Abstract
The current study compared the potency of naloxone versus 6-alpha-naloxol to precipitate opioid withdrawal under varying conditions of morphine pretreatment history using suppression of operant responding for food reward as the index of withdrawal. Male Wistar rats trained to respond on a lever for food reward received pretreatment with either Vehicle (Morphine-Naïve), a single subcutaneous (SC) injection of 5.6 mg/kg morphine (Single Morphine), or two morphine injections at 24 hr intervals (Repeat Morphine), with varying doses of naloxone or 6-alpha-naloxol injected SC 4 hr post-morphine and 5 min prior to the 30 min test session. When responding over the entire 30 min operant session was examined, naloxone was only 5-fold more potent than 6-alpha-naloxol in suppressing operant responding under Morphine Naïve conditions, but this increased to a 65-fold potency difference after Single or Repeat Morphine pretreatment. Examination of the relative potency of these antagonists in the Early Phase of operant testing (5–15 min post-antagonist) revealed an even greater 100-fold potency difference between naloxone and 6-alpha-naloxol, but in the Late Phase of testing (25–35 min post-antagonist), this had declined to a 9-fold potency difference, comparable to the relative potency of naloxone to 6-alpha-naloxol under Morphine-Naïve conditions. The results confirm a differential potency of naloxone to its reduced conjugate 6-alpha-naloxol in vivo, and extend the observation of this phenomenon to an acute (single) pretreatment with a low dose of morphine and an additional sign of opioid withdrawal to those previously used, but also indicate that delay in onset of action of 6-alpha-naloxol to opioid receptors in the central nervous system may contribute significantly to its reduced potency relative to naloxone under certain morphine pretreatment conditions.
Keywords: Opioid Dependence, Withdrawal, Abstinence, Addiction, Naloxone, Basal Signaling Activity, Opioid Receptors
1. Introduction
Leftward shifts in opioid antagonist dose-effect functions resulting from opioid agonist exposure are a well-established quantitative index of neuroadaptive changes associated with opioid dependence (Villereal and Castro, 1979; Way et al., 1969). Using this quantitative approach, numerous human and animal studies have revealed that even a single injection of an opioid agonist can elicit a state of “acute dependence” as measured by increased potency of opioid antagonists to precipitate a variety of withdrawal signs ranging from somatic/physiological to affective/subjective (Adams and Holtzman, 1990; Azar et al., 2003; Azorlosa et al., 1994; Bickel et al., 1988; Cheney and Goldstein, 1971; Easterling and Holtzman, 1997; Easterling et al., 2000; Harris and Gewirtz, 2005; Heishman et al., 1989a, b; Kalinichev and Holtzman, 2003; Liu and Schulteis, 2004; Parker and Joshi, 1998; Schulteis et al., 1997; Schulteis et al., 2004, 2003; Shoblock and Maidment, 2006, 2007; Wang et al., 2001; Wang et al., 2004; Young, 1986; Zhang and Schulteis, 2008). As would be expected if acute dependence reflects the early stages in the development of a chronicopioid dependence state, repeated treatments with morphine at daily or weekly intervals can progressively increase the severity of withdrawal-like signs elicited upon antagonist administration (Adams and Holtzman, 1990; Azorlosa et al., 1994; Liu and Schulteis, 2004; Schulteis et al., 1999; Schulteis et al., 2004, 2003; Zhang and Schulteis, 2008).
At first glance, the most plausible explanation for antagonist-induced precipitation of withdrawal from either acute or chronic morphine would appear to be displacement of agonist from opioid receptors. However, it has been demonstrated that significant somatic (body weight loss), endocrine (increased plasma corticosterone release) and aversive stimulus (conditioned place aversion) indices of withdrawal from acute or chronic morphine pretreatment can be elicited by antagonists given up to 24–48 hr post-morphine (Kishioka et al., 1995; Parker and Joshi, 1998; Schulteis et al., 1997; Shoblock and Maidment, 2006, 2007) at a time when there is negligible amounts of morphine present in the system (Kishioka et al., 1995). Recently a possible explanation of the ability of naloxone and naltrexone potency to elicit withdrawal signs in the absence of residual morphine has been offered, based on the observation that mu and delta opioid receptors, like other G-protein-coupled receptors, demonstrate some basal signaling activity as demonstrated by G-protein-coupled second messenger activity in the absence of agonist binding (Burford et al., 2000; Sadee et al., 2005). Pretreatment with agonist can increase the level of basal signaling activity of so-called constitutively active mu and delta opioid receptors (Liu and Prather, 2002, 2001; Wang et al., 2001; Wang et al., 2004; Wang et al., 2000). Some opioid antagonists such as the commonly used naloxone and naltrexone actually take on inverse agonist properties at the constitutively active opioid receptor, and are able to suppress basal second messenger activity after agonist pretreatment, even when no agonist remains to occupy the receptor (Sadee et al., 2005; Wang et al., 2001; Wang et al., 2004). In contrast, other antagonists such as the mu-selective peptide CTAP, and naloxone/naltrexone conjugates with a reduced C atom in position 6 of the ring portion of the antagonist molecule (6-alpha- and 6-beta-naloxol and naltrexol), remain neutral antagonists, capable of blocking agonist activity at the receptor, but not altering basal signaling in the absence of agonist (Bilsky et al., 1996; Raehal et al., 2005; Sadee et al., 2005; Wang et al., 2001; Wang et al., 2004).
Interestingly, the time course of increased potency of naltrexone to precipitate withdrawal jumping after 3 days of repeated intermittent morphine injection is correlated closely with the time course of increased basal mu opioid receptor signaling in brain tissues removed from mice treated with an identical morphine regimen (Wang et al., 2004). In contrast, the redox-modified naloxone and naltrexone conjugates which lack inverse agonist properties in vitro, precipitate withdrawal jumping after either single treatment with 100 mg/kg morphine or chronic morphine treatment (repeated injection 20–30 mg/kg or pellet implantation) only at times when agonist remains in the system (e.g. 2–10 hr post-morphine), but elicit little if any withdrawal jumping at extended time points (20–48 hr) where naloxone or naltrexone remain effective (Sadee et al., 2005; Shoblock and Maidment, 2006, 2007; Wang et al., 2001; Wang et al., 2004). Similar findings have been reported with additional somatic signs of withdrawal such as paw tremors, wet dog shakes, increased respiration, and increased defecation (Divin et al., 2008; Raehal et al., 2005), and with measures of the aversive motivational consequences of opioid withdrawal (e.g. conditioned place aversion; (Shoblock and Maidment, 2006, 2007). Based on these observations, Sadee and colleagues (2005) postulated that an increase in constitutively active mu opioid receptors in response to morphine pretreatment may be a primary factor in the leftward shift in potency of naloxone or naltrexone to precipitate opioid withdrawal.
However, whilst the inverse agonist properties of naloxone and naltrexone after morphine pretreatment versus neutral antagonist properties of 6-alpha- and 6-beta-naloxol and naltrexol as demonstrated in vitro are well-established (Bilsky et al., 1996; Sadee et al., 2005; Wang et al., 2001; Wang et al., 2004), it is not entirely clear that relative potency differences of these compounds to precipitate withdrawal in vivo are accounted for entirely by an agonist-induced increase in constitutively active opioid receptors. Some argue that constitutive opioid receptor activity is a “prerequisite mechanism involved in acute opioid withdrawal” (Freye and Levy, 2005), and there is evidence that weak inverse agonists or neutral antagonists exhibit little ability to precipitate somatic withdrawal at lower doses of morphine, but do elicit withdrawal after high dose morphine pretreatment (Walker and Sterious, 2005). However, others have argued that differential rate of access to opioid receptors in the central nervous system (CNS) may account for differences in potency of antagonists such as naltrexone and 6-beta-naltrexol in vivo (Divin et al., 2008).
The current study sought to further characterize the conditions under which the antagonists naloxone and 6-alpha-naloxol show differential potency in their ability to precipitate withdrawal following acute morphine pretreatment in vivo. Naloxone and its conjugate 6-alpha-naloxol were chosen based upon our laboratory’s extensive characterization of naloxone-precipitated opioid withdrawal as measured by numerous somatic (Criner et al., 2007; Schulteis et al., 1999, 1997) and negative emotional/aversive indices (Azar et al., 2003; Criner et al., 2007; Liu and Schulteis, 2004; Schulteis et al., 2004, 2003; Zhang and Schulteis, 2008). Because 6-alpha-naloxol was made available to us in only limited supply from the Research Resources Drug Supply System of the National Institute on Drug Abuse (NIDA, Bethesda, MD, USA), a single index of withdrawal, suppression of operant responding for food reward, was carefully chosen from among those we have characterized, based on the following advantages:
Suppression of operant responding has been repeatedly demonstrated as well suited to quantitative analysis of the relative magnitude of leftward shift in antagonist potency under varying pretreatment conditions (Adams and Holtzman, 1990; Schulteis et al., 1997; Schulteis et al., 1994; Schulteis et al., 2004, 2003; Young, 1986);
Antagonist-induced suppression of operant responding is observed after pretreatment with very low doses of morphine (1.0–5.6 mg/kg; (Schulteis et al., 1997; Schulteis et al., 2004, 2003) relative to those used in prior acute dependence studies (20–180 mg/kg; (Divin et al., 2008; Raehal et al., 2005; Sadee et al., 2005; Walker and Sterious, 2005; Wang et al., 2001; Wang et al., 2004)), thereby permitting a direct test of the hypothesis that weak inverse agonists or neutral antagonists cannot effectively precipitate withdrawal after low dose agonist pretreatment (Walker and Sterious, 2005); and
Collection of data in discrete epochs across the 30 min operant session enables examination of possible time-dependent differences in naloxone versus 6-alpha-naloxol potency (e.g. early [Min 1–10] versus late [Min 21–30] phases of testing), thereby providing further evaluation of the possibility that differential rate of access of these compounds to opioid receptors in the CNS may contribute to observed in vivo potency differences for withdrawal precipitation.
2. Material and methods
2.1 Animal Subjects
Male Wistar rats (n = 109, Harlan Labs, Livermore, CA, USA) weighing 300–400 g at the time of testing were used. All rats were group housed (2–3/cage) in a temperature- and humidity-controlled room with a 12 hour light/12 hour dark cycle (lights on at 06:00). Once operant training began, rats were maintained on 15 g/rat of standard rat chow per day in addition to the food pellets earned in the operant boxes (total food intake was approximately 20–22 g/rat/day), but had ad libitum access to water at all times except during the 30 min operant sessions. All training and testing took place between 12:00 and 16:30. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the VA San Diego Healthcare System, an AAALAC-accredited facility, and are in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (revised 1996).
2.2 Drugs
Morphine sulfate and 6-alpha-naloxol HCl were generously provided by the Research Resources Drug Supply System of the National Institute on Drug Abuse (NIDA, Bethesda, MD, USA), and naloxone HCl was purchased from Sigma (St. Louis, MO, USA). All drugs were prepared for injection in physiological saline, and all injections were made subcutaneously (SC) in a volume of 0.1 ml/100 g body weight. Morphine was administered at a dose of 5.6 mg/kg, selected from earlier work demonstrating effective induction of acute opioid dependence as measured by naloxone-precipitated withdrawal across a range of behavioral and somatic signs, including suppression of operant responding for food (Amitai et al., 2006; Azar et al., 2003; Liu and Schulteis, 2004; Schulteis et al., 2004, 2003; Zhang and Schulteis, 2008). Doses of all drugs are expressed as the salt.
2.3 Operant training and testing regimen
Fourteen operant chambers (Coulbourn Instruments, Columbus, OH, USA) served as the training and testing environments. Each chamber was housed inside a sound-attenuated cubicle and contained a food hopper located 4 cm above the grid floor, a lever located to the right of the food hopper, and a cue light located above the lever. Each time a rat completed a fixed-ratio (FR) component, the cue light was illuminated for 1 sec as a food pellet (45 mg) was delivered to the hopper. Rats were trained to lever press for food pellets in 30 min sessions five days a week, beginning on an FR-1 schedule and progressing to an FR-15 schedule (1 sec timeout).
Once stable baseline operant response rates were established (defined as less than 10% variation from the mean of three consecutive test days, rats were habituated to drug injection procedures prior to the onset of drug testing. During the habituation week, operant sessions on Monday and Tuesday were not preceded by any injections. However, on the final 3 days of the habituation week (Wednesday through Friday), rats received a subcutaneous (SC) injection of 0.9 % saline vehicle (0.1 ml/100 g body weight) 4 hr prior to the daily operant session. Five min prior to the operant session, each rat received an additional SC vehicle injection, and was immediately placed into the operant chambers. Levers were extended 5 min after placement into the test chamber and rats were allowed to respond for food pellets on the FR-15 schedule for 30 min, at which time levers were retracted to signal the end of food availability.
In the week following habituation to the injection regimen, an operant session was conducted on Monday without any injections, and rats were not tested on Tuesday. On Wednesday, rats again received vehicle both 4 hr and 5 min prior to the operant session. On Thursday, there were no operant sessions but rats were treated either with vehicle (Morphine Naïve and Single Morphine conditions) or 5.6 mg/kg of morphine (Repeat Morphine condition) at the same time of day that the early injection was given on all other days. On Friday, Morphine Naïve rats again received a vehicle injection, followed 4 hours later by a dose of naloxone (0.033 – 10 mg/kg) or 6-alpha-naloxol (0.33– 33 mg/kg) 5 min prior to an operant session. Rats in both the Single and Repeat Morphine conditions received 5.6 mg/kg morphine 4 hr prior to a dose of one of the antagonists and the operant session. This interval between morphine and naloxone has been shown to produce maximal withdrawal as measured by suppression of operant responding in prior studies of acute morphine dependence (Adams and Holtzman, 1990; Schulteis et al., 1997; Schulteis et al., 2004, 2003; Young, 1986). Also, increased basal signaling of mu opioid receptors appears to peak at 2–4 hr post-morphine treatment (Wang et al., 2001; Wang et al., 2004).
Response rates on the days of drug treatment were expressed as a percentage of the baseline response rate following vehicle pretreatment on the Wednesday prior to initial drug treatment. Each dose of naloxone or 6-alpha-naloxol was tested in a separate cohort of rats from the Morphine Naïve, Single Morphine, and Repeat Morphine groups. All cohorts tested with naloxone had a sample size of 5–6, but limited availability of the 6-alpha-naloxol compound from NIDA permitted 5–6 rats/cohort at lower doses (0.33, 1.0, 3.3 mg/kg) but dictated fewer animals tested (n=3–4) at the highest doses of the drug (10 and 33 mg/kg).
2.4 Data Analysis
Mean baseline response rates across treatment groups ranged from 77.05 ± 5.90 to 90.08 ± 9.57 responses/min. Data for each antagonist under each morphine pretreatment condition (Morphine Naïve, Single Morphine, Repeat Morphine) on the test days were expressed as percent of each group’s baseline response rate in 4 test epochs: 1) the entire 30 min test session; 2) Early test phase (Min 1–10 of operant session; 5–15 min post-antagonist); 3) Middle test phase (Min 11–20; 15–25 min post-antagonist); and 4) Late test phase (Min 21–30; min 25–35 post-antagonist). To minimize animal subject requirements and conserve our limited supply of 6-alpha-naloxol while ensuring that each drug was tested under the linear portion of its dose-effect function under all conditions possible, different dose ranges for naloxone and 6-alpha-naloxol were tested under different treatment conditions. Consequently, all dose response data could not be entered into a single overall ANOVA analysis with both drugs compared at all doses. Instead, quantitative probit dose-response analysis was conducted according to the method of Litchfield and Wilcoxon (Tallarida and Murray, 1987). This was done for data from the entire 30 min session, as well as data from each of 3 separate epochs of testing, Early, Middle and Late Phases. Using this procedure, ED50 values (and 95% confidence limits) for naloxone and 6-alpha-naloxol were calculated under each experimental condition, and potency ratios (with 95% confidence limits) served as the measure of statistical reliability of any observed differences in potency of the two antagonists under different pretreatment conditions and at different times post-administration. This same approach has been used previously in our laboratory in quantitative analyses of naloxone-precipitated withdrawal from acute (Azar et al., 2003; Schulteis et al., 2005; Schulteis et al., 2003) and chronic (Schulteis et al., 1994) morphine dependence.
3. Results
3.1 Relative potency of naloxone to 6-alpha-naloxol over the full 30 min operant session
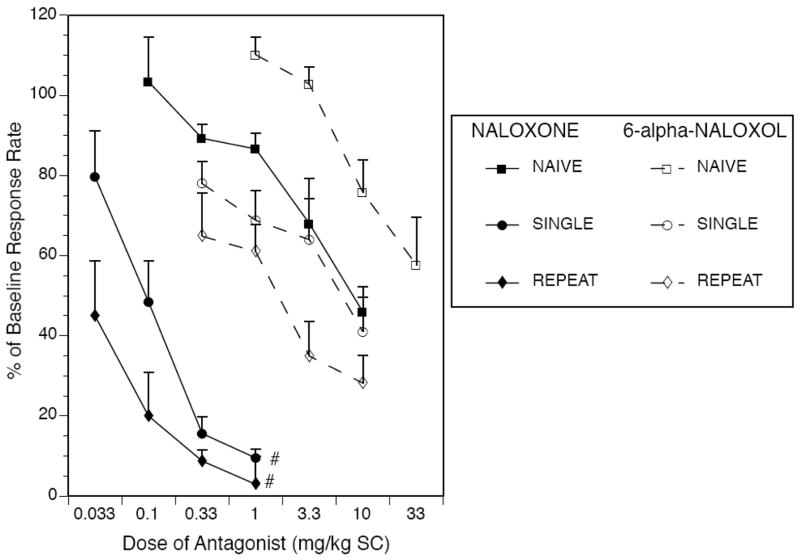
As shown in Figure 1, both naloxone and 6-alpha-naloxol produced an expected dose-dependent reduction in operant responding under all morphine treatment conditions, with left-ward shifts in the dose response curve for each antagonist as a function of morphine treatment history (i.e. from Morphine Naïve to Single Morphine to Repeat Morphine conditions). Calculation of ED50 values for each antagonist under each morphine treatment condition followed by relative potency analysis of naloxone to 6-alpha-naloxol (Table 1, 1st column of data) revealed that the potency of naloxone to suppress operant responding over the entire 30 min test session was slightly and non-significantly greater than that of 6-alpha-naloxol under Morphine Naïve conditions (5.3-fold difference, N.S.). However, there was a disproportionately greater shift in potency of naloxone versus 6-alpha naloxol following Single (65.2-fold, p < 0.05) and Repeat Morphine pretreatment (64.2-fold, p < 0.05).
Fig. 1.
Both naloxone (filled symbols, solid lines) and 6-alpha-naloxol (open symbols, dashed lines) produced dose-dependent suppression of operant responding under all treatment conditions over a 30 min operant session. A nonsignificant 5-fold difference in relative potency of naloxone to 6-alpha-naloxol under Morphine Naïve conditions grew to a significant 65-fold potency difference after Single or Repeat Morphine pretreatment (# p< 0.05, relative potency analysis by method of Litchfield and Wilcoxon using computer software of Tallarida and Murray, 1987). Exact ED50 values and potency ratios with 95% confidence limits derived during the relative potency analysis are provided in Table 1.
Table 1.
ED50 Valuesa, Potency Ratios of Naloxone to 6-Alpha-Naloxolb, and 95% Confidence Intervals for Antagonist-Induced Suppression of Operant Response Rates as a Function of Morphine Treatment History and Time Post-Antagonist.
| Entire 30 min session | Early (Min 1–10) | Middle (Min 11–20) | Late (Min 21–30) | |
|---|---|---|---|---|
| Morphine Naïve | ||||
| Naloxone ED50 | 8.1 (1.9 -- 34.7) | N.D. | 7.5 (1.1 -- 49.1) | 4.3 (0.9 -- 47.7) |
| 6-alpha-Naloxol ED50 | 42.8 (6.2 -- 298) | N.D. | N.D. | 27.8 (4.3 -- 195) |
| Potency Ratioc | 5.3 (0.5 -- 59.6), N.S | N.D. | N.D. | 8.4 (1.6 -- 43.8)* |
| Single Morphine | ||||
| Naloxone ED50 | 0.1 (0.04 -- 0.3) | 0.2 (0.1 -- 0.6) | 0.07 (0.03 -- 0.2) | 0.06 (0.02 -- 0.2) |
| 6-alpha-Naloxol ED50 | 6.5 (0.8 -- 55.9) | 22.5 (2.2 -- 225) | 4.5 (0.4 -- 52.5) | 0.6 (0.1 -- 2.6) |
| Potency Ratioc | 65.2 (6.0 -- 707)* | 105 (8.3 -- 1337)* | 68.6 (5.0 -- 943)* | 9.0 (1.5 -- 56.1)* |
| Repeat Morphine | ||||
| Naloxone ED50 | 0.02 (0.01 -- 0.1) | 0.05 (0.02 -- 0.2) | 0.02 (0.003 -- 0.10) | 0.01 (0.005 -- 0.1) |
| 6-alpha-Naloxol ED50 | 1.4 (0.2 -- 9.5) | 6.4 (1.4 -- 29.6) | 0.9 (0.3 -- 3.0) | 0.1 (0.02 -- 0.7) |
| Potency Ratioc | 64.2 (5.4 -- 759)* | 119 (16.3 -- 871)* | 50.3 (5.2 -- 491)* | 9.6 (1.9 -- 51.4)* |
ED50 values in mg/kg (95% confidence interval in parentheses)
Potency ratio of naloxone to 6-alpha-naloxol (95% confidence interval in parentheses)
N.D. = not determined, too few data points between ED16 and ED84 (linear portion of dose-response curve) to permit analysis
N.S. = potency ratio not significant
p < 0.05, potency ratio of naloxone to 6-alpha-naloxol
3.2 Change in potency of naloxone or 6-alpha-naloxol across Early, Middle, and Late phases of the operant session
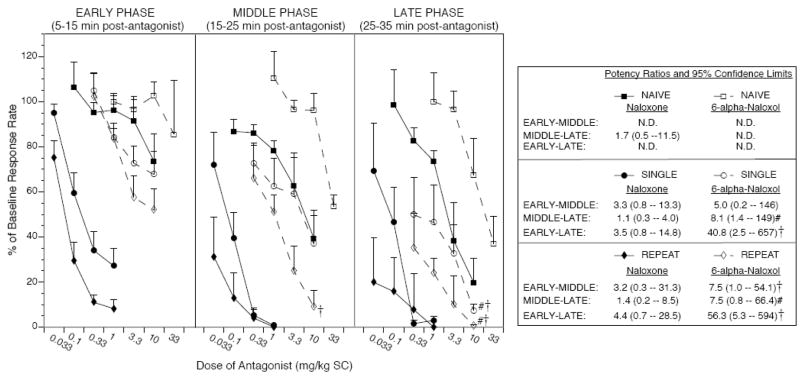
As shown in Figure 2, naloxone and 6-alpha-naloxol produced dose-dependent suppression of responding under Single and Repeat Morphine conditions across all phases of operant testing (10 min epochs of the operant session, Early, Middle, and Late). Both antagonists also produced dose-dependent suppression of responding in the Middle and Late Phases of operant testing in Morphine Naïve rats, but there was no clear dose-dependence for 6-alpha-naloxol in the Early Phase of testing up to the highest dose of employed (33 mg/kg). Calculation of ED50 values (Table 1, data columns 2–4) followed by relative potency analysis of each antagonist across phases of the test cycle (see inset to Figure 2 for details) revealed that only 6-alpha-naloxol showed significant increases in potency as the test session progressed. For example, 6-alpha-naloxol potency increased 41-fold from Early to Late Phase of testing after Single Morphine pretreatment, and 56-fold from Early to Late Phase of testing after Repeat Morphine pretreatment (p’s < 0.05). In contrast, naloxone potency ratios from Early to Late Phases of testing were 3.5 and 4.4 after Single and Repeat Morphine pretreatment, respectively, and these ratios were not statistically significant. Inability to calculate ED50 values in the Early phases of testing under Morphine Naïve conditions (too few doses tested fell within the linear portion of the dose-effect function, ED16 to ED84) precluded relative potency analysis in this treatment condition.
Fig. 2.
Suppression of responding by naloxone (filled symbols, solid lines) and 6-alpha-naloxol (open symbols, dashed lines) as a function of test phase. Under Single or Repeat Morphine conditions, 6-alpha-naloxol showed a significant 41–56-fold increase in potency from Early (5–15 min post-antagonist) to Late (25–35 min post-antagonist) phases of testing, whereas naloxone showed only a modest, nonsignificant increase in potency across these phases of testing. Potency ratios (method of Litchfield and Wilcoxon, see Tallarida and Murray, 1987) for the effects of each antagonist compared across phases of testing are shown in the legend on the right. †p < 0.05 vs. corresponding treatment condition in the Early Phase of testing; #p < 0.05 vs. corresponding treatment condition in the Middle Phase of testing. The differential increase of 6-alpha-naloxol but not naloxone potency as a function of time post-antagonist administration resulted in large potency differences between the two antagonists in the Early Phase of testing (105–119-fold after Single or Repeat Morphine, respectively, see Table 1 for details), but a much reduced potency difference between antagonists in the Late Phase of testing (roughly 9-fold after Single or Repeat Morphine, see Table 1 for details). The relative potency of naloxone to 6-alpha-naloxol in the Late Phase of testing under Morphine Naïve conditions was also about 9-fold (see Table 1), but potency ratios could not be reliably calculated for one or both antagonists in the earlier phases of testing because there were insufficient data points within the linear portion of the dose function (between ED16 and ED84) to permit ED50 estimation and relative potency analysis. (N.D. = not determined). Thus, the large (> 100-fold) potency difference between naloxone and 6-alpha-naloxol under Single and Repeat Morphine conditions observed Early in testing was almost entirely eliminated by the final 10 min of the operant session, consistent with a possible delayed access of the 6-alpha-naloxol compound relative to naloxone to opioid receptors in the central nervous system.
3.3 Relative potency of naloxone to 6-alpha-naloxol as a function of test phase
The significant time-dependent increase in potency of 6-alpha-naloxol but not naloxone as a function of test phase resulted in dramatic changes in relative potency of naloxone to 6-alpha-naloxol across 10 min epochs of the operant session. There was a progressive decline in the potency ratio of naloxone to 6-alpha-naloxol from the Early, Middle, and Late Phases of testing. Thus, in the first 10 min of the operant session (5–15 min post-antagonist administration), naloxone was 105- and 119-fold more potent than 6-alpha-naloxol after Single and Repeat Morphine, respectively (see Table 1). However, in the final 10 min of testing (25–35 min post- antagonist), naloxone was only about 9-fold more potent than 6-alpha-naloxol after either Single or Repeat Morphine pretreatment. Relative potency of naloxone to 6-alpha-naloxol in the Middle Phase of the test session (50–68-fold potency difference 15–25 min post-antagonist) was comparable to that observed with data from the entire 30 min session (Table 1 and Figure 1). It appears that time post-administration has a large effect on relative potency of naloxone to 6-alpha-naloxol, but that doubling the morphine exposure history does not significantly alter relative potency within any given phase of the operant session.
4. Discussion
The current findings extend the range of conditions under which morphine pretreatment produces greater apparent shifts in the potency of opioid antagonists with inverse agonist potential than neutral antagonists with nearly identical physicochemical properties to precipitate opioid withdrawal in vivo. As shown in Figure 1, both naloxone and 6-alpha-naloxol elicited significant dose-dependent suppression of responding when administered 4 hr after Single or two Repeat 5.6 mg/kg morphine injections. Naloxone was only 5-fold more potent than 6-alpha-naloxol in suppressing operant responding under Morphine-Naïve conditions, but was 65-fold more potent after Single or Repeat Morphine pretreatment (see Table 1), suggesting that morphine pretreatment contributes significantly to the in vivo relative potency difference between naloxone and its reduced conjugate. This outcome would be entirely consistent with the hypothesis of Sadee and colleagues (Raehal et al., 2005; Sadee et al., 2005; Wang et al., 2001; Wang et al., 2004), who argue that this relative potency difference is accounted for by a significant increase in constitutively active opioid receptors, at which naloxone can suppress basal signaling as an inverse agonist, whereas the neutral antagonist 6-alpha-naloxol can only block exogenous or endogenous opioid agonist binding. Notably, while in the present study there was a disproportionate shift in the relative potency of naloxone to 6-alpha-naloxol in the present study from Morphine Naïve to Single Morphine conditions, the relative potency of these compounds after Single versus Repeat Morphine pretreatment were identical (see Table 1). Moreover, earlier studies with chronic morphine treatment regimens indicated similar relative potency of naltrexone versus 6-beta-naltrexol (50–100 fold) to elicit somatic signs of opioid withdrawal (Raehal et al., 2005; Wang et al., 2004; Wang et al., 2000). This suggests that the relative potency of naloxone/naltrexone to their reduced conjugates to precipitate withdrawal may remain relatively constant across a range of opioid dependence induction conditions from acute (single-dose) to repeated intermittent to chronic exposure. Importantly, our observation that 6-alpha-naloxol exhibited dose-dependent suppression of operant responding after acute pretreatment with a low-dose (5.6 mg/kg) of morphine suggests that neutral antagonists can precipitate withdrawal across the full spectrum of conditions that support induction of acute or chronic morphine dependence, rather than just higher dose or chronic agonist treatment regimens as has been argued elsewhere (Walker and Sterious, 2005).
The current findings represent an extension beyond conditioned place aversion and somatic signs such as withdrawal jumping as reliable opioid withdrawal signs with which differential potency of naloxone/naltrexone versus their 6-alpha/beta reduced conjugates can be observed (Raehal et al., 2005; Shoblock and Maidment, 2006, 2007; Wang et al., 2001; Wang et al., 2004), thereby suggesting generality of the phenomenon across an expanding range of withdrawal indices. Future extension of such findings to additional negative emotional signs of withdrawal such as dysphoria-like elevations in brain reward thresholds (Easterling and Holtzman, 1997; Easterling et al., 2000; Liu and Schulteis, 2004), and anxiety-like behavior in the elevated plus maze (Zhang and Schulteis, 2008) could further confirm generality.
As shown in Figure 2, detailed analysis of the time course of antagonist effect across the operant session revealed that relative potency of naloxone to 6-alpha-naloxol in the present study varied inversely as a function of time post-injection (Figure 2, Table 1). This was not accounted for by a loss of naloxone potency over time; although naloxone administered after Single or Repeat Morphine was only modestly (about 4-fold, not significant by relative potency analysis) more potent in the Late Phase of operant testing than in the Early Phase, there was no evidence of a decrement in naloxone potency across the 30 min of testing, indicating that the duration of naloxone’s effect was sufficient to cover the entire test session. In contrast, the effects of 6-alpha-naloxol grew progressively from the Early to Middle to Late Phases of testing. There were significant 41- and 56-fold increases in 6-alpha-naloxol potency from Early to Late Phases of testing after Single or Repeat Morphine pretreatment, respectively. As a result of this differential time-dependent increase in 6-alpha-naloxol but not naloxone potency, naloxone exhibited more than 100-fold greater potency to suppress operant responding for food reward than its conjugate 6-alpha-naloxol in the first 10 min of the operant test session (i.e. 5–15 min post-antagonist injection), but only 9-fold greater potency in the final 10 min of the session (i.e. 25–35 min post-antagonist).
The initial relative potency of naloxone to 6-alpha-naloxol within 15 min of antagonist injection corresponds closely to observed 50–100-fold relative potency differences of naltrexone to 6-beta-naltrexol to precipitate withdrawal jumping, wet dog shakes, and paw tremors in mice after acute high dose or repeated/chronic morphine treatment; these signs were typically assessed in the first 20-min post-antagonist injection (Divin et al., 2008; Raehal et al., 2005; Wang et al., 2001; Wang et al., 2004). By the Late Phase of testing (25–35 min post-antagonist injection), the potency difference of naloxone to 6-alpha-naloxol was similar (about 9-fold) under Morphine Naïve, Single, and Repeat Morphine conditions. The similar potency ratios in Morphine Naïve and morphine-pretreated rats observed in the present study must be interpreted with caution, since naloxone and naltrexone effects in Morphine Naïve rats, including suppression of responding and discriminative stimulus effects, may not be mediated by action at opioid receptors, especially at higher doses needed to produce effects in the absence of morphine pretreatment (Carter and Leander, 1982; France and Woods, 1987). For example, there is evidence that naltrexone-induced suppression of operant responding at high doses of the antagonist administered to morphine naïve rats may be mediated at least in part by action at GABA receptors (Gewiss et al., 1994; Negus, in press; Schindler et al., 1992). It is nonetheless noteworthy that the observed 9-fold potency difference of naloxone to 6-alpha-naloxol in the Late Phase of testing following Single or Repeat Morphine is comparable to the roughly 10-fold differences reported in the literature for reversal of morphine antinociception and locomotor activity in non-dependent subjects by naltrexone versus 6-beta-naltrexol under conditions where the antagonists are administered just prior to morphine, or after peak morphine effect is reached (Divin et al., 2008; Raehal et al., 2005; Wang et al., 2001; Wang et al., 2004). This is consistent with a recent report in rhesus monkeys, wherein naltrexone, 6-alpha-naltrexol, and 6-beta-naltrexol showed similar relative potencies in a drug discrimination paradigm regardless of morphine pretreatment history (Li et al., 2008).
It has been reported that plasma concentrations of naltrexone and 6-beta-naltrexol at 10 min after intraperitoneal injection of 1 mg/kg of each compound are virtually identical, but 10-fold more of the reduced conjugate must be administered in order to achieve similar concentrations in the CNS at 10 min post-injection (Wang et al., 2004). Moreover, onset of antagonist-induced reversal of established antinociception by the long-lasting opioid agonist BU72 occurs 6-fold more rapidly with naltrexone than an equieffective dose of 6-beta-naltrexol (1 mg/kg), whilst a 10-fold higher dose of 6-beta-naltrexol produced a similar time course of reversal of morphine effect as 1 mg/kg of naltrexone (Divin et al., 2008). Combined with the present observations of progressive decline in relative potency of naloxone to 6-alpha-naloxol to precipitate suppression of operant responding in acute morphine dependence with increasing time post-administration, convergent data suggest that the reduced conjugates of naloxone and naltrexone may show a modest but significant delay in onset of action at CNS opioid receptors, and this may contribute in part to their observed 50–100-fold potency differences to precipitate withdrawal in the first 10–20 min post-injection.
In summary, the current findings are in agreement with earlier studies of 50–100-fold greater potency of naloxone/naltrexone than their 6-alpha-/beta-naloxol/naltrexol conjugates in precipitating opioid withdrawal after morphine pretreatment in the first 15–20 min post-antagonist administration. Although most prior studies have used considerably higher acute doses of morphine (e.g. 100 mg/kg), or a more chronic morphine treatment regimen, there has been a recent report in rhesus monkeys (Ko et al., 2006) that 6.4 mg/kg/day morphine for 3 days produced similar 100-fold potency difference in naltrexone and 6-beta-naltrexol to precipitate withdrawal as measured by increased respiratory parameters. In combination with the current findings, this suggests that 50–100 fold differential potency of naloxone/naltrexone to 6-alpha-/beta-naloxol/naltrexol is observed across a range of morphine pretreatment regimens from low-dose acute pretreatment to chronic dependence induction. However, the present findings also indicated that relative potency of naloxone to 6-alpha-naloxol was not constant across the time course of operant testing. By 25–35 min post-antagonist administration, naloxone was only 9-fold more potent than 6-alpha-naloxol in suppressing operant responding in rats receiving Single or Repeat Morphine pretreatment, identical to their relative potency in the absence of any morphine pretreatment (Morphine Naïve), suggesting that delayed onset of 6-alpha-naloxol displacement of residual agonist from opioid receptors in the CNS can account, at least in part, for the larger potency differences between naloxone and 6-alpha-naloxol in the first 10–20 min post-antagonist injection. In this regard, we reported previously that at 4 hr after SC administration of 5.6 mg/kg morphine, the time when all naloxone and 6-alpha-naoxol dose-response functions were determined in the present study, residual morphine levels (57.7 ± 8.3 ng/ml) reflect roughly 6% of peak plasma concentrations achieved 30 min post-morphine (Schulteis and Zhang, 2006).
Demonstrations of apparent time-dependent contributions to relative potency of naloxone (current study) and naltrexone (Divin et al., 2008) to their reduced conjugates, as summarized above, do not completely rule out a contribution of the inverse agonist properties of naloxone and naltrexone at constitutively active opioid receptors to precipitation of withdrawal under all conditions. The ability of morphine pretreatment to unmask inverse agonist properties of naloxone and naltrexone, but not their 6-alpha/beta-naloxol/naltrexol conjugates, as measured by suppression of basal signaling activity in vitro, or with ex vivo tissue derived from morphine pretreated animals, is well-established (Burford et al., 2000; Raehal et al., 2005; Sadee et al., 2005; Wang et al., 2001; Wang et al., 2004). It is possible that constitutively active opioid receptors may contribute more significantly to observed differences in potency of naloxone and naltrexone than neutral antagonists in vivo under high dose or chronic morphine pretreatment regimens (Raehal et al., 2005; Sadee et al., 2005; Shoblock and Maidment, 2006, 2007; Walker and Sterious, 2005; Wang et al., 2001; Wang et al., 2004). Another possibility is that constitutive opioid receptor activity at extended intervals post-agonist treatment may contribute to the ability of naloxone/naltrexone versus 6-alpha/beta-naloxol/naltrexol to elicit withdrawal jumping and conditioned place aversion up to 24–48 hr after cessation of chronic morphine treatment, at a time when no detectable morphine remains in the system, whereas even 100-fold higher doses of 6-alpha-naloxol or 6-beta-naltrexol are without significant effect beyond 4–8 hr post-morphine (Shoblock and Maidment, 2006, 2007; Wang et al., 2004). This cannot be easily reconciled with the view that precipitation of opioid withdrawal is strictly the result of displacement of residual morphine from the receptor. Thus, lack of significant precipitation of withdrawal by 6-alpha/beta- naloxol and naltrexol even after 100-fold greater doses than naloxone/naltrexone at 24–48 hr post-morphine appears more congruent with the observed time course of morphine-induced elevations in constitutive opioid receptor activity (Wang et al., 2004). Taken together, findings to date indicate that degree of morphine pretreatment, possible differential delays in onset of action in vivo, and induction of constitutively active opioid receptors are all factors interacting in determining the relative potency to precipitate opioid withdrawal of 6-alpha/beta-naloxol and naltrexol to the classic opioid antagonists naloxone and naltrexone. Further work to disentangle the relative contributions of these various factors will be crucial to proper identification of the potential for clinical use of 6-alpha/beta-naloxol and naltrexol in treatment of opioid overdose and long-term management of addiction (Sadee et al., 2005).
Acknowledgments
This study was supported by PHS grant DA10475 (National Institute on Drug Abuse) and a Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Award to GS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253:483–489. [PubMed] [Google Scholar]
- Amitai N, Liu J, Schulteis G. Discrete cues paired with naloxone-precipitated withdrawal from acute morphine dependence elicit conditioned withdrawal responses. Behav Pharmacol. 2006;17:213–222. doi: 10.1097/00008877-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Stitzer ML, Greenwald MK. Opioid physical dependence development: effect of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl) 1994;114:71–80. doi: 10.1007/BF02245446. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 1988;244:126–132. [PubMed] [Google Scholar]
- Bilsky EJ, Bernstein RN, Wang Z, Sadee W, Porreca F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J Pharmacol Exp Ther. 1996;277:484–490. [PubMed] [Google Scholar]
- Burford NT, Wang D, Sadee W. G-protein coupling of mu-opioid receptors (OP3): elevated basal signalling activity. Biochem J. 2000;348(Pt 3):531–537. [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Leander JD. Discriminative stimulus properties of naloxone. Psychopharmacology (Berl) 1982;77:305–308. doi: 10.1007/BF00432760. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Goldstein A. Tolerance to opioid narcotics: time course and reversibility of physical dependence in mice. Nature. 1971;232:477–478. doi: 10.1038/232477a0. [DOI] [PubMed] [Google Scholar]
- Criner SH, Liu J, Schulteis G. Rapid neuroadaptation in the nucleus accumbens and bed nucleus of the stria terminalis mediates suppression of operant responding during withdrawal from acute opioid dependence. Neuroscience. 2007;144:1436–1446. doi: 10.1016/j.neuroscience.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divin MF, Holden Ko MC, Traynor JR. Comparison of the opioid receptor antagonist properties of naltrexone and 6 beta-naltrexol in morphine-naive and morphine-dependent mice. Eur J Pharmacol. 2008;583:48–55. doi: 10.1016/j.ejphar.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther. 1997;281:188–199. [PubMed] [Google Scholar]
- Easterling KW, Plovnick RM, Holtzman SG. Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology (Berl) 2000;148:263–271. doi: 10.1007/s002130050050. [DOI] [PubMed] [Google Scholar]
- France CP, Woods JH. Beta-funaltrexamine antagonizes the discriminative stimulus effects of morphine but not naltrexone in pigeons. Psychopharmacology (Berl) 1987;91:213–216. doi: 10.1007/BF00217065. [DOI] [PubMed] [Google Scholar]
- Freye E, Levy JV. Constitutive opioid receptor activation: a prerequisite mechanism involved in acute opioid withdrawal. Addiction Biology. 2005;10:131–137. doi: 10.1080/13556210500123019. [DOI] [PubMed] [Google Scholar]
- Gewiss MV, Marley RJ, Thorndike EB, Goldberg SR, Schindler CW. GABA receptor-linked chloride channels and the behavioral effects of naltrexone in rats. Pharmacol Biochem Behav. 1994;49:589–597. doi: 10.1016/0091-3057(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology (Berl) 2005;178:353–366. doi: 10.1007/s00213-005-2155-0. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I. J Pharmacol Exp Ther. 1989a;250:485–491. [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J Pharmacol Exp Ther. 1989b;248:127–134. [PubMed] [Google Scholar]
- Kalinichev M, Holtzman SG. Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing morphine withdrawal: similarities and differences between acute and chronic dependence. J Pharmacol Exp Ther. 2003;304:603–609. doi: 10.1124/jpet.102.044206. [DOI] [PubMed] [Google Scholar]
- Kishioka S, Inoue N, Nishida S, Fukunaga Y, Yamamoto H. No relation of plasma morphine level to the severity of naloxone-induced withdrawal in acute morphine-dependent rats. Jpn J Pharmacol. 1995;69:187–193. doi: 10.1254/jjp.69.187. [DOI] [PubMed] [Google Scholar]
- Ko MC, Divin MF, Lee H, Woods JH, Traynor JR. Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther. 2006;316:772–779. doi: 10.1124/jpet.105.094409. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, France CP. Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology (Berl) 2008;195:479–486. doi: 10.1007/s00213-007-0914-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors. J Pharmacol Exp Ther. 2002;302:1070–1079. doi: 10.1124/jpet.102.035964. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol. 2001;60:53–62. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- Negus SS. Opioid antagonist effects in animal models related to opioid abuse: Drug discrimination and drug self-administration. In: Dean RL, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists: From Bench to Clinic. New York: Humana Press; in press. [Google Scholar]
- Parker LA, Joshi A. Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav. 1998;61:331–333. doi: 10.1016/s0091-3057(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadee W, Bilsky EJ. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Sadee W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005;76:1427–1437. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Marley RJ, Goldberg SR. Enhanced sensitivity to naltrexone is associated with an up-regulation in GABA receptor function. Life Sci. 1992;50:PL1–6. doi: 10.1016/0024-3205(92)90341-l. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–242. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology (Berl) 1997;129:56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J, Amitai N, Tzeng S. Context- and cue-conditioned potentiation of acute morphine dependence and withdrawal. Pharmacol Biochem Behav. 2005 doi: 10.1016/j.pbb.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Conditioning processes contribute to severity of naloxone-precipitated withdrawal from acute opioid dependence. Psychopharmacology (Berl) 2004;175:463–472. doi: 10.1007/s00213-004-1843-5. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76:493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Zhang Z. Increased anxiety-like behavior during naloxone-precipitated withdrawal from acute opioid dependence. 2006 Meeting of the College on Problems of Drug Dependence; Scottsdale, AZ. 2006. [Google Scholar]
- Shoblock JR, Maidment NT. Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology. 2006;31:171–177. doi: 10.1038/sj.npp.1300782. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. New York: Springer-Verlag; 1987. [Google Scholar]
- Villereal J, Castro A. A reformulation of the dual-action model of opioid dependence: Opioid-specific neuronal kindling. In: Beers RF, Bassett EG, editors. Mechanisms of Pain and Analgesic Compounds. New York: Raven Press; 1979. pp. 407–428. [Google Scholar]
- Walker EA, Sterious SN. Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol. 2005;145:975–983. doi: 10.1038/sj.bjp.0706247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadee W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadee W. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther. 2004;308:512–520. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Wang D, Surratt CK, Sadee W. Calmodulin regulation of basal and agonist-stimulated G protein coupling by the mu-opioid receptor (OP(3)) in morphine-pretreated cell. J Neurochem. 2000;75:763–771. doi: 10.1046/j.1471-4159.2000.0750763.x. [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Young AM. Effects of acute morphine pretreatment on the rate-decreasing and antagonist activity of naloxone. Psychopharmacology (Berl) 1986;88:201–208. doi: 10.1007/BF00652241. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schulteis G. Withdrawal from acute morphine dependence is accompanied by increased anxiety-like behavior in the elevated plus maze. Pharmacol Biochem Behav. 2008;89:392–403. doi: 10.1016/j.pbb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]