Abstract
Individuals affected by hypoxia experience a variety of immune-associated sickness symptoms including malaise, fatigue, lethargy and loss of interest in the physical and social environment. Recently, we demonstrated that the interleukin (IL)-1β arm of the neuroimmune system was critical to the sickness symptoms caused by hypoxia, and that IL-1 receptor antagonist (IL-1RA), IL-1β’s endogenous inhibitor, was critical to promoting sickness recovery. Here we report that leptin is key to recovery from hypoxia because it dramatically augmented IL-1RA production in mice. We found that hypoxia increased leptin in white adipose tissue (WAT) and serum, which in turn, caused a marked rise in serum IL-1RA. Interestingly, in vitro, leptin was a more potent inducer of IL-RA, in macrophages, than hypoxia. In leptin receptor defective (db/db) and leptin deficient (ob/ob) mice, sickness recovery from hypoxia was delayed 3-fold. Importantly, in ob/ob mice, leptin administration completely reversed this delayed recovery and induced a marked increase in serum IL-1RA. Finally, leptin administration to normal mice reduced hypoxia recovery time by 1/3 and dramatically increased WAT and serum IL-1RA. Leptin did not alter recovery from hypoxia in IL-1RA knock out mice. These results show that by enhancing IL-1RA production leptin promoted sickness recovery from hypoxia.
Keywords: hypoxia, leptin, IL-1RA, sickness, neuroimmunity
Introduction
As a term, hypoxia, in its most encompassing, indicates a diminished availability of oxygen. Systemic hypoxia occurs in a variety of conditions, diseases and accidents often precipitated by dysfunctional respiration (Braunwald, 2008), but loss of blood-based oxygen carrying capacity, reduced oxygen transportation from capillaries to tissue (Simon et al., 2006) and environmental oxygen loss (Basnyat and Murdoch, 2003) are also common precipitants. In the medical setting, hypoxia is often a component of congestive heart failure (Quaranta et al., 1997), heart attack (Burke and Virmani, 2007) and infectious and non-infectious lung diseases (Browne and Pitchumoni, 2006; Cottin et al., 1998). Additionally, hospital inpatient hypoxia accounts for up to 41% of calls for emergency response teams (Jones et al., 2006). Outside the primary care setting, pulmonary disease-associated hypoxia often significantly impacts well-being, as the acute and overt consequences of labored breathing, dyspnea, and dizziness significantly suppresses the performance of activities of daily living (Kelly et al., 2007). Interestingly, hypoxia can clinically mimic other conditions like acute alcoholism due to the symptom constellation of impaired judgment, motor in-coordination and confusion (Kress and Hall, 2008). Severe hypoxia impacts higher brain functions within minutes resulting in loss of purposeful behavior and consciousness (Pena and Ramirez, 2005). Less dramatic hypoxia precipitates a variety of symptoms that elicit the subjective feelings of sickness including malaise, fatigue, lethargy and loss of interest in the physical and social environment. These symptoms are especially common in those suffering from acute mountain sickness (Clarke, 2006) and can be experienced during both the hypoxic episode and after return of the individual to normal oxygenation (Virues-Ortega et al., 2006). In normal subjects, mixed venous oxygen saturation (SmVO2) is approximately 75% (Kress and Hall, 2008) with hypoxia-associated sickness symptoms usually manifesting at an SmVO2of 70% (Kelly et al., 2007).
Given the sickness symptoms induced by hypoxia, it is not surprising that in the last 10 years, the significance of hypoxia has extended beyond its somewhat straightforward metabolic bearing on highly oxygen reliant organs like heart (Fisher and Burggren, 2007; Ostadal et al., 1999) and brain (Pena and Ramirez, 2005). Hypoxia is now recognized as a powerful innate immune activator (Rius et al., 2008; Johnson et al., 2007; Kozak et al., 2006; Hu et al., 2005) that causes the elaboration of pro-inflammatory cytokines (Kozak et al., 2006; Basu et al., 2005; Gehrmann et al., 1992; Lazovic et al., 2005), which, in turn, influence the neuroimmune system (Johnson et al., 2007; Johnson et al., 2008; Dantzer, 2004; Dantzer et al., 2001). In C3H/HeN mice, exposure to 5% oxygen for 60 minutes causes a rapid increase in plasma tumor necrosis factor (TNF)-α and IL-6 (Knoferl et al., 2000). Mice exposed to 11% oxygen for 7 days demonstrate lethargy, hypophagia and decreased body weight, as well as elevated plasma IL-6 (Kozak et al., 2006). A critical reactant to hypoxia is the macrophage, which in response to hypoxia elaborates pro-inflammatory cytokines, especially IL-1β (Basu et al., 2005; Gehrmann et al., 1992; Lazovic et al., 2005). In addition, hypoxia appears to sensitize the innate immune system because macrophages from hypoxic mice are hyper-responsive to lipopolysaccharide (Ertel et al., 1995). This phenomenon is reflected in ischemic brain injury, due to carotid artery ligation, because such ligation leads to a marked brain-based increases in IL-1β (Hu et al., 2005). In IL-1 knockout mice, ischemic brain injury is reduced as is edema and immune cell recruitment (Lazovic et al., 2005). Whether this is due to loss of macrophage- or microglial-dependent elaboration of IL-1β due to hypoxia (Basu et al., 2005; Gehrmann et al., 1992; Lazovic et al., 2005) is still not clear. Finally, we have demonstrated that the IL-1β arm of the neuroimmune system is intimately involved in hypoxia-dependent-social withdrawal, especially as related to hypoxia recovery (Johnson et al., 2007). In addition, we have shown that IL-RA, the endogenous antagonist to IL-1 (Hannum et al., 1990; Dinarello., 2000), is critical to promoting recovery from hypoxia (Johnson et al., 2007).
Very recently, leptin has been shown to protect neurons from transient cerebral ischemia (Zhang et al., 2007). How leptin serves as a neuroprotectant is not defined. Leptin is produced by adipose tissue in proportion to body mass (Juge-Aubry and Meier, 2002) and is best known for its role in regulating satiety and energy stores. The effects of leptin are mediated through the leptin receptor, a member of the class I cytokine receptors, similar in structure to the IL-6 receptor (Fruhbeck, 2006). In general, leptin secretion from fat is relatively constant and plasma concentrations of leptin do not appear to be significantly impacted by meal ingestion and meal-induced increases in serum insulin (Molina, 2006). During hypoxia, however, adipocytes have been shown to markedly increase leptin gene expression and leptin protein production (Ambrosini et al., 2002; Grosfeld et al., 2002b). Furthermore, leptin appears to enhance IL-1RA expression in certain cell types (Gabay et al., 2001; Hosoi et al., 2002). Therefore, the question we ask here is whether leptin is critical to acute hypoxia recovery, leptin vis-à-vis its ability to up-regulate IL-1RA.
Materials and Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich except: fetal calf serum (FCS) (0.05 ng/mL, 0.48 units/mL endotoxin) which was purchased from Atlanta Biologicals, IL-1RA and Leptin Enzyme-linked ImmunoSorbent assay (ELISA) kits which were purchased from R&D Systems, and Leptin which was purchased from PreproTech.
Animals
Animal use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Johnson et al., 2007). C57BL/6J and C57BL/6J-leprdb/db(db/db) mice were bred in house. B6.V-Lepob/J (ob/ob) and B6.129S-Il1rntm1Dih/J (IL-1RA knockout) mice were purchased from The Jackson Laboratories. Mice were housed in standard shoebox cages at 72°(relative humidity of 45–55%). Light cycle was reversed with a 12/12 day/night interval. Food was NIH 5K52 LabDiet in pellet form, Purina Mills. Water was provided ad libitum. Mice used for experimentation were male and 8–12 wks old. Mice were sacrificed by C02 asphyxiation unless otherwise indicated.
Hypoxia
As we have described (Johnson et al., 2007), mice were placed in a 12 × 6 × 4 inch plastic container connected to a certified gas cylinder containing 8% oxygen and 92% nitrogen (S. J. Smith & Co., Cedar Rapids, IA). Normoxic controls were placed in a similar 12 × 6 × 4 inch plastic container, and atmospheric air was blown into the container at the same rate. Mice were kept in experimental oxygen for 2 hours and then returned to atmospheric oxygen.
Social exploratory behavior
Social exploratory behavior was measured as follows and as we have described (Johnson et al., 2007; Johnson et al., 2005; O’Connor et al., 2005). Juvenile and adult mice were individually housed for 18 h prior to experimentation. A novel 3– to 4-week-old conspecific juvenile mouse of the same sex (challenge mouse) was confined to a 7.62 × 7.62 cm wire mesh enclosure (with a perforated steel top and bottom) placed in the center of the home cage of the adult mouse (test mouse). The test mouse was exposed to the challenge mouse for 5 min immediately prior to hypoxia exposure (−2 h) and at the indicated times after return of the test mouse to atmospheric oxygen. A novel challenge mouse was supplied for each test mouse at every time point. Investigation (nose contact) of the challenge mouse by the test mouse was video-recorded. Time of the test mouse-initiated exploration of the challenge mouse was determined from video records. To control for mouse-to-mouse variability in baseline activity and to allow comparison of relative changes in exploration levels the pre-hypoxia exposure (−2 h) measurement was used as an internal control for each test mouse. Four independent studies were preformed to measure social exploratory behavior. Fig. 2A utilized 10 db/db and 10 C57BL/6J mice (n = 5/treatment group). Fig. 3A utilized 20 ob/ob mice (n = 5/treatment group), Fig. 4A utilized 20 C57BL/6J mice (n = 5/treatment group). Fig. 4D utilized 12 IL-1RA KO mice (n = 3/treatment group). Results were expressed as a percentage of the baseline measurement and shown as means ± SEM. Unrestricted access to food was provided at all times except during exposure to hypoxia and during video recording.
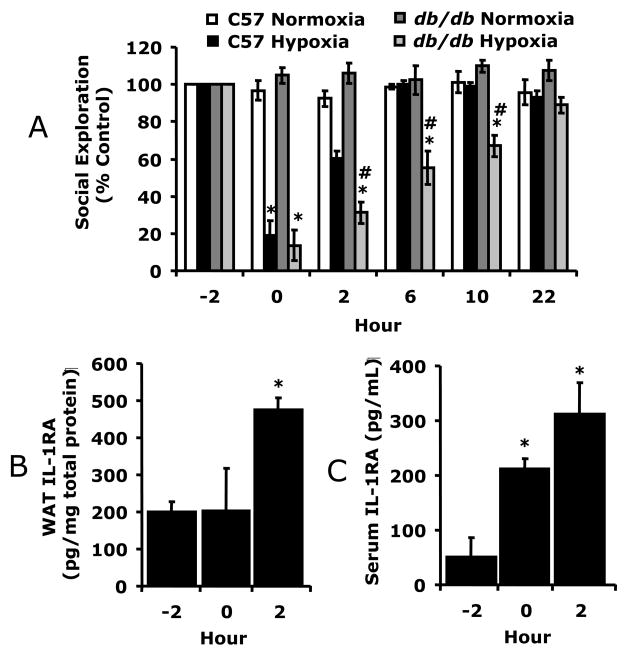
Fig. 2.
A, C57BL/6J and db/db mice were exposed to normoxia or hypoxia as indicated and social withdrawal was measured prior to hypoxia (−2 h) and at 0, 2, 6, 10 and 22 h after return of animals to normoxia. Results are expressed as percentages of the baseline measurement, means ± SEM; n = 5/treatment group; *p < 0.05, main effect of hypoxia vs normoxia, #p < 0.05, phenotype effect of C57BL/6J vs db/db. B/C, Db/db mice were treated as in A and IL-1RA was measured in WAT (B) and serum (C) by ELISA. D/E, Db/db mice were treated as in A and leptin measured in WAT (D) and serum (E) by ELISA. Results are expressed as means ± SEM; n = 4/time point; *p < 0.05 versus −2.
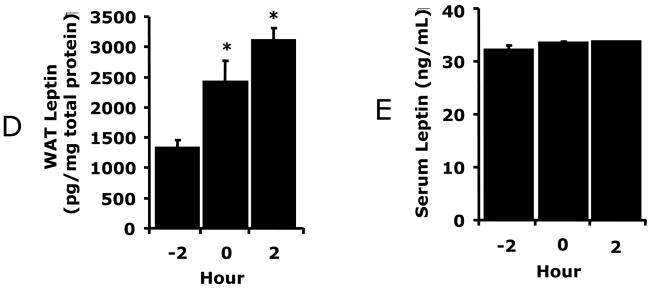
Fig. 3.
A, ob/ob mice were treated with or without leptin then exposed to normoxia or hypoxia as indicated and social withdrawal was measured prior to hypoxia (−2 h) and at 0, 2, 6, 10 and 22 h after return of animals to normoxia. Results are expressed as percentages of the baseline measurement, means ± SEM; n = 5/treatment group; *p < 0.05, main effect of normoxia vs hypoxia, #p < 0.05, treatment effect of leptin + hypoxia vs hypoxia. B, Ob/ob mice were treated with leptin as in A and IL-1RA was measured in serum by ELISA. Results are expressed as means ± SEM; n = 8/treatment; *p < 0.05. C, Macrophages from ob/ob mice were treated with or without leptin (620 nM) and/or normoxia or hypoxia for 2 h as indicated. Results are expressed as means ± SEM; n = 4; *p < 0.05 leptin vs PBS (normoxia and hypoxia), # p < 0.05 normoxia vs hypoxia (PBS and leptin).
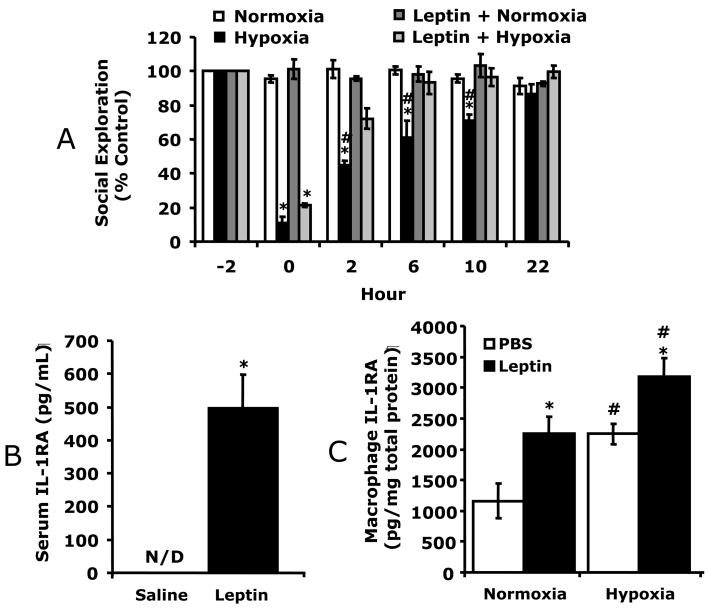
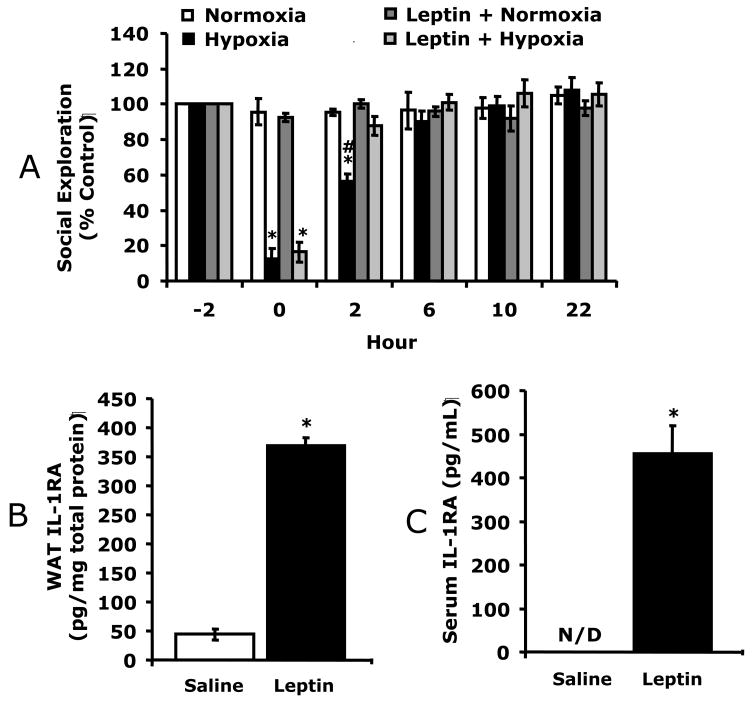
Fig. 4.
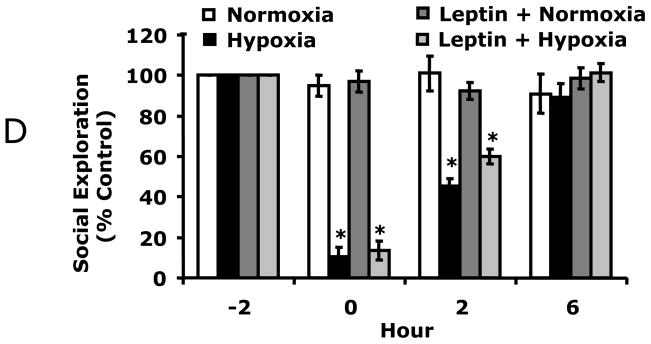
A, C57BL/6J were treated with or without leptin then exposed to normoxia or hypoxia as indicated and social withdrawal was measured prior to hypoxia (−2 h) and at 0, 2, and 6, 10 and 22 h after return of animals to normoxia. Results are expressed as percentages of the baseline measurement, means ± SEM; n = 5/treatment group; *p < 0.05, main effect of hypoxia vs normoxia, #p < 0.05 treatment effect of leptin + hypoxia vs hypoxia. B/C, Mice were treated with leptin as in A and IL-1RA measured in WAT (B) and serum (C) by ELISA. Results are expressed as means ± SEM; n = 8/treatment; *p < 0.05 vs leptin. D, IL-1RA knockout were treated with leptin, as in A, then exposed to normoxia or hypoxia as indicated and social withdrawal was measured prior to hypoxia (−2 h) and at 0, 2, and 6 h after return of animals to normoxia. Results are expressed as percentages of baseline measurement, means ± SEM; n = 3/treatment group; *p < 0.05, main effect of hypoxia vs normoxia.
Movement/alternation
Movement/alternation was measured in a 4-arm, black, Plexiglas cross maze (arms = 27.5 cm in length × 8 cm in width × 10 cm wall height: central platform = 8 cm × 8 cm) by methods previously described (Ragozzino et al., 1998). In brief, mice were placed on the center platform at the times indicated. Movement, as assessed by arm entries, was recorded over a 5 min period (from video records). The mouse was required to have all four legs in the arm for an arm entry to have occurred. The alternation score was quantified as a ratio of the actual alternations to total possible alternations (total arm entries minus 4). This study required the use of 6 C57BL/6J mice (n = 3/treatment group).
Macrophage isolation
As we have described (O’Connor et al., 2005; Sherry et al., 2007), peritoneal cells were collected by peritoneal lavage using 10 mL of ice-cold growth medium (RPMI 1640 supplemented with 10% FCS, 2 g/l sodium bicarbonate, 110 mg/l sodium pyruvate, 62.1 mg/l penicillin, 100 mg/l streptomycin, and 10 mM HEPES, pH 7.4). Lavage cells were pelleted and resuspended in 10 ml of hypertonic red blood cell lysis buffer (142 mM NaCl, 1 mM KHCO3 and118 mM NaEDTA, pH 7.4) at room temperature for 5 min then mixed 1:1 with growth medium. Cells were again pelleted and then resuspended at 37°C in growth media and plated on plastic at 0.5 × 106 cells/ml. After 60 min, plates (21 mm, 12-well for ELISA) were washed twice to remove non-adherent cells, resulting in >80% pure macrophages, confirmed by CD11b staining and morphology.
Serum and WAT cytokines
For all studies, an independent cohort of mice were used for each time point (−2, 0 and 2 h). IL-1RA and leptin were measured by ELISA for both serum and WAT. Fig. 1A–D utilized 12 C57BL/6J mice (n = 4/timepoint). Fig. 2B–E utilized 12 db/db mice (n = 4/timepoint). Fig. 3B utilized 16 ob/ob mice (n = 8/timepoint). Fig. 4B–C utilized 16 C57BL/6J mice (n = 8/timepoint). Serum was collected from the inferior vena cava, clotted at room temperature for 2 h, centrifuged at 2,000g (4°C) for 20 min. Retroperitoneal WAT was harvested into 0.5 ml of ice-cold homogenization buffer (100 mM NaCl, 50 mM NaF, 1% Triton-X, 50 mM Na3V04, 3.9 mg/ml benzamidine, 1 μl/ml Protease Inhibitor Cocktail Set III, 100 mM PMSF, 50 μM okadaic Acid and 50 mM Tris-HCl, pH 7.4) and homogenized with a Tissue Tearor (Biospec Products). Homogenates were centrifuged at 10,000g (4°C) for 10 min, and protein measured spectrophotometrically in the supernatant (Bio-Rad protein reagent).
Fig. 1.
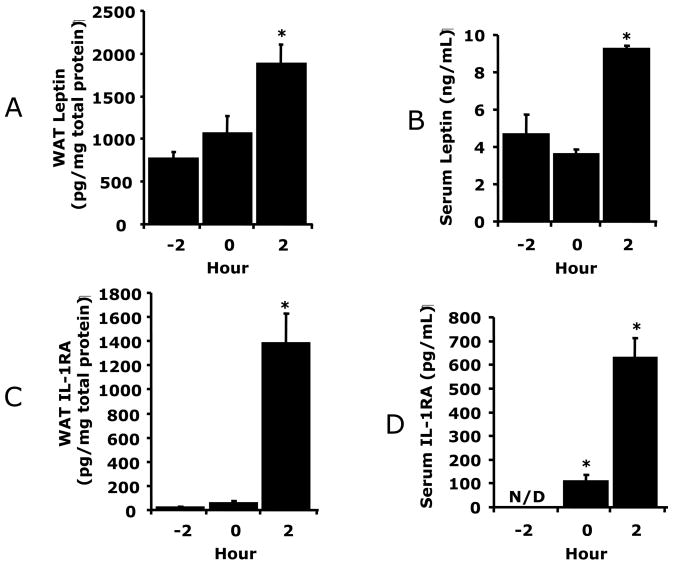
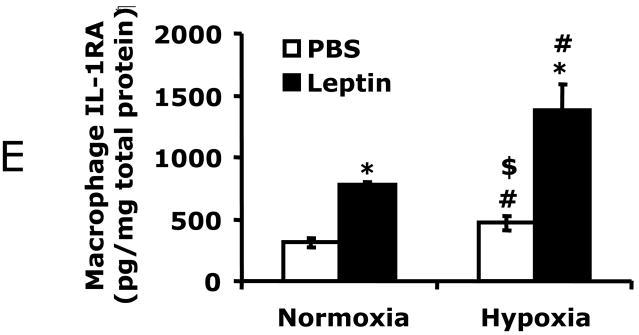
A/C, WAT was collected from hypoxic exposed C57BL/6J mice at the times indicated (−2 h = prior to hypoxia, 0 = immediately post hypoxia, 2 h = 2 h post hypoxia). Leptin (A) and IL-1RA (C) were measured in WAT tissue homogenates by ELISA. B/D, C57BL/6J mice were treated as in A/C and serum collected. Leptin (B) and IL-1RA (D) were measured by ELISA. Results are expressed as means ± SEM; n = 4/time point; *p < 0.05 vs −2 h. E, Macrophages were treated with or without leptin (620 nM) and/or normoxia or hypoxia for 2 h as indicated. Results are expressed as means ± SEM; n = 4; *p < 0.05 leptin vs phosphate buffered saline (PBS) (normoxia and hypoxia), # p < 0.05 normoxia vs hypoxia (PBS and leptin.), $ p <0.05 leptin normoxia vs PBS hypoxia.
Macrophage hypoxia
Peritoneal macrophages were isolated as described in macrophage isolation then placed in a Modular Incubator Chamber (Billups-Rothenberg, Del Mar, CA) containing an 8 % oxygen/92 % nitrogen environment at 37°C for 2 h. For leptin treatment, macrophages were incubated with 620 nM leptin (or PBS, as control) for 1 h prior to exposure to normoxia or hypoxia. IL-1RA was measured, by ELISA, in supernatants normalized for total protein. Two independent studies were conducted using in-vitro hypoxia on peritoneal macrophages. Fig. 1E utilized 4 C57BL/6J mice and macrophages isolated from each mouse were equally divided into four treatment groups. Fig. 3C utilized 4 ob/ob mice and macrophages isolated from each mouse were equally divided into four treatment groups.
Histology
As we have described (Johnson et al., 2007), an independent subset of mice were sacrificed by cervical dislocation. Brains were removed and perfused with ice-cold 10% neutral buffered formalin. Brains were paraffin embedded, serial sectioned and stained with hematoxylin and eosin (H&E).
Statistics
Data are presented as mean ± SEM. The experimental design for behavioral experiments was a completely randomized design with a 2 × 2 factorial arrangement of treatments (2 levels of pretreatment and 2 levels of treatment). All data were analyzed using SAS (Cary, NC) Inst PROC MIXED procedures of SAS. The statistical model for social withdrawal included the effects of leptin and hypoxia and time, with time as a repeated measure, and the interactions of leptin × hypoxia × time. Post-hoc comparisons of individual group means were performed with the Tukey test. Where indicated, experimental data were analyzed by ANOVA using SAS. Statistical significance was denoted at p<0.05.
Results
Leptin up-regulates IL-1RA during hypoxia
Figs.1A/B show that 2 h post hypoxia there was a 2.4-fold (765 ± 81 vs 1834 ± 225, pg/mg protein, F (1,6) = 25.07, p < 0.05) increase in WAT leptin and a 2-fold (5 ± 1 vs 9 ± 0.2, ng/ml, F (1,6) = 9.89, p < 0.05) increase in serum leptin from C57BL/6J mice. Fig. 1C demonstrates that 2 h post hypoxia, WAT IL-1RA was increased 60-fold (24 ± 3 vs 1382 ± 244, pg/mg protein, F (1,6) = 35.90, p < 0.05). In addition, serum IL-1RA was increased from undetectable, prior to hypoxia, to 102 ± 29 pg/ml and 629 ± 83 pg/ml immediately and 2 h post hypoxia, respectively (Fig. 1D). To determine the importance of leptin to hypoxia-dependent IL-1RA up-regulation, macrophages, a primary source of IL-1RA, were exposed to hypoxia and/or leptin. Fig. 1E shows that in-vitro macrophage treatment with hypoxia or leptin + hypoxia increased IL-1RA production 50% (314 ± 35 vs 473 ± 58, pg/mg protein, F (1,6) = 7.20, p < 0.05) and 338% (314 ± 35 vs 1376 ± 212, pg/mg protein, F (1,6) = 32.68, p < 0.05), respectively from control. Leptin was more effective at increasing macrophage-dependent IL-1RA production than hypoxia (314 ± 35 vs 777 ± 26, pg/mg protein, F (1,5) = 120.66, p < 0.05). Taken together, the Fig. 1 data indicate that hypoxia increases leptin and IL-1RA in both WAT and serum. In addition, leptin, more than hypoxia, appears responsible for the IL-1RA up-regulation seen.
Mice with defective leptin action have delayed recovery from hypoxia which leptin corrects
Fig. 2A shows that C57BL/6J mice had significantly improved recovery from hypoxia-induced depression of social exploratory behavior at 2 h, (60.4% ± 3.9% vs. 31.1% ± 5.8%), 6 h (100.2% ± 1.9% vs 55.1% ± 9.1%), and 10 h (99% ± 3.8 vs 67.2% ± 5.6%) as compared to db/db mice. Three-way ANOVA (phenotype × hypoxia × time) revealed a significant phenotype × hypoxia × time interaction [F (3,36) = 2.73, p < 0.05], hypoxia × time interaction [F (3,36) = 26.7, p < 0.0001], and a phenotype × hypoxia interaction [F (1,12) = 22.0, p < 0.0001). Figs.2B/C demonstrate that hypoxia-dependent IL-1RA up-regulation in WAT and serum were decreased 66% (474 ± 33 vs 1382 ± 244, pg/mg protein, F (1,6) = 15.63, p < 0.05) and 50% (312 ± 56 vs 629 ± 83, pg/ml, F (1,10) = 15.23, p < 0.05) 2 h post hypoxia, respectively, compared to wild type mice (see Figs.1C/D). Figs.2D/E show that db/db mice had greater basal WAT and serum leptin than wild type mice (1323 ± 138 vs 773 ± 81, pg/mg protein, F (1,6) = 13.99, p < 0.05; 32 ± 1 vs 5 ± 1, ng/ml, F (1,10) = 737.43, p < 0.05, respectively) (see Figs.1A/B) and that hypoxia increased WAT leptin 2.3-fold (1323 ± 138 vs. 3103 ± 204, pg/mg protein, F (1,6) = 60.54, p < 0.05) in db/db mice 2 h post hypoxia. Hypoxia did not significantly impact serum leptin, which was uniformly high (32 ± 1 ng/ml) in db/db mice. Ob/ob mice were used to determine if a failure of leptin bioaction was responsible for delayed recovery. Fig. 3A shows that ob/ob mice, like db/db mice, were delayed in their recovery from hypoxia. Leptin was administrated to ob/ob mice IP at 1 μg/mg twice a day for 2 d at 09:00 and 16:00 h prior to hypoxia. Leptin-treated ob/ob mice had significantly improved recovery from hypoxia-induced depression of social exploratory behavior at 2 h (72.2% ± 2.9% vs 44.6% ± 6.1%), 6 h (93.2% ± 6.7% vs 61.2% ± 9.6%), and 10 h (96.5% ± 5.2% vs. 70.9% ± 3.9%) as compared to saline treated ob/ob mice. Three-way ANOVA (leptin × hypoxia × time) revealed a significant hypoxia × time interaction [F (3,27) = 38.9, p < 0.0001] and leptin × hypoxia [F (1,9) = 16.9, p < 0.001], but no leptin × hypoxia × time interaction. Fig. 3B demonstrates that leptin increased ob/ob mouse serum IL-1RA from not detectable to 496 ± 101 pg/ml. Finally, in Fig. 3C, macrophages from ob/ob mice were treated in-vitro with hypoxia or leptin + hypoxia. Hypoxia or leptin + hypoxia increased IL-1RA production 93% (1160 ± 282 vs 2247 ± 165, pg/mg protein, F (1,6) = 12.63, p < 0.05), and 174% (1160 ± 282 vs 3178 ± 309, pg/mg protein, F (1,6) = 26.74, p < 0.05), respectively, from control. Leptin alone increased macrophage-dependent IL-1RA production 95% (1160 ± 282 vs 2254 ± 272, pg/mg protein, F (1,6) = 8.90, p < 0.05). Taken together, Figs.2/3 indicate that a functional leptin receptor is required for normal hypoxia recovery and that defects in leptin bioaction cause a blunted IL-1RA response that is corrected by leptin
Leptin improves recovery from hypoxia in normal but not in IL-1RA knockout mice
To determine if leptin would augment C57BL/6J mouse recovery from hypoxia, C57BL/6J mice were treated with leptin prior to hypoxia. Fig. 4A shows that leptin treated mice recovered 3 times faster than non-leptin treated mice. Leptin-treated C57BL/6J mice had significantly improved recovery from hypoxia-induced depression of social exploratory behavior at 2 h (87.9% ± 5.4% vs 56.4% ± 4.3%) as compared to saline treated C57BL/6J mice. Three-way ANOVA (leptin × hypoxia × time) revealed a significant hypoxia × time interaction [F (2,24) = 80.5, p < 0.0001] and leptin × hypoxia interaction [F (1,12) = 54, p < 0.05], but no leptin × hypoxia × time interaction. Fig. 4B demonstrates that leptin increased WAT IL-1RA 7.5-fold (44 ± 10 vs. 369 ± 13, pg/mg protein, F (1,5) = 622.78, p < 0.05) and serum IL-1RA from not detectable to 455 ± 65 pg/ml (Fig. 4C) in C57BL/6J mice. To prove that leptin-dependent recovery from hypoxia required IL-1RA, leptin was administered to IL-1RA KO mice. Fig. 4D demonstrates that leptin was ineffective at speeding recovery from hypoxia in these animals. Finally, the longer-term impact of hypoxia was examined in C57BL/6J mice. 14 d recovered animals demonstrated no deficit in social withdrawal (data not shown) or movement/alternation (Table 1). In addition, microscopic examination of paraffin embedded H&E stained brains revealed no identifiable neuronal damage or inflammatory changes (data not shown). Likewise, brains from mice 1 d and 10 d post hypoxia showed no morphologic changes (data not shown). Taken together these results indicate that leptin speeds recovery from hypoxia through a mechanism reliant on IL-1RA. In addition, the acute hypoxia used induced a mild sickness that allowed for recovery.
Table 1.
| Arm Entries | Alternations | % Alternations | ||||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| −2h | 53 ± 4.2 | 55.7 ± 0.4 | 26.7 ± 2.3 | 25.7 ± 5.9 | 50.4 ± 2.9 | 47.8 ± 11.4 |
| 0h | 41.3 ± 6.1 | 1.3 ± 0.4* | 23.7 ± 5.9 | 0 ± 0* | 62.2 ± 8.6 | 0 ± 0* |
| 2h | 24.7 ± 1.5 | 30.0 ± 2.4 | 11 ± 1.9 | 17 ± 1.4 | 52.8 ± 5.1 | 65.7 ± 3.3 |
| 4h | 24.3 ± 4.6 | 28.7 ± 1.5 | 12 ± 4.4 | 14.7 ± 1.1 | 56.8 ± 8.3 | 59.6 ± 3.9 |
| 7d | 72 ± 3.1 | 74 ± 7.1 | 34.7 ± 3.6 | 38 ± 5.5 | 50.9 ± 3.4 | 55.1 ± 9.2 |
| 14d | 54.3 ± 2.5 | 46.7 ± 9.0 | 29.7 ± 1.8 | 20 ± 4.3 | 62.8 ± 1.3 | 49.2 ± 10.3 |
Results are means ± SEM; n=3,
p < 0.05 normoxia vs hypoxia
Discussion
Even though the ob gene contains a hypoxia responsive element (Ambrosini et al., 2002; Grosfeld et al., 2002a) and leptin message and protein are increased under ambient and/or chemical hypoxia (Grosfeld et al., 2002b; Wang et al., 2007), the physiologic role of leptin in a low oxygen environment is unclear. Leptin is known to activate the IL-1RA promoter (Dreyer et al., 2003), increase IL-1RA message and protein (Gabay et al., 2001; Faggioni et al., 1999) and has recently been shown to protect against neuronal death (Zhang et al., 2007). Similar to leptin, IL-1RA reduces the adverse impact of focal, global, reversible, or permanent cerebral ischemia in rodents (Rothwell, 2003). Here we present novel data demonstrating that the beneficial effect of IL-1RA in hypoxia is dependent on leptin. Our previous work revealed that hypoxia-induced social withdrawal was worse in mice with defective leptin signaling. Incidentally, these same mice failed to up-regulate IL-1RA in response to hypoxia. Interestingly, administration of exogenous IL-1RA to these animals improved their recovery from hypoxia (Johnson et al., 2007). To determine if there was a causal link between leptin, IL-1RA and hypoxia-dependent social withdrawal, we further explored this interrelationship. In Fig. 1A/B, we demonstrate a doubling and near tripling of serum and WAT leptin, respectively, 2 h after exposure to hypoxia. These finding are consistent with several studies showing that hypoxia increases adipocyte leptin (Grosfeld et al., 2002b; Wang et al., 2007). Figs.1C/D show that hypoxia increased serum and WAT IL-1RA, with the likely source for this WAT IL-1RA being fat-based macrophages (Fain et al., 2006). Fig. 1E supports this contention in that leptin increased macrophage production of IL-1RA more than ambient hypoxia.
To further solidify the role of leptin in hypoxia recovery, mouse models defective in leptin signaling (db/db mouse) and production (ob/ob mouse) were exposed to hypoxia. Figs. 2A/3A show that db/db and ob/ob mice take 16 h longer to recover from hypoxia-induced depression of social exploratory behavior than wild type mice. In addition, db/db mice had significantly attenuated hypoxia-induced IL-1RA production (Figs.2B/C) as compared to wild type mice (Figs.1C/D). Furthermore, db/db mice were able to increase WAT leptin production in response to hypoxia, indicating that leptin itself was not the trigger for WAT to produce more leptin. Importantly, exogenously administered leptin returned ob/ob mice to normal recovery (Fig. 3A), and increased serum IL-1RA from undetectable to 500 pg/mL (a serum concentration nearly equivalent to that of wild type mice 2 h post hypoxia (Fig. 1D)). This is noteworthy because we (Johnson et al., 2007) and others (Rothwell, 2003) have shown that IL-1RA is protective in models of hypoxia and ischemia, respectively. As in wild type mice, leptin increased IL-1RA production from ob/ob macrophages by over 2-fold (Fig. 3C). However, in terms of IL-1RA production, ob/ob macrophages were much more responsive to hypoxia than wild type macrophages. This may be due to an unknown compensatory pathway that exists in ob/ob macrophages, possibly, because macrophages appear to produce their own leptin (Vernooy et al., 2006).
Given the beneficial effects of leptin in ob/ob mouse hypoxia recovery, we wanted to determine if leptin could affect recuperation in wild type mice. Fig. 4A shows that leptin hastened recovery in C57BL/6J mice by 4 h. This improvement was equivalent to that seen in MyD88 knockout mice and in mice administered intracerebroventricular caspase-1 inhibitor (Johnson et al., 2007). Both MyD88 knockout mice and caspase 1 inhibitor administered mice lack a functioning IL-1β arm of the neuroimmune system, which in aggregate implicate the importance of IL-1β to hypoxia-induced depression of social exploratory behavior (Johnson et al., 2007). The mechanism by which hypoxia up-regulates IL-1β is not entirely known but the IL-1β gene has multiple binding sites for the transcription factor, hypoxia inducible factor (HIF) (Hellwig-Burgel et al., 2005). Additionally, induction of IL-1β during hypoxia has been shown to be dependent on HIF, but independent of NF-κB (Zhang et al., 2006). Finally, leptin increased WAT and serum IL-1RA (Figs.4B/C). To confirm the requirement of IL-1RA to leptin-dependent enhancement of hypoxia recovery, leptin was administered to IL-1RA knockout mice. Fig. 4D demonstrates that these mice had no improvement in hypoxia recovery; underscoring that leptin, itself, does not promote hypoxia recovery unless IL-1RA is available. In terms of longer-term consequences of hypoxia exposure, mice had no movement, alternation or social interaction defects 2 wks after hypoxia nor did they develop neuronal death as measured by neuronal esosinophilia. These findings are not surprising because Prass et al. showed that mice exposed to 8% oxygen did not show significant neuronal death until nearly 5 h of exposure (Prass et al., 2003).
As a whole, our results are important because they demonstrate that in recoverable hypoxia, hypoxia-induced leptin causes an increase in IL-1RA that then counteracts sickness. It is possible that stress could have confounded our study, as exposure to low oxygen can induce increases in corticosterone in mice (Zoccal et al., 2007). Corticosterone pre-treatment and down-regulation of the hypothalamic pituitary adrenal axis, however, has been shown to be beneficial in hypoxic/ischemic brain damage models (Krugers et al., 1995) suggesting that increased corticosterone may actually be beneficial to hypoxia recovery. Moreover, absence of corticosterone in adrenalectomized mice potentiates IL-1β and lipopolysacchride-induced social withdrawal. In addition, administration of corticosterone to these animals reverses the changes caused by adrenalectomy (Johnson et al., 1996; Propes and Johnson, 1997). Such research underscores the similarity between hypoxia-dependent activation of the neuroimmune system and classically defined neuroimmune activators that are traditionally tied to infectious etiologies. Our current results also point to the difference between hypoxia-induced neuroimmune activation resulting in altered social behavior and that caused by ischemia, which results in unrecoverable brain injury that is difficult to ameliorate with almost all anti-inflammatory therapies (Chen et al., 2002). In sum, we demonstrate how leptin likely modulates the outcome of the low oxygen state by tying together the role of hypoxia-induced leptin and leptin augmentation of IL-1RA with the role of IL-1RA as a neuroprotectant.
Acknowledgments
This research was supported by grants from American Heart Association (Predoctoral Fellowship to C.L.S), and grants from the National Institutes of Health (DK64862 & NS58525 to G.G.F.) and University of Illinois Agricultural Experiment Station (to G.G.F.). This work was supported in part by a grant from the U.S. Department of Homeland Security, Assistance to Firefighters Grants Office, Research and Development Grants (EMW-2006-FP-02459). The Department of Homeland Security, Assistance to Firefighters Grants Office, Research and Development Grant is not responsible for the study design, implementation, results, or dissemination.
Abbreviations
- ELISA
Enzyme-linked ImmunoSorbent assay
- FCS
fetal calf serum
- IL
interleukin
- IP
intraperitoneal
- PBS
phosphate buffered saline
- RBC
red blood cell
- RPMI
Roswell Park Memorial Institute
- TNF
tumor necrosis factor
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem. 2002;277:34601–34609. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Hypoxia and Cyanosis. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JK, Loscalzo JL, editors. Harrison’s Principles of Internal Medicine. McGraw Hill Medical, United States of American; 2008. pp. 230–231. [Google Scholar]
- Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12:7087–7096. doi: 10.3748/wjg.v12.i44.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am. 2007;91:553–572. doi: 10.1016/j.mcna.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Chen SD, Lee JM, Yang DI, Nassief A, Hsu CY. Combination therapy for ischemic stroke: potential of neuroprotectants plus thrombolytics. Am J Cardiovasc Drugs. 2002;2:303–313. doi: 10.2165/00129784-200202050-00003. [DOI] [PubMed] [Google Scholar]
- Clarke C. Acute mountain sickness: medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad Med J. 2006;82:748–753. doi: 10.1136/pgmj.2006.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottin V, Donsbeck AV, Revel D, Loire R, Cordier JF. Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients. Am J Respir Crit Care Med. 1998;158:1286–1293. doi: 10.1164/ajrccm.158.4.9802119. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman JP, Laye P, Parnet P, Pousset F. Cytokine effects on behavior. In: Ader R, Felten L, Cohen N, editors. Psychoneuroimmunology. Academic Press; 2001. pp. 703–727. [Google Scholar]
- Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- Dreyer MG, Juge-Aubry CE, Gabay C, Lang U, Rohner-Jeanrenaud F, Dayer JM, Meier CA. Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor kappa B/PU.1 binding site. Biochem J. 2003;370:591–599. doi: 10.1042/BJ20021270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel W, Morrison MH, Ayala A, Chaudry IH. Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol. 1995;269:R160–6. doi: 10.1152/ajpregu.1995.269.1.R160. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–42. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- Fain JN, Tichansky DS, Madan AK. Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metabolism. 2006;55:1113–1121. doi: 10.1016/j.metabol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Burggren WW. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid Redox Signal. 2007;9:1339–1352. doi: 10.1089/ars.2007.1704. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Bonnekoh P, Miyazawa T, Hossmann KA, Kreutzberg GW. Immunocytochemical study of an early microglial activation in ischemia. J Cereb Blood Flow Metab. 1992;12:257–269. doi: 10.1038/jcbfm.1992.36. [DOI] [PubMed] [Google Scholar]
- Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002a;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- Grosfeld A, Zilberfarb V, Turban S, Andre J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia. 2002b;45:527–530. doi: 10.1007/s00125-002-0804-y. [DOI] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y. Leptin induces IL-1 receptor antagonist expression in the brain. Biochem Biophys Res Commun. 2002;294:215–219. doi: 10.1016/S0006-291X(02)00486-2. [DOI] [PubMed] [Google Scholar]
- Hu X, Nesic-Taylor O, Qiu J, Rea HC, Fabian R, Rassin DK, Perez-Polo JR. Activation of nuclear factor-kappaB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J Neurochem. 2005;93:26–37. doi: 10.1111/j.1471-4159.2004.02968.x. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Sherry CL, York JM, Freund GG. Acute Hypoxia, Diabetes, and Neuroimmune Dysregulation: Converging Mechanisms in the Brain. Neuroscientist. 2008;14:235–239. doi: 10.1177/1073858407309544. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Dantzer R, Freund GG. Inhibition of vagally mediated immune-to-brain signaling by vanadyl sulfate speeds recovery from sickness. Proc Natl Acad Sci USA. 2005;102:15184–15189. doi: 10.1073/pnas.0507191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Propes MJ, Shavit Y. Corticosterone modulates behavioral and metabolic effects of lipopolysaccharide. Am J Physiol. 1996;270:R192–8. doi: 10.1152/ajpregu.1996.270.1.R192. [DOI] [PubMed] [Google Scholar]
- Jones D, Duke G, Green J, Briedis J, Bellomo R, Casamento A, Kattula A, Way M. Medical emergency team syndromes and an approach to their management. Crit Care. 2006;10:R30. doi: 10.1186/cc4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge-Aubry CE, Meier CA. Immunomodulatory actions of leptin. Mol Cell Endocrinol. 2002;194:1–7. doi: 10.1016/s0303-7207(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Kelly PT, Hlavac M, Beckert LE. Episodic hypoxemia in an airline passenger with chronic respiratory failure on supplemental oxygen. Aviat Space Environ Med. 2007;78:712–715. [PubMed] [Google Scholar]
- Knoferl MW, Jarrar D, Schwacha MG, Angele MK, Cioffi WG, Bland KI, Chaudry IH. Severe hypoxemia in the absence of blood loss causes a gender dimorphic immune response. Am J Physiol Cell Physiol. 2000;279:C2004–10. doi: 10.1152/ajpcell.2000.279.6.C2004. [DOI] [PubMed] [Google Scholar]
- Kozak W, Wrotek S, Walentynowicz K. Hypoxia-induced sickness behaviour. J Physiol Pharmacol. 2006;57(Suppl 8):35–50. [PubMed] [Google Scholar]
- Kress JP, Hall JB. Critical Care Medicine: Respiratory Critical Care. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JK, Loscalzo JL, editors. Harrison’s Principles of Internal Medicine. McGraw Hill Medical; United States of American: 2008. pp. 1678–1680. [Google Scholar]
- Krugers HJ, Knollema S, Kemper RH, Ter Horst GJ, Korf J. Down-regulation of the hypothalamo-pituitary-adrenal axis reduces brain damage and number of seizures following hypoxia/ischaemia in rats. Brain Res. 1995;690:41–47. doi: 10.1016/0006-8993(95)00585-e. [DOI] [PubMed] [Google Scholar]
- Lazovic J, Basu A, Lin HW, Rothstein RP, Krady JK, Smith MB, Levison SW. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke. 2005;36:2226–2231. doi: 10.1161/01.STR.0000182255.08162.6a. [DOI] [PubMed] [Google Scholar]
- Molina PE. Endocrine Physiology. McGraw-Hill; Ohio: 2006. pp. 257–261. [Google Scholar]
- O’Connor JC, Satpathy A, Hartman ME, Horvath EM, Kelley KW, Dantzer R, Johnson RW, Freund GG. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–4997. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- Ostadal B, Ostadalova I, Dhalla NS. Development of cardiac sensitivity to oxygen deficiency: comparative and ontogenetic aspects. Physiol Rev. 1999;79:635–659. doi: 10.1152/physrev.1999.79.3.635. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol. 2005;32:251–283. doi: 10.1385/MN:32:3:251. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Propes MJ, Johnson RW. Role of corticosterone in the behavioral effects of central interleukin-1 beta. Physiol Behav. 1997;61:7–13. doi: 10.1016/s0031-9384(96)00350-2. [DOI] [PubMed] [Google Scholar]
- Quaranta AJ, D’Alonzo GE, Krachman SL. Cheyne-Stokes respiration during sleep in congestive heart failure. Chest. 1997;111:467–473. doi: 10.1378/chest.111.2.467. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17:152–157. doi: 10.1016/s0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Sherry CL, O’Connor JC, Kramer JM, Freund GG. Augmented lipopolysaccharide-induced TNF-α production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 Map kinase. J Immunol. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- Simon BA, Moody EJ, Johns RA. Therapeutic gases: oxygen, carbon dioxide, nitric oxide, and helium. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill Medical; United States of America: 2006. pp. 390–391. [Google Scholar]
- Vernooy JHJ, Cloots RHE, Dentener MA, Wouters EFM. Suppressed pulmonary expression of leptin in lipopolysaccharide-induced acute and chronic lung inflammation. European Respiratory Review. 2006;15:207–208. [Google Scholar]
- Virues-Ortega J, Garrido E, Javierre C, Kloezeman KC. Human behaviour and development under high-altitude conditions. Dev Sci. 2006;9:400–410. doi: 10.1111/j.1467-7687.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhang W, Petrovic JM, Callaghan D, Jones A, Cui H, Howlett C, Stanimirovic D. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Antunes-Rodrigues J, Machado BH. Plasma corticosterone levels is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci. 2007;134:115–117. doi: 10.1016/j.autneu.2007.01.004. [DOI] [PubMed] [Google Scholar]