Abstract
Objectives/Hypothesis
Normal human vocal fold fibroblast (hVFF) primary cell lines are unavailable commercially and are very difficult to acquire, subsequently little is known about their characteristics. The purpose of this study was to compare the morphologic and proliferation characteristics and gene expression of hVFFs from different aged donors.
Study Design
In vitro. Methods We developed 3 normal hVFF primary cell lines from donors aged 21 (21T), 59 (59T) and 79 (79T) years. We characterized their morphologic features, proliferative abilities, telomere lengths and their functional gene expression by quantitative real-time PCR.
Results
The 21T line maintained a typical spindle shape until passage 14 whereas 59T and 79T changed morphology to wider, shorter cells at passage 7. Proliferation rates were constant for the 21T through passage 14; 59T’s proliferative half-life was passage 9, whereas 79T maintained lower proliferation rates from passage 4. Gene expression levels for fibronectin, collagen I, collagen VI, procollagen I and elastin demonstrated similar patterns for all lines, however, relative amounts decreased with the age of donor. Telomere lengths did not show differences related with donor age.
Conclusions
hVFF primary cultures have limited proliferative capacity. The morphology, proliferation, differentiation and gene expression levels of VFF can be affected by age, but senescence patterns were similar across the ages.
Keywords: aging, age-related, human vocal fold fibroblast, morphology, proliferation, functional gene expression, telomere length, senescence
INTRODUCTION
Human adult vocal folds consist of five layers: epithelium, superficial, intermediate and deep layers of the lamina propria of the mucosa and the vocalis muscle. It has been well documented that human vocal fold fibroblasts (hVFF), the main cellular component of the lamina propria, are responsible for the maintenance, development and repair of ECM of the lamina propria1–6. hVFF in the lamina propria migrate and proliferate in response to injury and are thought to produce ECM components for wound healing of damaged lamina propria.7
Numerous investigations have described the age-related anatomic, physiological and acoustic changes of the voice and the larynx, 8–10 histopathology (light and electron microscopes) of the aging larynx 6,11–17 and more recent studies have delved into the gene expression19,20 and protein level of aged fibroblasts.18 Ding et al reported that gene expressions of collagens I, III, IV, V, MMP-2 (collagen-degrading proteinases) and tropoelastin, elastase (the proteins affecting the amount of elastin) in rat vocal folds varied with age. 19,20 Hammond et al15,16 reported changes in collagen and elastin protein levels as measured by histology across the age span in human cadevar tissue. There are little if any reports in the literature that utilize molecular methodologies to improve our understanding the changes across the lifespan in humans, nor improve understanding of the mechanisms responsible for these changes. One of the main reasons for this limitation is the scarce availability of hVFF cell lines for investigation.
The purpose of the present study was to develop three hVFFs primary cell lines, in three different age adults. Once established, we were able to compare characteristics in morphological, proliferative, gene expression and telomere lengths as a preliminary investigation to better understand vocal fold senescence.
MATERIALS AND METHODS
Normal vocal folds of three human adults (two males and one female) obtained from autopsy or surgical cases (ages, 21, 59 and 79 years) were used in this study. In all cases, the larynx and vocal folds were normal and did not have disease. For the surgical cases, larynges were received immediately after excision from the donor and for the autopsy case the larynx was received within 4 hours of death.
Primary Cell Culture
For primary cell culture, true vocal fold tissue (epithelium and lamina propria) was cut into small pieces and suspended in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.01 mg/ml streptomycin sulfate and 1x NEAA (all from Sigma, USA). Cells were grown on uncoated plastic tissue culture dishes (Focal) at 37°C in 5% CO2-humidified atmosphere. After 14 days, the adherent confluent hVFFs were trypsinized and passaged. Fibroblast categorization and identification has previously been reported with this culture methodology.21 These cells were considered as population doubling level 0 (0 PDs).
Cell Proliferation Assay
The growth curves of hVFFs were determined by the quadruplicate cell counts of trypsinized cells at daily intervals. To determine cell PD, the cells were sub-cultured until they reached post-mitotic stage. PD of the cells was calculated at the end of each passage by the formulation 2N=(Cf/Ci), where N denotes PD, Cf denotes the total cell number harvested at the end of a passage, and Ci denotes the total cell number of attached cells at seeding.
Gene Expression Analysis
Total cellular RNA was isolated from cells using an RNA extraction kit, RNeasy Mini Kit (Qiagen, CA) according to the manufacturer’s instructions. cDNA was synthesized from 1 µg of total RNA using a QuantiTect Reverse Transcription Kit (Qiagen, CA). The genes in mRNA level were quantified by real-time PCR method by using LightCycler System (Roche, IN), with amplification of β-actin as control. The primer sequences used and product sizes are listed in Table 1.
Table 1.
Primer Sequences and Products of RT-PCR
| Gene | GenBank # | Forward Primer | Reverse Primer | Size of PCR product |
|---|---|---|---|---|
| Elastin | NM_000501 | 5’-AAGCAGCAGCAAAGTTCGGT-3’ | 5’-ACTAAGCCTGCAGCAGCTCCATA-3’ | 187bp |
| Fibronectin | NM_002026 | 5’-ACCTACGGATGACTCGTGCTTTGA-3’ | 5’-CAAAGCCTAAGCACTGGCACAACA-3’ | 116bp |
| Procollagen I α-1 | NM_000088 | 5’-AATGGTGCTCCTGGTATTGCTGGT-3’ | 5’-ACCAGTGTCTCCTTTGCTGCCA-3’ | 141bp |
| Collagen I α -2 | NM_000089 | 5’-AACAAATAAGCCATCACGCCTGCC-3’ | 5’-TGAAACAGACTGGGCCAATGTCCA- 3’ | 101bp |
| Collagen VI α -3 | NM_057167 | 5’-GAAGAAGGAGTCAGCCATCG-3’ | 5’-ATTCGAGAGAACTGCCTGGA-3’ | 189bp |
| β-Actin | NM_001101 | 5’-ACGTTGCTATCCAGGCTGTGCTAT-3’ | 5’-CTCGGTGAGGATCTTCATGAGGTAGT-3’ | 188bp |
Amplification was carried out for 45 cycles, each of 95C, 10s, 55C, 5s, and 72C 8s in a 20ul reaction mixture containing 2µl cDNA, 0.5 µM of each primer, 2–3 mM MgCl2 (dependent on the target gene), dNTPs and Tag DNA polymerase from LightCycler FastStart DNA Master SYBR Green I(Roche, IN) by the LightCycler 1.5 System. Results were analyzed by the mRNA ng/µl normalized by β-actin house keeping gene.
Telomere Length Assay
Telomere length was determined by Southern blot analysis of telomere restriction fragments using the TeloTAGGG Telomere length Assay kit (Roche, IN). Genomic DNA was extracted from primary 21T, 59T, and 79T hVFFs at different time points, respectively by using DNA extraction Kit (Roche, IN) according to manufacturer’s instructions. Two micrograms of genomic DNA was digested with the restriction enzymes HinfI and RsaI, the fragmented DNA were then electrophoresed through a 0.8% agarose gel in 1 × TAE buffer at 5V/cm for 4 h. The gel was then twice denaturized in 1.5 M NaCl, 0.5 M NaOH buffer for 15 min at room temperature, and twice neutralized in 3M NaCl, 0.5 M Tris-HCl, pH 7.5 for 15 min, then transferred to positively charged nylon membrane using capillary transfer with 20 × SSC transfer buffers overnight. The transferred DNA was fixed by UV-crosslinking (120mJ/cm2) and hybridized to a digoxigenin (DIG)-labeled probe, specific for telomeric repeats. The average telomere length is determined by comparing the signals relative to a molecular weight standard according to the formula, mean TRF=Σ(ODi)/Σ(ODi/Li), where ODi is the chemiluminescent signal and Li is the length of TRF(terminal restriction fragment) at position i.
RESULTS
Morphological Characterization
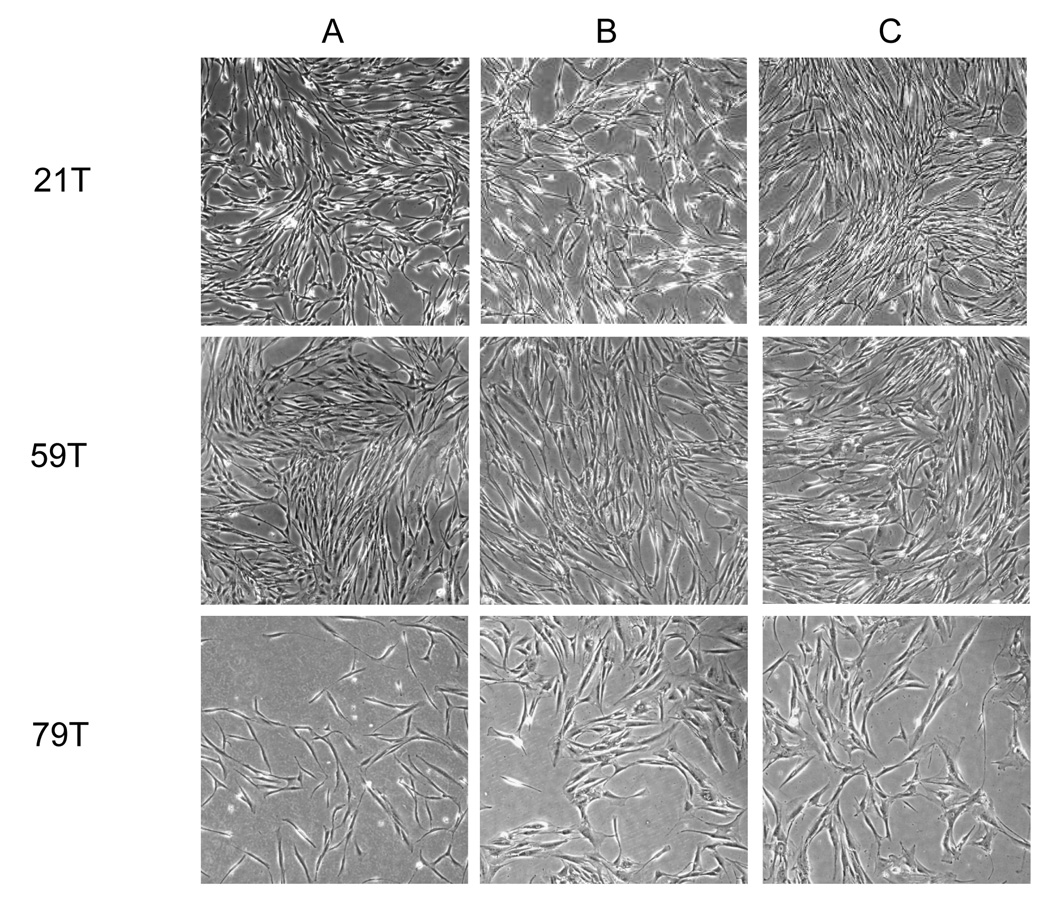
The hVFF morphology of 21T, 59T and 79T were spindle-shaped typical of fibroblasts (Figure1A). However, by passage 7, 59T and 79T cells became to shorter and wider, typical of fibroblast aging, while the 21T line maintained the fibroblastic spindle shape until passage 16 (Fiqures 1 B and C).
Figure 1. Morphology of primary hVFF from different aged-donors (21T, 59T and 79T).
Cells are shown at passage 3 (A), passage 8 (B) and passage 16 (C). The hVFF of 21T, 59T and 79T were spindle-shaped typical of fibroblasts. However, after passage 7, 59T and 79T cells became to shorter and wider in shape, while 21T line maintained the fibroblastic spindle shape until passage 16.
Growth Kinetics
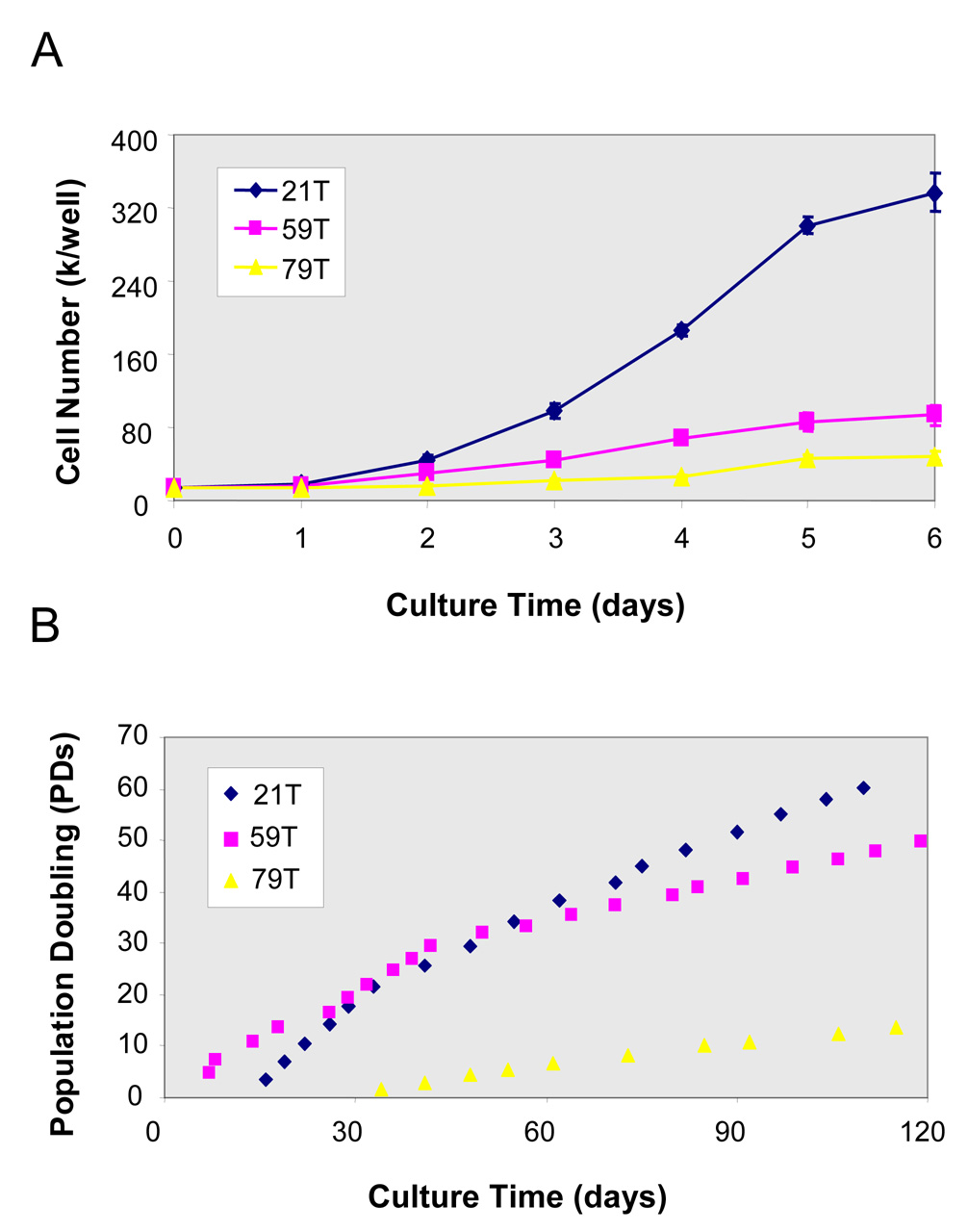
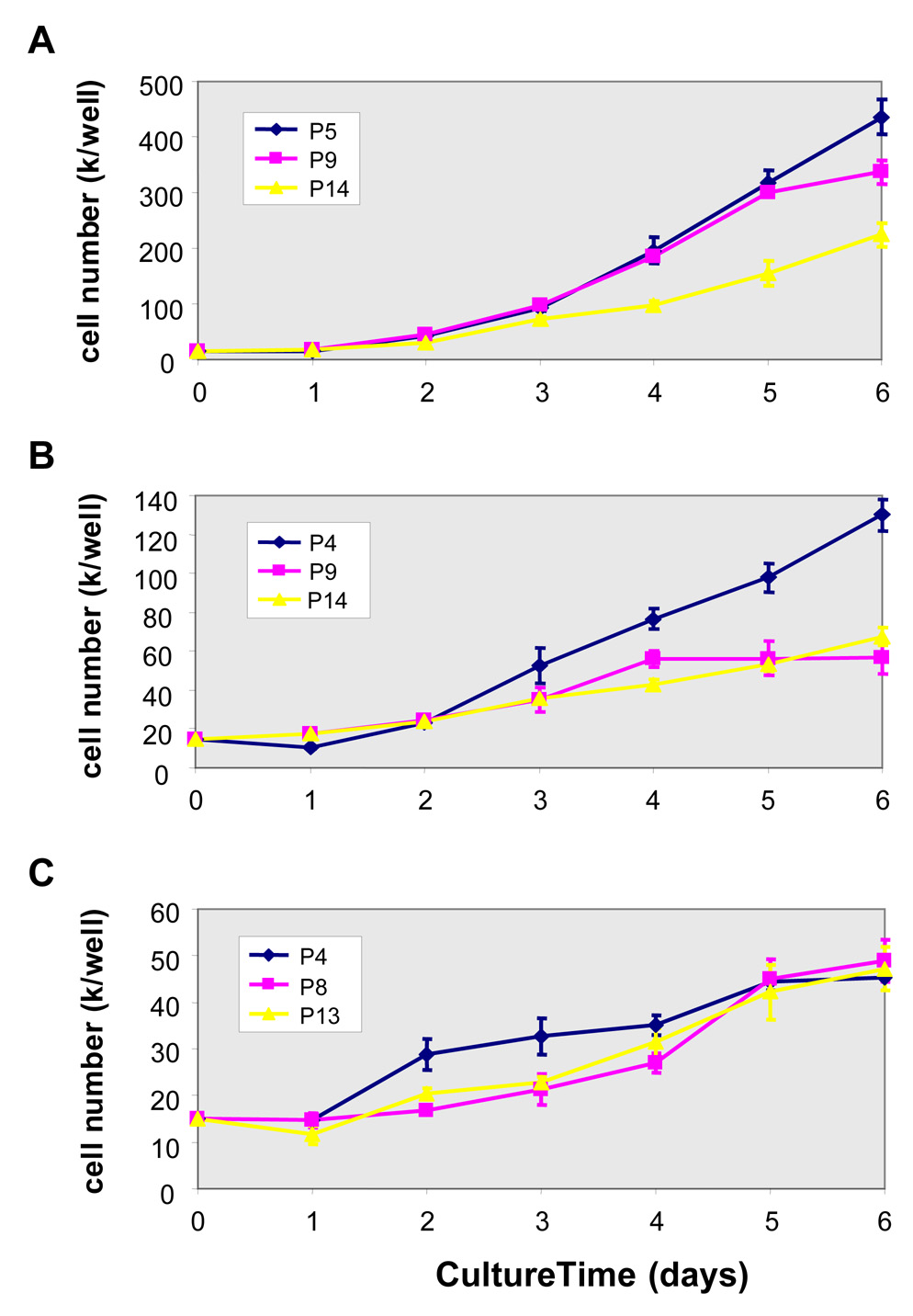
The growth kinetics of hVFFs of different aged-donors (21T, 59T and 79T) was determined by daily cell counts of trypsinized cells at passage 8–9 (Figure 2A). 21T cell line had a higher proliferation rate than the 59T and 79T lines. The long-term growth curves (population doubling level) of 21T, 59T and 79T (Figure 2B) demonstrates that the population of 21T and 59T doubled up to the end point of the study (day 120), whereas 79T cells from aged-adult had lower cell population doubling (13 PDs). Comparing the growth curves of these three cell lines, 21T and 59T cells showed high proliferation rates until passage 9 and 4, respectively (Figures 3A and 3B), while 79T maintained a lower proliferation rate from passage 4 until passage 13 (Figure 3C).
Figure 2. Growth curves of hVFFs (21T, 59T and 79T).
(A) Growth curves of hVFFs (21T, 59T and 79T) at passage 8–9. The cells were seeded onto 24-well plates as a density of 1.5 × 104 cells/well, the cell counts of parallel quadruplicate wells were made on daily basis. Values are the mean ± SD of results of 4 wells. (B) Long-time growth curves--Population doubling level (PDs) of 21T, 59T and 79T hVFFs are shown over 120 days of culture. The PD time was estimated in the logphase of growth between days 0 and 5.
Figure 3. Growth curves of 21T, 59T and 79T during different growth periods.
(A) Growth curves of 21T hVFFs at passage 5, 9, and 14. (B) Growth curves of 59T hVFFs at passage 4, 9, and 14. (C) Growth curves of 79T hVFFs at passage 4, 8, and 13. The cells were seeded onto 24-well plates as a density of 1.5 × 104 cells/well, the cell counts of parallel quadruplicate wells were made on daily basis. Values are the mean ± SD of results of 4 wells.
Genes Expression Analysis
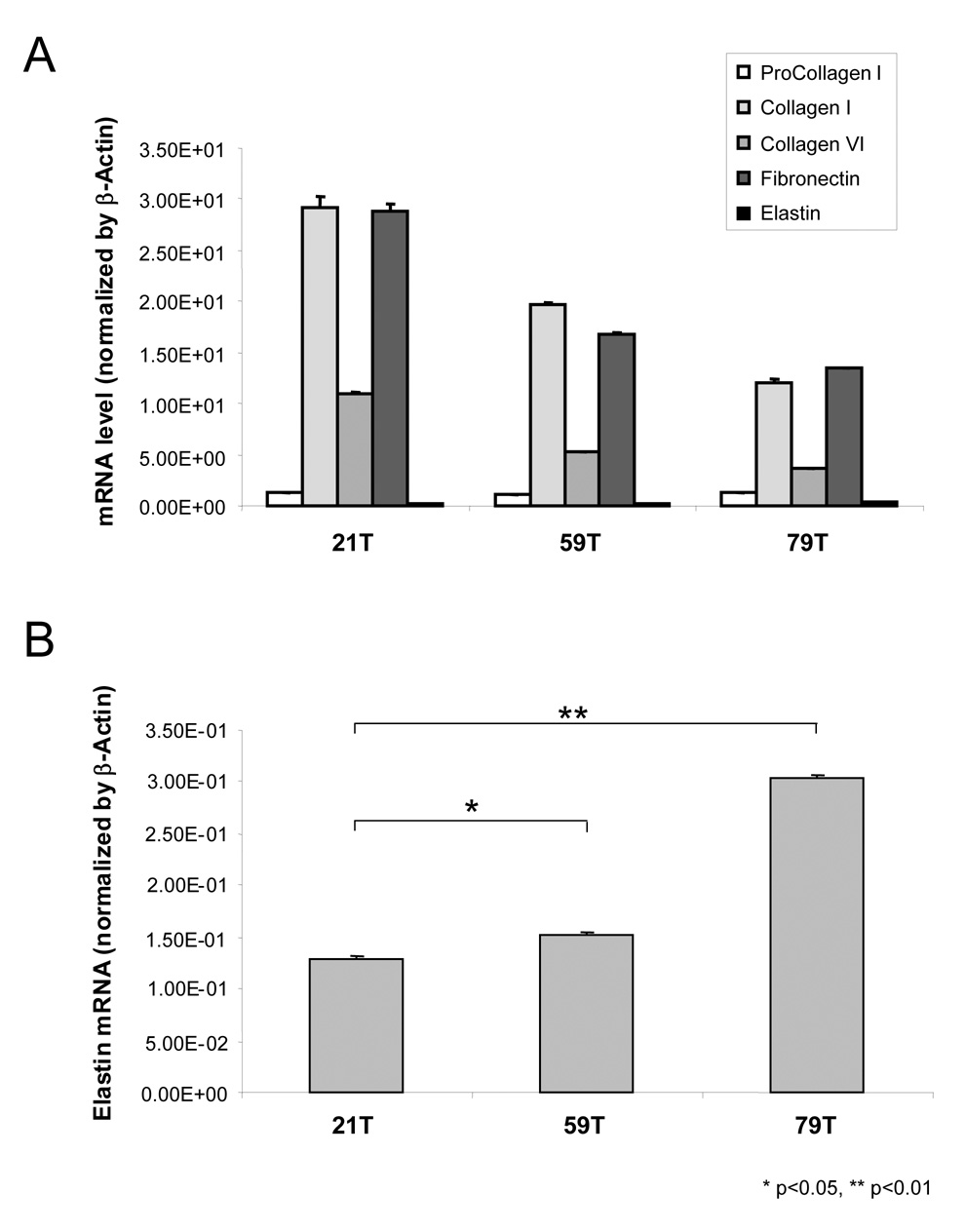
For 21T, 59T and 79T hVFFs, mRNA expression demonstrated similar patterns but with decreased mRNA levels of procollagen, collagen I, collagen VI and fibronectin genes as the donors were older (Figure 4A). Conversely, elastin mRNA levels from the eldest donor’s hVFFs increased to 238% and 201% of 21T and 59T levels, respectively (Figure 4B).
Figure 4. Gene expression of 21T, 59T and 79T hVFF.
(A) Expression of procollagen I α-1, collagen I α-2, collagen VI α-3, finbronectin and elastin genes in 21T, 59T and 79T hVFF cells. (B) Gene expression of elastin in 21T, 59T and 79T hVFF cells. All of data were normalized to β-Actin. The graph shows the ratios of each gene expression in 21T, 59T and 79T. Values are the mean ± SD of results of triplicate test.
Telomere Length Analysis
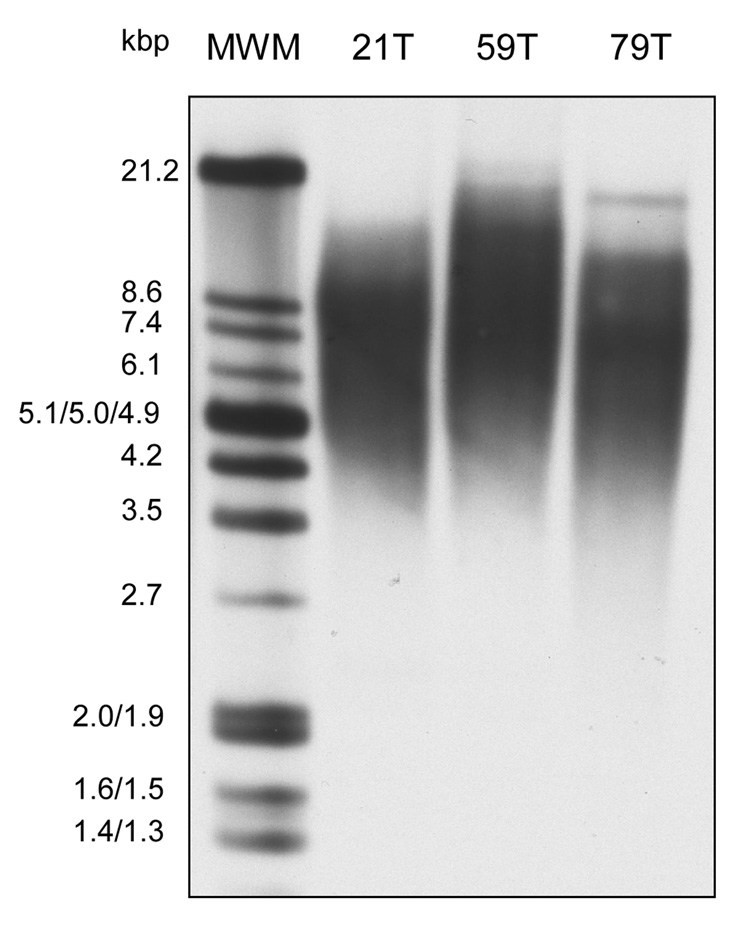
As shown in Figure 5, at passage 7, the telomere lengths from 21T, 59T and 79T hVFFs were 8.6, 8.7 and 7.0 kbp, respectively, although their proliferation rates were very different. There was a loss of telomeric DNA within each cell line across serial passages (Figure 5).
Figure 5.
Terminal restriction fragment (telomere) length in cultures human vocal fold fibroblasts (21T, 59T and 79T) during passage 7 to 18 by Southern blot analysis. Genomic DNA was isolated from hVFF of passage 7, 13 and 18, and hybridized with a telomeric probe to visualize the TRF. The first lane shows the molecular weight marker in kb. A. This experiment was repeated in triplicate.
DISCUSSION
The most important function of human vocal fold fibroblasts (hVFF) in the lamina propria is to produce intercellular substances such as collagen, elastin, fibronectin and glycoaminoglycans. It is also known that hVFF produce more intercellular substances, when in their active phase.6 The results of the present study, when taken as a whole, indicate that hVFF from a young adult are more active (as measured by population doublings), producing more collagen and fibronectin than those hVFF from a aged-adult or geriatric vocal folds. Previously, it was reported that hVFF in geriatric vocal folds may be less active than in adults of younger ages because of the degree of development of their rough endoplasmic reticulum (rER) and Golgi apparatus (GA). 11 Our results corroborate these findings that indeed younger show higher growth, proliferation and differentiation rates than hVFF from an aged adult or geriatric donor.
There exists, an interest, in the literature regarding collagen and elastin, their change in expression across the lifespan and correlating these to changes measured in vocal quality. In a previous rat study, mRNA levels of collagen I, III, IV, and V significantly decreased with age.20 Our present study confirmed this phenomenon for procollagen 1, collagen I and VI, in humans, utilizing similar methodology. In contrast, quantitative histological studies observed increased collagen in the vocal folds of an elderly population.14 Our results demonstrates that less collagen is being made, but perhaps hypothetically, less collagen is being broken down by the enzyme collagenase, as it too may have decreased gene expression levels. If less collagenase is present than collagen may not break down as quickly, corresponding to increased levels measured histologically. The increased levels of collagen measured in the elderly maybe explain in part stiffness that has been reported 20 that occurs with aging in human vocal fold. Our findings of increases in elastin gene expression as the age of the donor increases complements previous studies that have measured increases in protein levels of elastin vocal folds with increases with age, in humans16 and in rats.19 As discussed in Hammond et al16 the increases levels of elastin in geriatric samples may not necessarily imply as increase in elasticity.
Collagen VI distribution and function in the vocal folds has not been previously reported. Recently, high levels of collagen VI mRNA has been measured in muscle interstitial fibroblasts, implying that collagen VI may play an important role in the pathogenesis of some muscular diseases.22 We found that the hVFF expressed rather high levels of collagen VI. As a first step, immunohistochemical studies need to be completed to determine if there is posttranslational modification of collagen VI such that it is a component of the vocal fold lamina propria.
Human cells can undergo only a limited number of divisions in vitro. This phenomenon is termed replicative senescence. Senescence in primary human cells may represent a checkpoint rather than an absolute limit to cell proliferation. 28 Telomere shortening could be one of the signals that trigger a checkpoint to cause cell cycle exit. Telomeres are specialized structures at the ends of eukaryotic chromosomes, consisting of proteins and simple repeated DNA sequences which are highly conserved throughout evolution. 29 Shorter length is representative of increasing age, as cells have undergone multiple rounds of replication. In this study, we found that although there were different proliferation abilities of hVFF across age their telomere lengths were similar. This result is similar to our previous work, which demonstrated that donor age was not significantly associated with telomere length across different tissue types. 30 There was loss of telomeric DNA across passages, within cell lines. This can be explained by incomplete copying of the template strands at the 3’ terminal and incomplete replication, degradation of termini (specific or nonspecific), and unequal recombination coupled to selection of cells with shorter telomeres. 31
CONCLUSIONS
This study demonstrates that hVFFs have limited proliferative capacity, differing morphology and different gene expression levels depending upon the donor age. This preliminary investigation suggests that these may play an important role in the senescence of vocal fold and the deterioration of the human voice.
Acknowledgments
This work was supported by a grant R21DC008428, from the National Institute of Deafness and other Communication Disorders. This paper will be presented at the 128th American Laryngological Association Meeting in Orlando, FL, 2008. The Institutional Review Board at the University of Wisconsin-Madison approved this investigation.
REFERENCES
- 1.Kahane JC. Connective tissue changes in the larynx and their effects on voice. J Voice. 1987;1:27–30. [Google Scholar]
- 2.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histological observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 3.Gray SD, Trize IR, Chan R, Hammond TH. Vocal fold proteoglycnas and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Hirano M. Histologic investigation of the macula flava of the human vocal fold. Ann Otol Rhinol Laryngol. 1995;104:138–143. doi: 10.1177/000348949510400210. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Hirano M. Histologic investigation of the macula flava of the human newborn vocal fold. Ann Otol Rhinol Laryngol. 1995;104:556–562. doi: 10.1177/000348949510400710. [DOI] [PubMed] [Google Scholar]
- 6.Hirano M, Sato K, Nakashima T. Fibroblasts in human vocal fold mucosa. Acta Otolaryngol. 1999;119:271–276. doi: 10.1080/00016489950181800. [DOI] [PubMed] [Google Scholar]
- 7.Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: a rat study. Ann Otol Rhinol Laryngol. 2006;115:135–143. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Stathopoulos ET. Vocal efficiency as a function of vocal intensity: a study of children, woman, and men. J Acoust Soc Am. 1995;97:1885–1892. doi: 10.1121/1.412062. [DOI] [PubMed] [Google Scholar]
- 9.Connor NP, Lee K, Leverson G, Ford CN. Laryngeal-respiratory kinematics are impaired in aged rats. Ann Otol Rhinol Laryngol. 2002;111:684–689. doi: 10.1177/000348940211100805. [DOI] [PubMed] [Google Scholar]
- 10.Hygen P, Lyons GD, Nuss DW. Dysphonia in the elderly: diagnosis and management of age-related voice changes. Southern Medical Journal. 1996;89:204–207. doi: 10.1097/00007611-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hirano M, Sato K, Nakashima T. Fibroblasts in geriatric vocal fold mucosa. Acta Otolaryngol. 2000;120:336–340. doi: 10.1080/000164800750001215. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Hirano M, Nakashima T. Age-related changes in vitamin A-storing stellate cells of human vocal folds. Ann Otol Rhinol Laryngol. 2004;113:108–112. doi: 10.1177/000348940411300204. [DOI] [PubMed] [Google Scholar]
- 13.Filho JAX, Tsuji DH, Nascimento PH, Sennes LU. Histologic changes in human vocal folds correlated with aging: a histomorphometric study. Ann Otol rhinol Laryngol. 2003;112:894–899. doi: 10.1177/000348940311201012. [DOI] [PubMed] [Google Scholar]
- 14.Abdelkafy WM, Smith JQ, Henriquez OA, Golub JS, Xu J, Rojas M, Brigham KL, Johns MM. Age-related changes in the murine larynx: initial validation of a mouse model. Ann Otol Rhinol Laryngol. 2007;116:618–622. doi: 10.1177/000348940711600810. [DOI] [PubMed] [Google Scholar]
- 15.Hammond TH, Zhou R, Hammond EH, Pawlak A, Gray SD. The intermediate layer: a morphologic study of the elastin and hyaluronic acid constituents of normal human vocal folds. J Voice. 1997;11:59–66. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- 16.Hammond TH, Gray SD, Bulter J, Zhou R, Hammond E. Age- and gender-related elastin distribution changes in human vocal fold. Otolaryngol Head Neck Surg. 1998;119:314–322. doi: 10.1016/S0194-5998(98)70071-3. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Hirano M, Nacashima T. Age-related changes of collagenous fibers in the human vocal fold mucosa. Ann Otol Rhinol Laryngol. 2002;111:15–20. doi: 10.1177/000348940211100103. [DOI] [PubMed] [Google Scholar]
- 18.Hirano S, Bless DM, del Rí AM, Connor NP, Ford CN. Therapeutic potential of growth factors for aging voice. Laryngoscope. 2004;114:2161–2167. doi: 10.1097/01.mlg.0000149450.37640.db. [DOI] [PubMed] [Google Scholar]
- 19.Ding H, Gray SD. Senescent expression of genes coding tropoelastin, elastase, lysyl oxidase, and tissue inhibitors of metalloproteinases in rat vocal folds: comparison with skin and lungs. J Speech Lang Hear Res. 2001;44:317–326. doi: 10.1044/1092-4388(2001/026). [DOI] [PubMed] [Google Scholar]
- 20.Ding H, Gray SD. Senescent expression of genes coding collagens, collagen-degrading metalloproteinases, and tissue inhibitors of metalloproteinases in rat vocal folds: comparison with skin and lungs. J Gerontol A Biol Sci Med Sci. 2001;56:B145–B152. doi: 10.1093/gerona/56.4.b145. [DOI] [PubMed] [Google Scholar]
- 21.Thibeault S, Li W. A Method for Identification of Vocal Fold Lamina Propria Fibroblasts in Culture. Otolaryngology Head and Neck Surgery. doi: 10.1016/j.otohns.2008.09.009. (In review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y, Zhang RZ, Sabatelli P, Chu ML, Bönemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008 Jan 18; doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]
- 23.Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1998;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 24.Fuji TJ, Ostrem EM, Probst-Fuja MN, Titze IR. Differential cell adhesion to vocal fold extracellualr matrix constituents. Matrix Biol. 2006;25:240–251. doi: 10.1016/j.matbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Hirano S, Bless DM, Rousseau B, Welham N, Scheidt T, Ford CN. Fibronectin and adhesion molecules on canine scarred vocal folds. Laryngoscope. 2003;113:966–972. doi: 10.1097/00005537-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 27.Li-Korotky HS, Hebda PA, Lo CY, Dohar JE. Age-dependent differential expression of finbronectin variants in skin and airway mucosal wounds. Arch Otolaryngol Head Neck Surg. 2007;133:919–924. doi: 10.1001/archotol.133.9.919. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein S. Replicative Senescence: The Human Fibroblast Comes of Age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 29.Blackburn EH, Szostak JK. The Molecular Structure of Centromeres and Telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- 30.Thibeault SL, Glade RS, Li W. Comparison of telomere length of vocal folds with different tissues: a physiological measurement of vocal senescence. J Voice. 2006;20:165–170. doi: 10.1016/j.jvoice.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Harley CB, Futcher AB, Grelder CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]