golgi along the long axis of the cell, we believe that the cell motility structures played a role in increasing cell polarity for this shape.
We then characterized the response of cells on a rainbow shape that presented multiple local and global geometric cues. The upper and lower arc of the rainbow presented a broad curvature cue that opposed the moderate 2:1 aspect ratio of the shape. The corners of the rainbow (Figure 6 J), however, presented highly convex curvature cues that might provide strong cues for lamellipodia and therefore align polarity in the direction of aspect ratio. On these shapes, cells generated large lamellipodia that extended across the entire convex arc (Figure 6 G). Lamellipodia were also observed on the straight edges at the bottom of the rainbow, particularly at the outside corner that presents a region of high convex curvature. Lamellipodia were excluded from the concave center of the shape, which was instead supported by stress fibers. The localization of lamellipodia and stress fibers on these shapes was consistent with the dominant influence that local shape cues play in organizing actin-based structures, as described above. Indeed, measurement of the alignment of the Golgi relative to the nucleus showed that 30 ± 6% (CI = 95%) of the cells were polarized in the horizontal direction indicated by aspect ratio and local curvature, and not in the vertical direction indicated by the broad upper convex feature of the shape. The existence of geometric cues which support opposing forces partially explains the diminished polarity seen in the rainbow, compared to cells on the dumbbell shape. Nonetheless, both shapes indicate that local ordering of cytoskeletal structures with local shape cues and their forces could amplify cell responses to global properties of the adhesive island, such as aspect ratio.
Acto-Myosin Contractility is Required for Transmission of Global Cues
We tested the role that actin contractility plays in the response of adherent cells to shapes with global, local, or both geometric cues. We allowed B16F10 cells to attach, spread, and organize their cytoskeletal structures in response to the shape of adhesive islands before treating the cells with blebbistatin, a toxin that blocks the contractile acto-myosin skeleton and leads to a loss of pronounced stress filaments (Zhang and Rao 2005). Non-patterned cells that are treated with blebbistatin form lamellipodia along the entire perimeter of the cell (Kolega 2006). We also evaluated the response of spread B16F10 cells to nocodazole, a microtubule depolymerizing drug that also increases acto-myosin contractility and leads to a greater number of stress fibers (Liu et al. 1998).
For a population of cells adherent to rectangular shapes with a 4:1 aspect ratio, 43 ± 10.5% (CI = 95%) of the cells were polarized along the long axis of the shape. Treatment with blebbistatin weakly broadened the localization of cortactin (Figure 6 B), and significantly reduced the presence of stress fibers (data not shown). With the loss of stress fibers, we found that a decreased dependence of cell polarization on aspect ratio. We measured the distribution of Golgi apparatus and found that 31 ± 12.5% (CI = 95%) of the blebbistatin-treated cells showed global polarization. In contrast to treatment with blebbistatin, nocodazole significantly increased the number of stress fibers in each cell and sharply reduced the amount of cortactin displayed at the cell perimeter. While there was diminished cortactin overall, (Figure 7 C), there was a slight preference of cortactin for the short ends of the shape. In contrast to untreated controls, cells treated with nocodazole had a higher polarization along the long axis, observed in 68 ± 7% (CI = 95%) of cells. Cell polarity was lowest in populations treated with blebbistatin, which had low contractility; similarly, polarity was highest in cells treated with nocodazole, which had high contractility. ANOVA tests established that cells treated with blebbistatin or with nocodazole were significantly different populations (p-value of <0.001). This trend was confirmed with Tamhane's post-hoc test (p-value of <0.001). In this shape, which had an aspect ratio but no local curvature cues, polarity was dependent on actomyosin contractility.
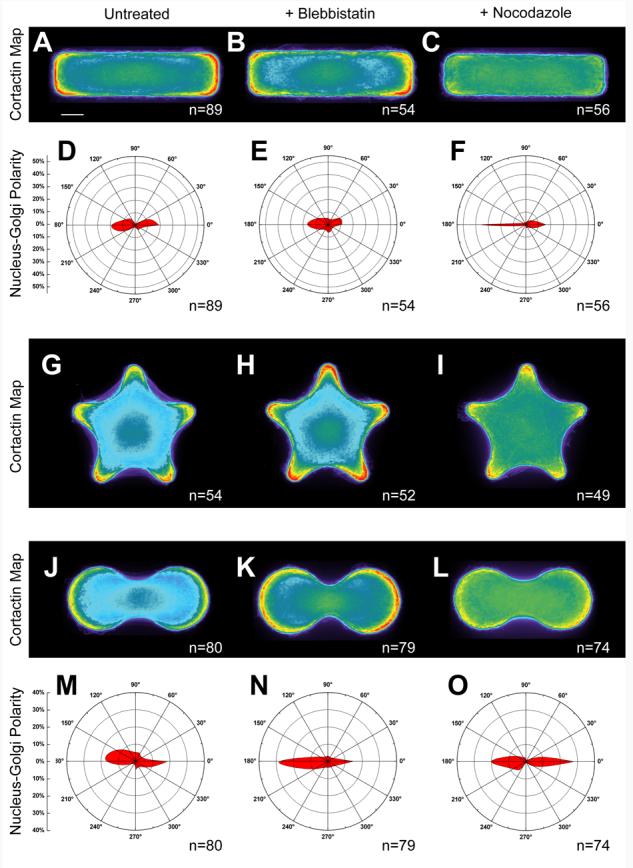
We next tested the effect that blebbistatin and nocodazole had on the response of cells on shapes with only local geometric cues (Figure 7 G-I). We found that cells assembled lamellipodia at the corners of the star under all treatments, but that the size of the cortactin-rich regions increased with contractility. Lamellipodia in cells treated with blebbistatin were larger, while those in cells treated with nocodazole were more localized. Heat maps revealed that cortactin is most concentrated at regions of high convexity for all cases.
Finally, we examined cells that were patterned on the dumbbell shape (Figure 7 J-O), which had both moderate aspect ratio and regions of strong local curvature. Cells treated with blebbistatin retained their spatial segregation of lamellipodia in the absence of stress fibers. Lamellipodia remained strongly restricted to convex curves and were absent from the concave regions at the center of the dumbbell. The exclusion of lamellipodia from the concave regions, even in the absence of stress fibers, demonstrates the strength of this local cue in preventing lamellipodia formation. The distribution of the Golgi structure, however, was not strongly affected by blebbistatin. In untreated populations, 40 ± 10% (CI = 95%) of the cells were aligned with the aspect ratio of the shape. Polarity increased, but not significantly, in cells treated with either blebbistatin or nocodazole; 54 ± 10% (CI = 95%) of cells were polarized after blebbistatin, while 55 ± 10.5% (CI = 95%) of cells polarized along the long axis after nocodazole treatment. The segregation of lamellipodia through curvature provided a local cue to cells that allowed consistent polarization irrespective of the contractility state of a cell. ANOVA comparison of the three populations established that drugs did not create a significant change in the three populations (p-value = 0.119). This conclusion was supported by Tamhane's test, which did not reveal statistical significance between any of the groups discussed.
DISCUSSION
This study demonstrates that local and global geometric cues in the extracellular environment have a strong influence on the localization of distinct actin-based cytoskeletal structures in adherent cells, which in turn contribute to the establishment of polarity. We established that polarity in B16F10 cells depends on shape and is sensitive to both global aspect ratio and local curvature. Further, studies using the inhibitors blebbistatin and nocodazole reveal that the influence of local geometric cues, but not aspect ratio (a global shape cue), in directing lamellipodial distribution and cell polarity is not dependent on the assembly of strong stress fibers. Collectively, this work advances an understanding of the roles by which both global and local geometric cues lead to a segregation of cytoskeletal structures global cell polarity in adherent cells.
The relationship between cell shape and behavior was historically difficult to assay, but can now be addressed using micropatterning methods. In the current work, we used microcontact printing to pattern self-assembled monolayers of alkanethiolates on gold into regions that either promoted or prevented the adsorption of protein and cell attachment (Mrksich et al. 1997). The high spatial fidelity of this technique enables reliable, quantitative assays for examining cell populations. A challenge in these experiments derives from the heterogeneity that is intrinsic to cell cultures, particularly with the metastatic cell lines used in this paper. While micropatterned surfaces remove variations in the sizes and shapes of cells, they do not influence the heterogeneous behavior of large cell populations. In this paper, we used statistical analyses to improve the sensitivity of polarity assays on micropatterned cells.
We first confirmed that cell polarity is sensitive to aspect ratio. This finding is consistent with earlier studies reporting that the migration of cells released from a pattern is influenced by shapes having a high aspect ratio (Jiang et al. 2005). By quantitating populations of cells that are adherent to shapes with moderate and high aspect ratio, we find that global aspect ratio is a strong, and graded, cue for polarity. We used a series of shapes to further study the role of local shape cues in promoting distinct actin-based cytoskeletal structures in the cells. As reported by Bornens (Thery et al. 2006a), concave edges were strong local cues for the formation of stress fibers. We also find that convex edges are strong cues for the formation of lamellipodia, and that the effects of these cues are largely independent of the overall shape of the pattern.
The correlation between cytoskeletal architecture and cell polarity suggested that both lamellipodia and stress fibers contribute to cell polarity and is consistent with the previous finding that the number and position of strong stress fibers can direct polarity (Thery et al. 2006b). To test the dependence of polarity on stress fibers, we treated cells with blebbistatin, which is known to disrupt the contractile actin filaments and results in the loss of stress filaments and an increase in lamellipodial structures (Kolega 2006). We also treated cells with nocodazole, which causes microtubule depolymerization and increases cell contractility. In unpatterned cells, nocodazole does not alter cell polarity, as measured by the position of the nucleus relative to the Golgi structure (Magdalena et al. 2003). Treatment of cells on rectangular shapes with blebbistatin leads to a loss of polarity whereas treatment with nocodazole leads to a larger fraction of the population displaying global polarity, showing that polarity on these shapes was dependent on stress fiber contractility. When cells were allowed to spread onto shapes with both moderate aspect ratio and local curvature cues, the fraction of cells that were polarized was less dependent on stress fiber contractility. In the presence and absence of stress fibers, lamellipodia remained localized onto the convex ends of the shape. The robustness of polarity on these shapes suggests that the segregation of cytoskeletal structures in response to local curvature cues provides a subtle but significant contribution to cell polarity.
The role of actin and microtubules in orienting cell polarity in response to geometric cues is still uncertain. The relationship demonstrated in this study between actin architecture and whole cell polarity is consistent with studies that suggest actin modulates cell polarity (Charest and Firtel 2006). Devreotes has shown that the actin cytoskeleton provides feedback to polarity pathways (Devreotes and Janetopoulos 2003) when chemotactic cells are presented with chemical cues. Chemotactic cells polarize in response to extracellular chemical cues by localizing phosphatidylinositol 3-kinase (PI3K) to the front and PTEN to the sides and rear of the cell. This separation of activities leads to an intracellular gradient of PtdIns(3,4,5)P3 (PIP3), which is highest at the leading edge of a chemotactic cell. PIP3 promotes the localization of RhoA at the trailing edge of the cell, which then serves to induce the assembly of contractile stress fibers. The gradient of PI3K and PTEN also promotes localization of Rac at the front of the cell which, together with Cdc42, induces lamellipodial protrusion (Van Keymeulen et al. 2006). In the absence of an intact cytoskeleton, PI3K accumulation at the front of the cell is significantly diminished, suggesting a positive feedback cycle by which the cytoskeleton amplifies PI3K and PIP3 at the leading edge (Sasaki et al. 2004). The link between cytoskeletal architecture and polarity pathways in chemotactic cells is consistent with our findings that segregation of stress fibers and lamellipodia can dictate cell polarity in a population.
Acknowledgments
This work was completed in the Department of Chemistry, University of Chicago, Chicago, IL
REFERENCES
- Ballestrem C, Wehrle-Haller B, Imhof BA. Actin dynamics in living mammalian cells. J Cell Sci. 1998;111(Pt 12):1649–58. doi: 10.1242/jcs.111.12.1649. [DOI] [PubMed] [Google Scholar]
- Brock A, Chang E, Ho CC, LeDuc P, Jiang X, Whitesides GM, Ingber DE. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir. 2003;19(5):1611–7. doi: 10.1021/la026394k. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16(4):339–47. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307(2):355–61. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–61. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- Cramer D, Howitt D. The SAGE Dictionary of Statistics. Sage Publications Ltd.; London: 2004. [Google Scholar]
- Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278(23):20445–8. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci U S A. 2005;102(4):975–8. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. The role of myosin II motor activity in distributing myosin asymmetrically and coupling protrusive activity to cell translocation. Mol Biol Cell. 2006;17(10):4435–45. doi: 10.1091/mbc.E06-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc P, Ostuni E, Whitesides GM, Ingber DE. Methods in Cell-Matrix Adhesion. In: Adams JC, editor. Methods in Cell Biology. Elsevier; 2002. pp. 385–401. [DOI] [PubMed] [Google Scholar]
- Liu BP, Chrzanowska-Wodnicka M, Burridge K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes Commun. 1998;5(4):249–55. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- Madeja Z, Szymkiewicz I, Zaczek A, Sroka J, Miekus K, Korohoda W. Contact-activated migration of melanoma B16 and sarcoma XC cells. Biochem Cell Biol. 2001;79(4):425–40. [PubMed] [Google Scholar]
- Magdalena J, Millard TH, Machesky LM. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J Cell Sci. 2003;116(Pt 4):743–56. doi: 10.1242/jcs.00288. [DOI] [PubMed] [Google Scholar]
- Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp Cell Res. 1997;235(2):305–13. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16(10):1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167(3):505–18. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–8. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Thery M, Pepin A, Dressaire E, Chen Y, Bornens M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil Cytoskeleton. 2006a;63(6):341–55. doi: 10.1002/cm.20126. [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Piel M, Pepin A, Dimitrov A, Chen Y, Sibarita JB, Bornens M. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc Natl Acad Sci U S A. 2006b;10352:19771–6. doi: 10.1073/pnas.0609267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174(3):437–45. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Suo Z. Long-distance propagation of forces in a cell. Biochem Biophys Res Commun. 2005;328(4):1133–8. doi: 10.1016/j.bbrc.2005.01.070. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11(5):370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46(11):4130–8. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]