Abstract
We investigated the circadian function of Drosophila dopamine receptors by using a behaviorally active decapitated preparation that allows for direct application of drugs to the nerve cord. Quinpirole, a D2-like dopamine receptor agonist, induces reflexive locomotion in decapitated flies. We show that the amount of locomotion induced changes as a function of the time of day, with the highest responsiveness to quinpirole during the subjective night. Furthermore, dopamine receptor responsiveness is under circadian control and depends on the normal function of the period gene. The head pacemaker is at least partly dispensable for the circadian modulation of quinpirole-induced locomotion, because changes in agonist responsiveness persist in decapitated flies that are aged for 12 h. This finding suggests a role for the period-dependent molecular oscillators in the body in the modulation of amine receptor responsiveness.
Circadian rhythms are genetically determined biological oscillations with a period of close to 24 h evident in the physiology and behavior of most organisms (1, 2). One of the most obvious behaviors controlled by the circadian pacemaker in a variety of organisms is the rest–activity cycle, which can be clearly seen in Drosophila (3, 4). Components of the circadian pacemaker responsible for rhythm generation were first identified in Drosophila, but recent data from other organisms, including humans, indicate a conservation in the molecular mechanisms underlying circadian rhythms (5–10).
The first genetically identified circadian mutant, period (per), encodes one of the essential elements involved in the transcription/translation-based autoregulatory loop of the cellular circadian pacemaker (3, 6). In Drosophila, the role of per in a group of brain neurons, the lateral neurons, is essential for the expression of circadian locomotor rhythm, thus pointing to these neurons as the site of the circadian pacemaker (11–14). However, studies in Drosophila indicate that circadian rhythm generators are not exclusively neural. In flies, per expression is also present in nonneural tissues, such as the Malpighian tubules, gut, testes, ovaries, and the chemosensory cells of the antennae (15–18). Most of these cells appear to contain autonomous per-based circadian oscillators (16), but only in the case of the antennal chemosensory cells have these peripheral oscillators been linked to a functional output (18).
Control of motor behaviors in both vertebrates and invertebrates has been linked to the biogenic amines, found in the central and peripheral nervous system (19–23). Dopamine receptors with greatest similarity to the mammalian D1-like G-protein-coupled receptors have been cloned in Drosophila (24–27). Although a D2-like dopamine receptor has not been identified at the molecular level in Drosophila or other insects, several lines of evidence support its existence (28–30).
Our laboratory has described a behaviorally active preparation of decapitated Drosophila that allows for direct application of drugs to the nerve cord (28). Biogenic amines stimulate locomotion, grooming, and hyperactive behaviors in these otherwise immobile preparations in similar but distinct manners. Here we focus our attention on a specific class of dopamine receptors and their role in circadian behavior. We measure behavioral responses to a D2-like dopamine agonist, quinpirole, which in decapitated flies stimulates locomotion. The fact that decapitated flies are behaviorally responsive but lack the brain circadian pacemaker makes them ideal for determining the role of body oscillators in the control of circadian behavior.
Materials and Methods
Decapitation of Flies and Behavioral Assays.
w1118 wild-type and pero flies were raised at 23°C under a 12-h light/12-h dark cycle. Two- to four-day-old flies were decapitated as described (28) and tested shortly thereafter, with modifications as noted. Flies were decapitated by using Dewecker Iris scissors (Fullam, Schenectady, NY) under CO2 anesthesia and then were placed in a humidified container for either 30 min (no delay) or 12 h (12-h delay) before the addition of quinpirole. Only flies that showed an upright posture and a grooming response after stimulation of a thoracic bristle were used in the further studies. Each fly was placed on a grid of 1-mm graph paper under the microscope. The quinpirole solution was made in 10 mM phosphate buffer (pH 7) and applied at the exposed nerve cord at the anterior notum as a droplet with a micropipet, maintaining the contact for 2–3 s. Green food color (3 μl) was added to 100 μl of quinpirole solution to confirm that the drug and vehicle diffused into the preparation. Locomotion and grooming starts immediately after the drug application and was video-recorded for 2 min. Locomotion was quantified counting the grid crossings, assigning a value of 1.5 mm for diagonal crossings. Flies (n = 30–50) were used for each time point. To establish if there is a statistically significant difference in the average amount of locomotion as a function of time of day, one-way ANOVA was performed by using the Microsoft excel data analysis package. For pairwise comparisons between different time points, Microsoft excel Student's t test was used.
In the constant light experiments, light/dark (LD)-entrained, intact flies were released into constant dim light at dusk and decapitated 25, 31, 37, 43, 49, and 55 h of constant light. Assuming that the circadian period is not significantly changed on release under constant conditions, the 25-, 31-, 49-, and 55-h time points fall within the subjective day, and the 37- and 43-h time points in the subjective night. Subjective day and night are defined as times that would have been light and dark had the flies continued in LD. By convention, time in LD is expressed as Zeitgeber time (ZT). In the 12-h delay experiments, under the LD conditions, flies were decapitated 7 h after lights on (ZT 7) or 7 h after lights off (ZT 19), and were aged for 12 h in the humidified containers under the same LD schedule. In the 12-h delay experiment in constant light (LL), flies were decapitated after 31 h (subjective day) or 43 h (subjective night) of LL, and tested with quinpirole 12 h later (43 or 55 h of LL).
Traditionally, constant dark instead of LL is used to characterize the circadian properties of a rhythm. In our experiments, we used constant dim light, instead, to facilitate manipulation and data collection from the decapitated flies. Although prolonged exposure to bright light eventually will lead to a behavioral arrhythmicity, the effect is not immediate. After release into LL, a robust cycling in per mRNA and protein persists for at least 58 h (31). Additionally, to minimize the arrhythmic-inducing effects of LL, we used very dim light (13 × 1010 μW/cm2). Consistent with observations in intact flies (31), we detect robust amplitude in the modulation of the quinpirole sensitivity during the second day in LL (Fig. 1B).
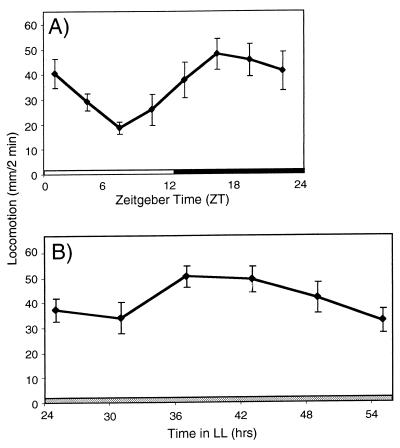
Figure 1.
Quinpirole-sensitive receptors are under circadian control. w1118 flies were decapitated at different time points during the 24-h period. Locomotion is expressed in mm per 2-min observation period starting immediately after 5 mM quinpirole application. Data are presented as mean locomotion of 30–50 flies tested at each time point (± SEM). (A) LD-entrained flies assayed 30 min after decapitation. One-way ANOVA shows a significant effect of the time of day on the amount of quinpirole-induced locomotion, (P < 0.001). (B) LD-entrained flies released into LL and tested after 25, 31, 37, 43, 49, and 55 h of constant light. One-way ANOVA shows the significant effect of the subjective time of day on the amount of induced locomotion (P < 0.001).
Results
Quinpirole-Induced Locomotion Changes as a Function of the Time of Day and Persists Under Constant Conditions.
Decapitated flies maintain an upright posture with a low level of grooming and show no locomotion without the addition of biogenic amines or dopamine agonists (28). Distinct and reproducible behaviors can be evoked by mechanical or pharmacological stimulation. Application of the dopamine agonist, quinpirole, to the exposed nerve cord of decapitated Drosophila induces locomotion (28). Because in mammals, neurotransmitters reported to be involved in regulation of motor activity exhibit circadian modulation in their levels as well as the corresponding receptor densities, we decided to test if quinpirole sensitivity changes during 24 h. When flies are kept under a LD schedule, and are decapitated at 3-h intervals during LD cycle, the amount of quinpirole-induced locomotion changes as a function of the time of day (Fig. 1A). Quinpirole was least effective during the light portion of the LD cycle, inducing the least locomotion at ZT 7, and the most at ZT 19 (Fig. 1A). Because quinpirole was applied shortly after flies were decapitated, we expect that the locomotor output measured is indicative of the dopamine agonist responsiveness at the time of decapitation.
To determine whether the modulation of the dopamine agonist responsiveness is under the control of the circadian pacemaker, we performed similar measurements after transferring LD-entrained flies into LL. The locomotor responses to quinpirole continue to be rhythmic for at least 2.5 days of constant conditions, indicating that responsiveness to agonist is modulated by an endogenous circadian pacemaker (Fig. 1B). Similar to the behavior observed in LD conditions, agonist responsiveness to quinpirole was significantly higher during the subjective night (37 and 43 h) than during the subjective day (25, 31, 49, and 55 h) (P < 0.001). We find that similar to the described dampening of 24-h molecular rhythms of per and cryptochrome (cry) expression under constant conditions in intact flies (32–34), modulation of quinpirole-responsiveness is lower in LL than in LD. We observe that a 2.3-fold peak-to-trough amplitude is in LD (Fig. 1A), versus a 1.7-fold in LL (Fig. 1B).
Quinpirole-Induced Locomotion Is per Dependent.
If the modulation of dopamine agonist responsiveness is under the control of the circadian pacemaker, we expect that circadian modulation of responsiveness to quinpirole would depend on a functional per gene. We tested per-null mutants, (per0), which lack per protein and are functionally arrhythmic under constant conditions. These mutants, however, do show rhythmic locomotion if they are kept in LD (3, 35).
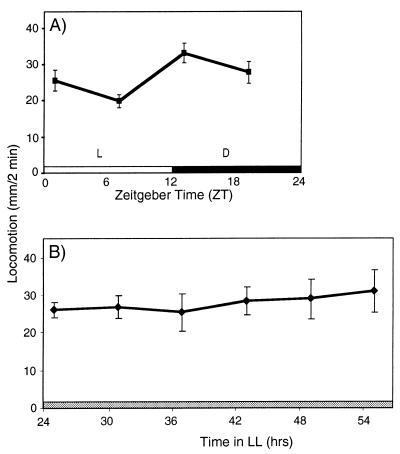
We find that the rhythmic properties of the behaviors evoked in our decapitated preparation show parallels with circadian behaviors of the intact per0 flies. Decapitated per0 flies show significant modulation of quinpirole-induced locomotion when kept in LD conditions (Fig. 2A), however with a lower peak-to-trough amplitude (1.7-fold) than the wild-type flies. This indicates that rhythmic effects of LD have a similar effect on the modulation of quinpirole-induced locomotion in our decapitated preparation as it has on the locomotor activity of intact per0 flies. In contrast, when the quinpirole challenge was performed on per0 flies kept in LL, this modulation disappears (Fig. 2B). This result shows that modulation of responsiveness to the dopamine agonist is under per-dependent circadian control and that the modulation of quinpirole-responsive dopamine receptors is likely downstream of per.
Figure 2.
Circadian changes in quinpirole-induced locomotion are per dependent. LD-entrained pero flies were decapitated and then placed into (A) LD or (B) LL light conditions, and tested by application of 5 mM quinpirole at the indicated times. Data are presented as mean locomotion of 30–50 flies tested at each time point (± SEM). In LD, one-way ANOVA shows a significant effect of the time of day on locomotion (P < 0.002), but in LL, the modulation of quinpirole-induced locomotion disappears (P = 0.96).
Body Oscillators Can Modulate Quinpirole-Induced Locomotion in the Absence of the Head Pacemaker.
We used decapitated flies to determine whether the brain pacemaker is required for the circadian modulation of quinpirole-induced locomotion. Specifically, we were interested to learn whether the clock in the body can support the modulation of agonist responsiveness after removal of head input. In the following experiments, flies were decapitated and aged for 12 h before receiving quinpirole. Although decapitated flies can live and remain responsive to touch up to 3 days, we find a 12-h time interval optimal, because at longer aging times, a significant number of flies becomes unresponsive to drug because healing of the exposed tissue prevents successful drug diffusion into the nerve cord (data not shown).
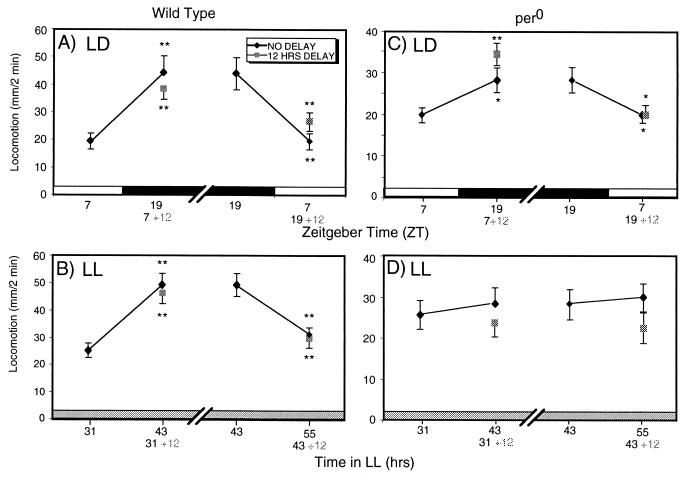
Our data indicate that the brain circadian pacemaker is at least partly dispensable for the continued modulation of nerve cord dopamine receptor responsiveness (Fig. 3). Fig. 3A compares average quinpirole-induced locomotion from the control flies, decapitated and tested at ZT 7 and ZT 19 (no delay) with flies that were tested with quinpirole 12 h after decapitation (12-h delay). If flies are decapitated at ZT 7, but tested 12 h later (corresponding to ZT 19), their responses are strikingly similar to the flies that are decapitated and tested at ZT 19. To determine whether the modulation of agonist responsiveness is a property of an oscillator capable of modulating receptor sensitivity under constant conditions, we performed the same experiments in LL. Again, the response to quinpirole in flies decapitated and aged for 12 h changes with the expected directionality. Flies that were decapitated and tested after 43 h of LL show similar locomotion to flies decapitated after 31 h of LL and tested 12 h later (Fig. 3B). Similar modulation is obtained from flies decapitated at the peak of the quinpirole sensitivity (43 h of LL) and tested 12 h later (55 h of LL), indicating that the amount of induced locomotion either increases or decreases depending on the receptor sensitivity at the time of decapitation.
Figure 3.
Circadian modulation of nerve cord dopamine receptor responsiveness continues after decapitation. Wild-type w1118 (A or B) or pero (C or D) flies were LD entrained. Flies were decapitated at ZT 7 or ZT 19, and then assayed with 5 mM quinpirole either 30 min (no delay) or 12-h (12-h delay) later. A Student's t test for samples, assuming equal variance, was performed comparing the no delay and 12-h delay means to the means for flies decapitated and tested 12 h earlier. (*, P < 0.05; **, P < 0.01.) (A) Wild-type flies decapitated and tested in LD. (B) Wild-type flies decapitated and tested in LL. (C) pero flies decapitated and tested in LD. (D) pero flies decapitated and tested in LL.
The body oscillators that modulate agonist responsiveness are per dependent, because the modulation of quinpirole responsiveness subsequent to decapitation is abolished in per0 flies in LL (Fig. 3D). In contrast, when per0 flies were kept under LD conditions, modulation of the quinpirole-sensitive dopamine receptors persists without the brain input (Fig. 3C), showing that the bodies of per0 flies are photosensitive. Taken together, these observations indicate that the dopamine receptor circuits in the body, presumably in the nerve cord, are per dependent, and show at least a degree of independence in circadian modulatory functions from the brain circadian oscillators. Technical limitations preclude the determination of whether these body-localized circuits can show true circadian oscillatory behavior when independent of the brain.
Discussion
We used a behaviorally active preparation of decapitated D. melanogaster to demonstrate circadian modulation of quinpirole-sensitive dopamine receptor responsiveness in the ventral nerve cord. We show that quinpirole-induced locomotion changes as a function of time of day, is modulated by per in a circadian manner, and persists in at least partial function in the absence of the brain circadian pacemaker. We propose that quinpirole-sensitive amine receptors are a component of the output pathway from the molecular pacemaker involved in the circadian control of locomotor activity.
The Relationship Between Modulation of Quinpirole-Stimulated Locomotion in the Nerve Cord and Circadian Modulation of Locomotion in Vivo.
The circadian variation in locomotor output of the Drosophila nerve cord in response to dopamine agonist stimulation shows two interesting differences from the pattern of locomotor responsiveness in living flies. First, the rhythms of quinpirole-stimulated nerve cord responsiveness are in opposite phase with in vivo locomotor activity patterns, reaching peak levels during the subjective night, at the time when living flies are least active. Second, there are subtle differences in the activity profiles of the intact and decapitated flies during the light-to-dark transitions.
The out-of-phase nature of in vivo vs. nerve cord locomotion is most readily explained if the nerve cord responses are modulated as compensatory postsynaptic effects as has been observed in vertebrates (36) and Drosophila (61). Postsynaptic dopamine receptors can compensate for differences in the amount of presynaptic release, decreasing sensitivity when dopamine release is high, and increasing when low. In this latter study, constitutive overexpression of a stimulatory Gα subunit in the dopamine and serotonin neurons, which is expected to increase amine release, results in a decrease in postsynaptic responsiveness to quinpirole (61). Reciprocally, overexpression of the inhibitory Gα subunit or tetanus toxin results in a increased responsiveness to quinpirole. We speculate that increased dopamine release during the subjective day would stimulate locomotor behaviors, with decreased postsynaptic receptor sensitivity acting as a partially effective compensatory mechanism. At night when dopamine release is presumed to be lower, up-regulation of receptor responsiveness would mediate the enhanced response to quinpirole. Although this model postulates that dopamine release is under circadian control, dopamine synthesis is not, because we find no variation in brain dopamine content as a function of the 24-h day (data not shown). If modulated dopamine release sets the responsiveness of the quinpirole-sensitive receptors, it could occur even in flies lacking brain input. In invertebrates, the ventral cord, unlike the higher vertebrate spinal cord, contains aminergic cell bodies (37). Thus, a rhythm of aminergic release under the control of body modulatory circuits could set the responsiveness of quinpirole-sensitive receptors. In intact flies, circadian behavior could be under coordinated control of both the body oscillators and the pacemaker in the brain, because additional dopamine input to the ventral cord originates in the brain and reaches the nerve cord through descending dopamine fibers (37, 38).
Alternatively, the observed circadian modulation of quinpirole sensitivity could be under more direct circadian control. By this scenario, modulation of dopamine receptor sensitivity or other signaling components downstream of the receptor would be modulated independently of the magnitude of dopamine release. More complicated models are certainly possible, in which dopamine receptor activation could be part of a negative regulatory input acting on a neuronal locomotor circuit. Further mechanistic studies will depend on isolation of the relevant quinpirole-sensitive receptor, and examination of its neuronal connectivity and mutant phenotypes.
The second difference between nerve cord responsiveness and circadian locomotor patterns in vivo comes from the shape of the day/night activity profiles. Living flies are most active at times surrounding the day/night transitions in LD conditions. On transfer to constant conditions, these peaks of activity gradually merge into a single peak of activity that occurs during the subjective day (35, 39). In contrast, the activity profiles of the quinpirole-stimulated decapitated flies show a rather smooth and nearly sinusoidal transition in either LD or LL, with no hint of activity spikes or depression at the LD transition times. This behavior is most likely a result of a degree of independence of the body modulatory circuits from direct photic input from the eyes. These nerve cord activity profiles rather closely mimic variations in olfactory sensory neuron responsiveness, which show both a similarly smooth LD variation, and enhanced responsiveness during the subjective night (18). It thus seems likely that the modulation of the nerve cord amine receptor is linked more closely to the day vs. night bias in activity, rather than precisely tracking in vivo activity levels.
Partial Autonomy of the Nerve Cord Oscillators Controlling Dopamine Receptor Responsiveness.
Whereas it is clear that the lateral neurons within the brain contain the central circadian pacemaker (13, 40, 41), several lines of evidence suggest that circadian oscillators in the periphery are required for normal circadian behaviors. The existence of independent circadian oscillators has been demonstrated by findings of rhythmic daily transcription of per and timeless in peripheral sensory tissues (16), and in nonneural organs such as Malpighian tubules (15, 17). Protein and mRNA production of per and timeless genes remains rhythmic in Malpighian tubules of decapitated flies (15). Light can entrain and phase shift per transcriptional rhythms in cultured body parts (16). Cryptochrome, a blue light-sensitive photoreceptor, is proposed to be the major photoreceptor involved in resetting the brain circadian clock, and may be involved in transducing light information to circadian oscillators outside the head (42). Furthermore, unlike head-specific opsin expression, cryptochrome mRNA is present in the body where it is rhythmic and in phase with the expression in the head (34).
Functional roles for these peripheral oscillators have been demonstrated only for two processes: (i) localized per expression is required for circadian variation in olfactory sensitivity of antennal chemosensory cells (18), and (ii) the normal ultradian rhythm of the male courtship song, which depends on per expression in thoracic ganglia (43).
Our results show that when decapitated flies are aged for 12 h before application of quinpirole, locomotor responses continue to be modulated with the expected directionality. Technical limitations of our system preclude the demonstration that this modulation results from fully independent nerve cord oscillators, but our results suggest that the nerve cord can maintain at least a degree of independence from the central circadian oscillators in the brain. Because this independent nerve cord modulation is both per dependent and light sensitive, it shares modulatory aspects characteristic of true circadian behaviors. The potential relevance of these nerve cord regulatory circuits to in vivo circadian modulation of locomotory activity is indicated by the transgenic line per017,2:2 where per expression is restricted only to the laternal neurons with no detectable expression in the nerve cord (40). These flies show a longer than normal period of locomotion and lower power, a measure of robustness of the rhythm, indicating that although per expression in the lateral neurons is necessary, it is not sufficient for a completely wild-type pattern of circadian locomotion. These flies lack per expression in glia surrounding the brain lateral neurons, leading those authors to propose that the lack of per expression in these cells might be responsible for the altered phenotypes (40). It remains possible that the altered rhythmicity in these transgenics is caused by the lack of peripheral per expression, leading to reduced robustness of locomotor modulation.
In insects other than Drosophila, there is additional evidence indicating that the brain pacemaker may not be the only structure capable of controlling circadian outputs. Sperm release in the gypsy moth is controlled by a light-sensitive circadian pacemaker located in the reproductive system (44). Circadian cuticular deposition in the cockroach persists after complete ablation of optic lobes and shows light independence (45, 46). In Leuchopaea maderae, a temperature-sensitive oscillator located outside the optic lobes can control locomotor activity (47).
Quinpirole-Activated Dopamine Receptors: A Component of the Circadian Organization in Drosophila.
In mammals, dopamine is an important component of the input pathway to the pacemaker during a restricted phase of development (48–50). Dopamine, acting through a D1 receptor that is highly expressed before birth, conveys nonphotic information mediating maternal–fetal entrainment (48, 49). D1 receptors continue to be expressed in the adult circadian pacemaker; however, they do not seem to play a role in photic control, a major form of entrainment of the adult circadian clock (50).
Our results are most simply consistent with a role for quinpirole-activated dopamine receptors acting in the output pathway from the brain circadian pacemaker. However, none of our results preclude a role for these as of yet unidentified receptors in modulating intercellular responses between cells of the brain circadian pacemaker. Biogenic amines have been implicated in the control of motor behaviors in vertebrates and invertebrates, both in the central and peripheral nervous system (19–23). In humans, the importance of dopamine in motor control is most evident in Parkinson's disease, where degeneration of dopamine cell bodies in substantia nigra results in movement disorders. Interestingly, some Parkinson's disease patients display variations in circadian activity patterns, whereas other studies show daily oscillations in the severity of the symptoms, indicating potential communication between the dopamine system and circadian clock (51). In spinal cats, where the neural axis has been transected, monoaminergic systems are involved in initiation and modulation of locomotion (52). In arthropod species, injections of dopamine, serotonin, or octopamine into the central nervous system evokes distinct motor postures, suggesting that they are released endogenously to mediate behavior (53, 54).
Additional evidence consistent with a role of aminergic signaling in the Drosophila circadian output pathway comes from studies of mutants in the fly catalytic subunit of cAMP-dependent protein kinase gene (PKA), DCO, whose gene product functions downstream of the brain circadian pacemaker (55). DCO mutant flies display arrhythmic locomotor activity, but per protein and mRNA in the brain shows normal daily oscillations, indicating that PKA functions in the flow of information between the pacemaker cells and the output pathways (56). In vertebrates, D2-like dopamine receptors are negatively coupled to adenylyl cyclase, resulting in inhibition of cAMP production (57). If the analogous situation holds in flies, mutations in PKA would be epistatic to regulation of a dopamine receptor.
Our data indicate a role for modulation of dopamine receptor responsiveness in circadian behavior. Modulation of dopamine receptor sensitivity is involved in modulating responses to the indirect amine agonist cocaine both in vertebrates and in flies (58, 59). Cocaine functions as a stimulator of reflexive motor and locomotor behaviors both in flies and in vertebrates (60). It is thus not totally surprising that modulation of responsiveness to cocaine in Drosophila crucially depends on the normal function of a subset of the circadian genes (59). Given this overlap in functions, it seems likely that there will be altered circadian functions in other mutants showing altered cocaine responses.
Acknowledgments
We thank E. Blumenthal, C. Green, E. Herzog, and M. Sankovic for helpful comments on the manuscript, and the members of our laboratory for helpful suggestions throughout. This work was supported by National Institutes of Health Grant GM/DA 27318 to J.H.
Abbreviations
- per
period gene
- LD
light/dark
- ZT
Zeitgeber time
- LL
constant light
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pittendrigh C S. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 2.Dunlap J C. Annu Rev Genet. 1996;30:579–601. doi: 10.1146/annurev.genet.30.1.579. [DOI] [PubMed] [Google Scholar]
- 3.Konopka R J, Benzer S. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson R B. In: Circadian Rhythm Mutants of Drosophila. Leland J, Edmunds N, editors. New York: Dekker; 1993. pp. 91–122. [Google Scholar]
- 5.Reppert S M. Neuron. 1998;21:1–4. doi: 10.1016/s0896-6273(00)80234-2. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap J. Science. 1998;280:1548–1549. doi: 10.1126/science.280.5369.1548. [DOI] [PubMed] [Google Scholar]
- 7.Zylka M J, Shearman L P, Levine J D, Jin X, Weaver D R, Reppert S M. Neuron. 1998;21:1115–1122. doi: 10.1016/s0896-6273(00)80628-5. [DOI] [PubMed] [Google Scholar]
- 8.Zylka M J, Shearman L P, Weaver D R, Reppert S M. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 9.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Nature (London) 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z S, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee C C. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 11.Helfrich-Forster C, Homberg U. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 12.Helfrich-Forster C. Proc Natl Acad Sci USA. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helfrich-Forster C. J Comp Physiol A. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 14.Helfrich-Forster C, Stengl M, Homberg U. Chronobiol Int. 1998;15:567–594. doi: 10.3109/07420529808993195. [DOI] [PubMed] [Google Scholar]
- 15.Giebultowicz J M, Hege D M. Nature (London) 1997;386:664. doi: 10.1038/386664a0. [DOI] [PubMed] [Google Scholar]
- 16.Plautz J D, Kaneko M, Hall J C, Kay S A. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 17.Hege D M, Stanewsky R, Hall J C, Giebultowicz J M. J Biol Rhythms. 1997;12:300–308. doi: 10.1177/074873049701200402. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan B, Dryer S E, Hardin P E. Nature (London) 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- 19.Kravitz E A, Glusman S, Harris-Warrick R M, Livingstone M S, Schwarz T, Goy M F. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- 20.Harris-Warrick R M, Kravitz E A. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloley B D, Juorio A V. Int Rev Neurobiol. 1995;38:253–303. doi: 10.1016/s0074-7742(08)60528-0. [DOI] [PubMed] [Google Scholar]
- 22.Grillner S, Wallen P. Annu Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- 23.Grillner S, Wallen P, Viana di Prisco G. Cold Spring Harbor Symp Quant Biol. 1990;15:779–789. doi: 10.1101/sqb.1990.055.01.073. [DOI] [PubMed] [Google Scholar]
- 24.Gotzes F, Balfanz S, Baumann A. Recept Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- 25.Feng G, Hannan F, Reale V, Hon YY, Kousky C T, Evans P D, Hall L M. J Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugamori K S, Demchyshyn L L, McConkey F, Forte M A, Niznik H B. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- 27.Han K-A, Millar N S, Grotewiel M S, Davis R L. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 28.Yellman C, Tao H, He B, Hirsh J. Proc Natl Acad Sci USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger N A, Sturgis S L, Ebersohl R, Geng C, Sparks T C. Arch Insect Biochem Physiol. 1996;32:449–466. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<449::AID-ARCH17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Davis J P, Pitman R M. J Exp Biol. 1991;155:203–217. doi: 10.1242/jeb.155.1.203. [DOI] [PubMed] [Google Scholar]
- 31.Marrus S B, Zeng H, Rosbash M. EMBO J. 1996;15:6877–6886. [PMC free article] [PubMed] [Google Scholar]
- 32.Hardin P E. Mol Cell Biol. 1994;14:7211–7218. doi: 10.1128/mcb.14.11.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan E S, Franklin T M, Hilderbrand-Chae M J, McNeil G P, Roberts M A, Schroeder A J, Zhang X, Jackson F R. J Neurosci. 1999;19:3665–3673. doi: 10.1523/JNEUROSCI.19-10-03665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emery P, So W V, Kaneko M, Hall J C, Rosbash M. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler D A, Hamblen-Coyle M J, Dushay M S, Hall J C. J Biol Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 36.Chen J F, Aloyo V J, Weiss B. Neuroscience. 1993;54:669–680. doi: 10.1016/0306-4522(93)90238-b. [DOI] [PubMed] [Google Scholar]
- 37.Lundell M J, Hirsh J. Dev Biol. 1994;165:385–396. doi: 10.1006/dbio.1994.1261. [DOI] [PubMed] [Google Scholar]
- 38.Nassel D R, Elekes K. Cell Tissue Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 39.Majercak J, Sidote D, Hardin P E, Edery I. Neuron. 1999;24:219–230. doi: 10.1016/s0896-6273(00)80834-x. [DOI] [PubMed] [Google Scholar]
- 40.Frisch B, Hardin P E, Hamblen-Coyle M J, Rosbash M, Hall J C. Neuron. 1994;12:555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 41.Ewer J, Frisch B, Hamblen-Coyle M J, Rosbash M, Hall J C. J Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay S A, Rosbash M, Hall J C. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 43.Konopka R J, Kyriacou C P, Hall J C. J Neurogenet. 1996;11:117–139. doi: 10.3109/01677069609107066. [DOI] [PubMed] [Google Scholar]
- 44.Giebultowicz J M, Joy J E. J Biol Rhythms. 1992;7:203–212. doi: 10.1177/074873049200700302. [DOI] [PubMed] [Google Scholar]
- 45.Lukat R. Experientia. 1987;34:447. [Google Scholar]
- 46.Wiedenmann G, Lukat R, Weber F. J Insect Physiol. 1986;32:1019–1027. [Google Scholar]
- 47.Page T L. J Insect Physiol. 1985;31:235–242. [Google Scholar]
- 48.Viswanathan N, Weaver D R, Reppert S M, Davis F C. J Neurosci. 1994;14:5393–5398. doi: 10.1523/JNEUROSCI.14-09-05393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver D R, Reppert S M. Brain Res Mol Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 50.Bender M, Drago J, Rivkees S A. Brain Res Mol Brain Res. 1997;49:271–277. doi: 10.1016/s0169-328x(97)00161-7. [DOI] [PubMed] [Google Scholar]
- 51.Chokroverty S. Neurol Clin. 1996;14:807–826. doi: 10.1016/s0733-8619(05)70286-3. [DOI] [PubMed] [Google Scholar]
- 52.Barbeau H, Rossignol S. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- 53.Wood D E. J Comp Physiol A. 1995;177:335–349. doi: 10.1007/BF00192422. [DOI] [PubMed] [Google Scholar]
- 54.Wood D E, Gleeson R A, Derby C D. J Comp Physiol A. 1995;177:321–333. doi: 10.1007/BF00192421. [DOI] [PubMed] [Google Scholar]
- 55.Levine J D, Casey C I, Kalderon D D, Jackson F R. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 56.Majercak J, Kalderon D, Edery I. Mol Cell Biol. 1997;17:5915–5922. doi: 10.1128/mcb.17.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gingrich J A, Caron M G. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 58.Nestler E J, Aghajanian G K. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 59.Andretic R, Chaney S, Hirsh J. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 60.McClung C, Hirsh J. Curr Biol. 1998;8:109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- 61.Li, H., Chaney, S., Forte, M. & Hirsh, J. (2000) Curr. Biol., in press. [DOI] [PubMed]