Abstract
Objective
Supporting patients' self care could have a major effect on the management of long‐term conditions, which has led to worldwide interest in effective self care interventions. In England, self care support is being developed through the “Expert Patients Programme”, which provides lay‐led generic courses to improve patients' self care skills. However, the clinical and cost effectiveness of such courses remains unclear.
Methods
Two‐arm pragmatic randomised controlled trial design with waiting list control in community settings in England. 629 patients with a wide range of self‐defined long‐term conditions were studied. The lay‐led self care support group involved 6‐weekly sessions to teach self care skills. Primary outcomes were self‐efficacy, reported energy and routine health services utilisation at 6 months. A cost‐effectiveness analysis was also conducted.
Results
Patients receiving immediate course access reported considerably greater self‐efficacy and energy at 6‐month follow‐up, but reported no statistically significant reductions in routine health services utilisation over the same time period. The cost‐effectiveness analysis showed that patients receiving immediate course access reported considerably greater health related quality of life, and a small reduction in costs. If a quality adjusted life year was valued at £20 000 ($39 191; €30 282), there was a 70% probability that the intervention was cost effective.
Conclusions
Lay‐led self care support groups are effective in improving self‐efficacy and energy levels among patients with long‐term conditions, and are likely to be cost effective over 6 months at conventional values of a decision‐maker's willingness to pay. They may be a useful addition to current services in the management of long‐term conditions.
The global burden of disease is shifting to long‐term conditions,1 and there is worldwide interest in the development of models of service delivery to manage these changing needs.2 The National Health Service (NHS) policy envisages care for long‐term conditions based on three tiers: case management for patients with multiple, complex conditions; disease management for patients at some risk, through guideline‐based programmes in primary care;3,4 and self care support for low‐risk patients (70–80% of those with long‐term conditions). self care has been defined as “the care taken by individuals towards their own health and well being: it comprises the actions they take to lead a healthy lifestyle; to meet their social, emotional and psychological needs; to care for their long‐term condition; and to prevent further illness or accidents”.5
self care support in England is being provided through a broad initiative called the “Expert Patients Programme” (EPP).6 One key priority of this initiative is the development of effective interventions to increase patients' self care skills. Effective disease‐specific programmes have been designed for heart disease and arthritis.7,8,9,10 However, commonalities in the approaches used to manage long‐term conditions have led to interest in generic programmes,11,12 which may be more efficient to provide.
One generic programme developed in the US, the chronic disease self management programme (CDSMP),6 has been adapted for use in England. This is a group intervention, led by trained lay people with experience of long‐term conditions, designed to enable participants to develop appropriate self care skills.11
There is preliminary evidence for the effectiveness of the CDSMP in the US and China.11,13,14 However, deficiencies in the evidence remain. Effectiveness may be influenced by local health service context, and published results may not generalise. A recent study has been completed in England, but that was restricted to ethnic minorities with particular conditions recruited directly from primary care.15 In addition, there is significant interest in the potential for effective self care to reduce demand on health services.16 There is some preliminary evidence that the CDSMP is associated with reductions in health service use,11 but these analyses have been limited in scope, and have not explicitly modelled the relationship between costs and outcomes.17
Methods
The Research into Expert Patients—Outcomes in a Randomised Trial (REPORT) was a two‐arm trial comparing the clinical and cost effectiveness of the lay‐led self care support programme with a waiting list control. The evaluation of the lay‐led self care support programme within REPORT was conducted in parallel with a wider national implementation of the programme within the EPP.
Participants
REPORT was a pragmatic trial, designed to maximise external validity by recruiting a representative sample of patients and providing treatments in a way that reflects routine delivery. The EPP is informed by philosophies of social inclusion and patient empowerment,18 and access is not based on medical definitions, but on self‐defined long‐term conditions. The trial was designed to reflect the broader programme, and no specific inclusion or exclusion criteria were used beyond a self‐defined long‐term condition. Recruitment was carried out in all 28 Strategic Health Authorities in England.
Intervention
The lay‐led self care support programme was an anglicised version of the CDSMP developed by researchers at Stanford University in the USA.11 The course involved six 2.5 h group sessions held weekly. Attendance at four or more was required for a patient to be considered a “completer”. Groups comprised 8–12 participants, taught by a pair of lay trainers or volunteer tutors who were trained and subject to quality assurance.19 Meetings were generally held in non‐NHS premises. The intervention was conducted according to a written manual, and included sessions on relaxation, diet, exercise, fatigue, breaking the “symptom cycle”, managing pain and medication, and communication. Trainers are taught to act as “role models”. Goal setting and action planning sessions form a key part of the training course and each leader and participant is expected to set out a goal with specific actions they plan to undertake during the coming week. The plans are based on something that the individual wants to do which is reasonable, action‐specific and state What? How much? When? and How often? they will undertake the action. They state their level of confidence that they will achieve this goal. This activity is intended to increase self‐efficacy as participants are taught how to refine their plans until they are confident they will be able to achieve them; the following week they report on their success and the group attempts to deal with any difficulties encountered. The theoretical model underlying the course was categorised by a recent systematic review as “social learning”.10
Patients in the waiting list control could access the intervention after 6 months.
Outcomes
The causal mechanisms of “complex interventions”20 such as the current intervention are potentially multifaceted. However, previous work within health psychology and the CDSMP21,22 suggests a theoretical model (fig 1) where the primary causal mechanism is change in self‐efficacy cognitions (ie, patients' confidence in managing their condition), with changes in self care behaviour secondary. Changes in self‐efficacy are hypothesised to lead directly to changes in health status, which in turn influences healthcare utilisation.22 Outcome assessments reflected this model, and were based on validated self‐report scales used in previous studies23 (fig 1). These were supplemented by self‐reported measures of healthcare utilisation (including medication, primary, secondary and community care) and patient‐borne costs. Finally, health‐related quality of life was measured using the EuroQol, which assesses patients' health state across five dimensions (self care, mobility, anxiety/depression, usual activities and pain/discomfort) and ascribes each state a utility value based on a population tariff.24 All measures were collected at baseline, 6 months (from randomisation) and 12 months, although only 6‐month follow‐up data are presented as the 12‐month follow‐up lacked an untreated control group.
Figure 1 Theoretical framework for outcomes measurement
Procedures
REPORT was approved by the Multicentre Research Ethical Committee (03/8/2). Patients were recruited through EPP and primary care trust staff, press releases and the EPP webpage. Patients were only recruited when random assignment to the waiting list control group did not risk insufficient participants being available to run a group.25 Patients interested in participating were sent information sheets, consent forms and baseline questionnaires.
Patients were the unit of randomisation. A computer generated minimisation procedure was used, using the following variables: Strategic Health Authority, general health, main condition, gender, age, ethnicity and accommodation status. Seasonal change between date of recruitment and 6‐month follow‐up was an additional, uncontrolled a priori prognostic factor.
To ensure concealment of allocation, patient details were passed to another member of the research team not involved with individual patient recruitment. The randomisation ratio was 1:1. Patient preferences were measured.25,26 Follow‐up was by postal questionnaire with telephone reminders. Some group meetings were delayed or cancelled, resulting in variability in elapsed times between randomisation, start of the intervention and follow‐up for patients in the intervention group. A computer program was used to calculate follow‐up dates with comparable variability for control patients and to maintain distributional equivalence between the trial arms.
Statistical analysis
A published systematic review of disease‐specific chronic disease management interventions3 reported an overall standardised effect size of 0.25. Pretrial sample‐size calculations, assuming ranges of values for several unknown parameters (eg, intraclass correlation, attrition and baseline‐follow‐up covariance), estimated that 500–900 patients were needed for 90% power to detect a standardised effect size of 0.25. A second power calculation was conducted when values of these unknown parameters could be estimated from the data, and this suggested that 600 patients were required. Recruitment ended with the entry of 629 patients into the trial.
Three primary outcomes were defined a priori (fig 1). The first was the mean of scores on four self‐efficacy scales. The second was a measure of health status. Multiple health status measures were available, but the energy scale was chosen as the most relevant measure applicable across a heterogenous patient group. The third primary outcome was a measure of routine health service utilisation, based on the sum of self‐reported general practitioner consultations, practice nurse appointments, accident and emergency attendances and outpatient visits. The measure provided only a partial view of overall utilisation (excluding aspects such as inpatient stays and medication), and a conventional cost‐effectiveness analysis was also conducted using the full range of eligible costs (see below).
Secondary outcomes included other measures of health status and measures of self care behaviour.
Analyses were conducted in Stata V.8 according to a prespecified plan and on an intention‐to‐treat basis, using analysis of covariance with robust estimates of variance. The primary analysis used the baseline outcome values and all minimisation variables as covariates, with the exception of Strategic Health Authority, as the number of authorities was large relative to sample size.27 Although the utilisation outcome was markedly skewed, the sample was large enough for this to not be a problem in the analysis.
An α level of 5% was used throughout. Each primary outcome dealt with a different question and analysis of secondary outcomes was exploratory, therefore adjustment for multiple testing was not applied.28 Standardised effect sizes were calculated to allow comparison with previous studies. Post‐hoc subgroup analyses examined differential effects of the intervention associated with types of long‐term condition.
Sensitivity analyses
Three sensitivity analyses were conducted to assess the robustness of the results to the choice of covariates: (a) adding Strategic Health Authority to the model; (b) adding variables to adjust for seasonal change and variable times between baseline and follow‐up assessments; and (c) an analysis unadjusted for any covariates other than the baseline value of the outcome.29
Cost‐effectiveness analysis
A cost‐effectiveness analysis was conducted alongside the clinical trial. Only a summary of the main findings are reported, and full details will be presented elsewhere. Data on resource use were combined with unit cost data to provide estimates of overall costs per patient. The costs included the full range of costs associated with the self care support programme (estimated at £250 ($489; €378) per patient by the Department of Health), which was applied to all patients who were randomised to the course, irrespective of attendance or loss to follow‐up. The EuroQoL EQ‐5D instrument was used to calculate quality‐adjusted life years (QALYs).24 The primary analysis used the net benefit approach.30 Net benefit at the patient level was calculated by multiplying each patient's QALY score by decision‐makers' assumed maximum willingness‐to‐pay for a QALY and then subtracting that patient's costs. These estimates can then be used to generate the probability that the intervention is cost‐effective over a range of values of willingness‐to‐pay for a QALY.
Results
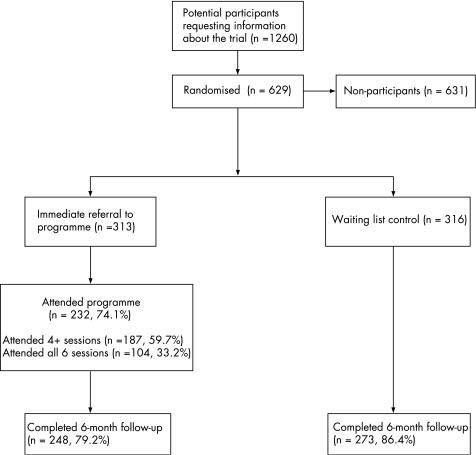
In total, 629 patients were recruited between April 2003 and March 2005 (fig 2). We have discussed some of the reasons for not taking part in REPORT elsewhere.25,31 Reasons included problems with access (no local course available, course times unsuitable, lack of provision for those with disabilities); poor current health state; a feeling that they were already effective self‐managers; dislike of the group approach; or insufficient motivation to commit to a 6‐week course. A total of 521 (83%) patients returned 6‐month assessments (although completion of individual scales varied slightly). There were no marked differences in baseline characteristics (table 1). Approximately one third of patients reported preferences to enter the EPP immediately, half were indifferent, and the remainder reported preferences for the wait list control.
Figure 2 Consolidated Standards of Reporting Trials diagram showing patient flow through the Research into Expert Patients—Outcomes in a Randomised Trial.
Table 1 Baseline demographic and health characteristics.
| Characteristic | Intervention n = 313 | Control n = 316 |
|---|---|---|
| Age (SD) | 55.5 (13.6) | 55.3 (13.6) |
| Gender: Female | 219 (70.0%) | 220 (69.6%) |
| Ethnicity: White | 298 (95.2%) | 299 (94.6%) |
| Marital status: | ||
| Lives alone | 82 (26.2%) | 93 (29.4%) |
| Lives with spouse/partner | 188 (60.1%) | 190 (60.1%) |
| Educational qualifications: | ||
| None | 77 (24.6%) | 61 (19.3%) |
| Degree | 51 (16.3%) | 53 (16.8%) |
| Accommodation: Owner‐occupied | 214 (68.4%) | 214 (67.7%) |
| Work situation: | ||
| In paid work | 58 (18.5%) | 66 (20.9%) |
| Unable to work due to condition | 111 (35.5%) | 106 (33.5%) |
| Retired | 110 (35.1%) | 111 (35.1%) |
| Self‐reported main long‐term health condition* | ||
| Musculoskeletal | 106 (33.9%) | 107 (33.9%) |
| Endocrine | 37 (11.8%) | 37 (11.7%) |
| Circulatory | 20 (6.4%) | 24 (7.6%) |
| Myalgic encephalitis /chronic fatigue | 22 (7.0%) | 25 (7.9%) |
| Respiratory | 23 (7.4%) | 17 (5.4%) |
| Mental health | 19 (6.1%) | 19 (6.0%) |
| Neurological | 20 (6.4%) | 18 (5.7%) |
| Other | 66 (21.1%) | 69 (21.8%) |
| Self‐reported general health | ||
| Very good/excellent | 32 (10.2%) | 34 (10.8%) |
| Good | 90 (28.8%) | 92 (29.1%) |
| Fair | 122 (39.0%) | 111 (35.1%) |
| Poor | 69 (22.0%) | 79 (25.0%) |
| Self‐reported baseline health characteristics | ||
| Self‐efficacy (SD) | 45.9 (21.5) | 47.7 (22.3) |
| Energy (SD) | 32.6 (19.5) | 33.3 (20.1) |
| Service utilisation (SD) | 8.6 (7.3) | 9.1 (8.1) |
| Classification of primary care trust locality† | ||
| Predominantly rural | 94 (30.0%) | 93 (29.4%) |
| Some rural and mixed | 114 (36.4%) | 118 (37.3%) |
| Major and large urban | 105 (33.6%) | 105 (33.2%) |
| Seasonal change between recruitment and follow‐up‡ | ||
| Follow‐up at a “worse” month in cycle than recruitment | 21 (6.7%) | 18 (5.7%) |
| Follow‐up at a “better” month in cycle than recruitment | 31 (9.9%) | 45 (14.2%) |
| No difference | 28 (9.0%) | 24 (7.6%) |
| No seasonal pattern | 233 (74.4%) | 229 (72.5%) |
| Time between recruitment and follow‐up: (mean days, SD) | 219 (40.6) | 209 (37.1) |
Values are represented as n (%) unless otherwise specified.
*Post‐hoc classification. The classification used in minimisation was: musculoskeletal, diabetes, heart disease, other.
†Definition taken from Department of Environment, Food and Rural Affairs classification of Primary Care Trusts in England (September 2005).
‡Based on informant ratings of each calendar month, collected at baseline.
There was differential attrition, with 79.2% of 6‐month assessments returned by the intervention group and 86.4% by the controls (difference 7.2%, 95% CI 1.3% to 13%). Stepwise logistic regression analysis was used to estimate the probability of return on the basis of patient characteristics. Return was significantly more likely from patients who were older, had the condition longer, owned their own home or had certain types of condition (musculoskeletal, circulatory, respiratory). The inverse of these probabilities was assigned as weights.
On primary outcomes (table 2), the intervention patients reported considerably higher scores for overall self‐efficacy and energy, but reported no differences in healthcare utilisation. On secondary outcomes, intervention patients reported considerably fewer social role limitations, better psychological well‐being, lower health distress, more exercise and relaxation, and greater partnership with clinicians. There were no differences between groups on general health, pain, diet, use of complementary products or information seeking.
Table 2 Outcomes at 6‐month follow‐up.
| Outcome | Unadjusted intervention scores Mean (SD; n) | Unadjusted control scores Mean (SD; n) | Adjusted difference (95% CI)* | p Value | Effect size† |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Self‐efficacy | 60.3 (19.6; 237) | 52.1 (21.2; 267) | 8.9 (6.2 to 11.5) | 0.000 | 0.44 |
| Energy | 37.7 (21.4; 247) | 35.0 (20.8; 273) | 3.7 (1.2 to 6.3) | 0.004 | 0.18 |
| Health care visits‡ | 6.29 (7.4; 248) | 6.77 (7.5; 273) | −0.20 (−1.35 to 0.95) | 0.732 | 0.03 |
| Secondary outcomes | |||||
| General health‡ | 2.64 (0.9; 247) | 2.75 (0.9; 273) | −0.10 (−0.22 to 0.01) | 0.083 | 0.11 |
| Social role limitations‡ | 45.4 (29.9; 248) | 51.4 (30.4; 273) | −5.6 (−9.2 to −2.0) | 0.002 | 0.19 |
| Pain‡ | 62.6 (26; 237) | 64.8 (24.5; 267) | −2.4 (−5.4 to 0.7) | 0.129 | 0.10 |
| Psychological well‐being | 64.8 (20.5; 247) | 61.2 (20.9; 272) | 5.1 (2.7 to 7.6) | 0.000 | 0.25 |
| Health distress‡ | 41.3 (26.2; 247) | 46.8 (25.8; 272) | −5.1 (−8.4 to −1.7) | 0.003 | 0.20 |
| Exercise | 160.2 (132.6; 247) | 152.6 (155; 273) | 18.8 (0.3 to 37.3) | 0.047 | 0.13 |
| Partnership with clinicians‡ | 56.5 (23.5; 236) | 62.6 (23; 267) | −5.7 (−9.5 to −1.9) | 0.003 | 0.25 |
| Diet | 2.3 (0.6; 234) | 2.3 (0.7; 267) | 0.08 (−0.02 to 0.17) | 0.126 | 0.12 |
| Complementary products | 1.6 (0.6; 234) | 1.6 (0.7; 266) | −0.03 (−0.12 to 0.07) | 0.562 | −0.05 |
| Relaxation | 2.1 (0.5; 226) | 2.0 (0.6; 257) | 0.11 (0.02 to 0.21) | 0.018 | 0.20 |
| Information seeking | 2.3 (0.7; 229) | 2.2 (0.7; 261) | 0.09 (−0.02 to 0.19) | 0.096 | 0.13 |
*Values adjusted for baseline outcome values and all minimisation variables as covariates (general health, main condition, gender, age, ethnicity and accommodation status).
†Effect size based on adjusted difference in means divided by the pooled standard deviation. Positive effect size represents favourable outcome for intervention.
‡Low scores indicate favourable outcome.
To explore whether the intervention effect, when significant, varied between conditions, patients were classified into eight conditions (table 1) and the interaction between condition and trial group added to the primary analysis. The trial was not powered to detect interactions, so a criterion of p<0.15 was used to warrant further investigation.29 There were no significant differences between subgroups on any primary outcomes, and only one secondary outcome met the criterion (partnership with clinicians, p = 0.13).
None of the results relating to the primary outcome were influenced substantively by the sensitivity analyses. In the secondary outcomes, the sensitivity analysis with seasonality and time between recruitment and follow‐up as additional covariates, the intervention group scored significantly higher on general health than the controls (p = 0.037). In the sensitivity analysis with Strategic Health Authority as an additional covariate, the increase in exercise in the intervention group was no longer statistically significant (p = 0.068).
Data on all major categories of health services utilisation are provided in table 3, with conventional measures of significance and effect size statistics for comparative purposes. There was a difference in QALYs of 0.02 (95% CI 0.007 to 0.034, adjusted for baseline characteristics) in favour of the intervention group, and a reduced cost of £27 ($53; €41) (95% CI £368 ($721; €557) to £422 ($827; €639)). Thus the intervention group were associated with a better QALY profile as well as a small reduction in costs. Although there is considerable uncertainty around the estimates of costs and QALYs, if decision‐makers are willing to pay £20 000 ($391 91 and €30 282) per QALY,32 there is a 70% probability that the intervention is cost effective. Full details of the economic analysis will be presented elsewhere, including appropriate sensitivity analyses.
Table 3 Mean resource use over the 6‐month period*.
| Resource type | Intervention group Mean (SD; n) | Proportion of total costs | Control group Mean (SD; n) | Proportion of total costs | Adjusted difference (95% CI)† | Effect size‡ |
|---|---|---|---|---|---|---|
| Inpatient days | 0.80 (5.31; 246) | 12.9% | 1.59 (5.81; 272) | 28.9% | −0.75 (−1.71 to 0.21) | 0.13 |
| Medication costs | £426 (785; 243) | 22.3% | £450 (933; 267) | 23.2% | −0.06 (−108.4 to 108.3) | 0.00 |
| Number of outpatient appointments | 2.73 (6.30; 248) | 14.4% | 2.91 (5.07; 273) | 15.2% | −0.06 (−1.01 to 0.89) | 0.01 |
| General Practitioner visits (surgery) | 3.36 (3.09; 246) | 3.7% | 3.44 (3.44; 269) | 3.7% | −0.03 (−0.54 to 0.48) | 0.01 |
| Patient out‐of pocket expenditure§ | £493 (1278; 235) | 25.8% | £378 (964; 263) | 19.5% | 119.4 (−73.8 to 312.6) | −0.11 |
| Intervention costs¶ | £250 (0; 248) | 13.1% | £0 (0; 0) | 0% | NA | NA |
| Number of day case appointments | 0.91 (2.96; 247) | 4.6% | 1.33 (3.39; 272) | 6.5% | −0.43 (−0.98 to 0.12) | 0.13 |
| Number of counsellor visits | 0.64 (2.91; 237) | 1.1% | 0.60 (2.78; 268) | 1.0% | 0.06 (−0.34 to 0.46) | −0.02 |
*Table includes all utilisation accounting for >1% of total costs.
†Values adjusted for baseline utilisation, gender and age.
‡Effect size based on adjusted difference in means divided by the pooled standard deviation. Positive effect size represents favourable outcome for intervention.
§Includes expenditure such as dietary supplements, gym classes, yoga and exercise, equipment and house alterations.
¶Includes Expert Patients Programme staff salaries and expenses, travel expenses, assessment and quality assurance, venue hire, consumables and other materials.
Discussion
The lay‐led self care support programme was responsible for significant increases in self‐efficacy and energy, but did not affect routine health‐services utilisation. Patients in the intervention groups reported increased health‐related quality of life, and the programme was cost effective at conventional levels of a decision makers' willingness to pay.
Internal validity issues
Internal validity was increased through procedures to ensure concealment of allocation.33 Attrition was relatively low given the national sample and the consequent restriction to postal follow‐up. Delivery of the intervention was outside the control of the REPORT team, and the intervention was being tested within REPORT at the same time as it was being implemented routinely, and it is possible that initial delivery was suboptimal, although central quality assurance procedures were in place.
Pragmatic trials such as REPORT use large sample sizes and limited controls on patient entry, and these methods have been criticised.34 The heterogenous sample means that variation in prognosis and capacity to benefit within groups is high; care must be taken in applying the results to individual patients and particular patient groups. Although the subgroup analysis did not find that effects were moderated by health condition, such analyses were post hoc and underpowered, and classification of patient problems was based on self‐report only. The results of REPORT may have greater implications for overall health service policy concerning long‐term conditions than for individual clinical decisions. It should be noted that variability in the quality of delivery of the course and in the types of patients entering the trial would generally make it more difficult to show a statistically significant effect.
Patients were randomised to the intervention or a wait list control, and there was potential for patient preferences to affect outcomes through “resentful demoralisation” of the control group. However, measures at baseline showed that initial preferences were mixed, and qualitative data suggested that a proportion of patients were ambivalent about the intervention.25 Furthermore, a recent systematic review showed a lack of clear evidence that preferences and resentful demoralisation are significant predictors of outcome in trials.26
External validity issues
REPORT was designed to maximise external validity, by recruiting a patient population similar to that accessing EPP. However this approach was only partly successful. Patients volunteered through various sources, and as with many self care studies,10 it proved impossible to estimate the proportion of eligible patients who declined. To ensure that REPORT did not compromise routine provision, only a proportion of patients who entered the programme routinely were approached.25
Data on the participants in REPORT were compared with data from the Health Survey for England, a representative annual cross‐sectional survey which identified nearly 7000 adults with at least one long‐standing condition.35 The trial sample involved a higher proportion of women (70% compared with 56% in the Health Survey), and they were generally less active (20% in paid employment vs 48%) and reported greater numbers of long‐term conditions (2.6 vs 1.8). There were smaller differences in the proportion living alone (28% vs 23%), and the proportions of ethnic minorities (5% vs 6%) and owner occupiers (68% vs 72%) were similar.
What is already known
The burden of long‐term conditions is significant, and there is interest in the potential for increasing the role of patient self care in the management of these conditions.
Effective self care may improve outcomes and reduce health service utilisation.
In England, significant resources have been invested in the development of a lay‐led self care group support programme for patients, but the clinical and cost effectiveness of that programme delivered in routine National Health Service contexts is unclear.
What this paper adds
The trial shows that a lay‐led self care group support programme improves patient self‐efficacy and self‐reported energy.
Although the programme does not have a significant effect on routine health service utilisation over 6 months, overall it is associated with improvements in health related quality of life at no increased cost, and is likely to be cost effective.
The programme may be a useful addition to current provision for long‐term conditions.
All these issues raise questions about external validity. As noted above, REPORT was conducted in parallel with the national implementation of the EPP self care initiative, and the Department of Health collected data on patients who took part in the lay‐led self care support programme as part of the wider initiative, but did not participate in the trial. Comparison of populations in and outside the trial showed broad similarities in characteristics.36 The EPP faces significant challenges in reaching out to a broader population of patients with chronic disease in the community, and it is unclear whether the results of REPORT will generalise to that population should the EPP be successful in increasing participation. However, it is likely that the results of the trial can be generalised to the population currently accessing the intervention.
Other studies of the CDSMP have not always provided relevant data for effect size calculations, but two reported a somewhat different pattern of results, with comparatively smaller effects on self‐efficacy and psychological well‐being in Hispanic and Chinese populations, larger effects on self‐rated health and health service utilisation, and similar effects on social role limitations, pain, and partnership with the doctor.13,14 Clearly there are differences in patient populations and health service contexts that could account for these variations. Such variations do highlight the need for primary studies of imported interventions, because results in one population cannot be generalised. However, the current results broadly replicate previous studies.11,13,14,15 They also extend them with a comprehensive assessment of patient quality of life and economic outcomes.
Interpretation of the outcomes
In terms of the model in fig 1, the intervention shows the largest relative effect on self‐efficacy, and the effect decreases further down the causal pathway. The pattern of results accords with the model in fig 1, where the effects are mediated through self‐efficacy. The self‐efficacy concept has a significant theoretical and empirical basis,21 but it is not a conventional health outcome and was originally seen as a mediator of other outcomes rather than an outcome per se. The REPORT team is conducting a discrete choice experiment37 to investigate the value placed on self‐efficacy by patients compared with other outcomes of the intervention.
Energy was the primary measure of health status because it was an outcome which could be potentially modified by the intervention across all patients. Although other dimensions were available, these were less relevant to all types of patient (for example, pain in patients with diabetes). However, the circumscribed nature of the health status measure must be considered when interpreting the results.
The lack of effect on utilisation may reflect the short nature of the follow‐up, which may not have provided enough time for patterns of usage to change, or the fact that the intervention was not linked explicitly with wider care provision. Inpatient use was reduced (which supports a previous study of the CDSMP11) although the difference was not statistically significant. However, the overall reduction in utilisation meant that the costs of provision of the programme were offset, and there was a small net reduction in costs. The formal cost‐effectiveness analysis showed that the programme is likely to be cost effective when compared with a wait list control.
Several research questions remain. The exact mechanisms by which the intervention achieves its effects are unclear, and may be due to the structured nature of the intervention or more non‐specific mechanisms such as the group experience. The group approach may not be acceptable to a proportion of patients, and the benefits of the intervention need to be compared with other approaches, such as individual interventions, on line delivery or the training of health professionals in the facilitation of self management.
Conclusion
In conclusion, the results indicate that provision of a lay‐led self care support programme to patients with long‐term conditions results in significant increases in self‐efficacy and energy, and is likely to be cost effective. The programme may be a useful addition to current provision for long‐term conditions.
Policy implications
The Expert Patients Programme is a major Department of Health policy initiative designed to introduce effective self care into the National Health Service, and has been given a high priority.
Self care is suggested as being a way to improve patient outcomes and quality of life, and reduce demand on the health service, thus ensuring that the future provision of health care is affordable.
This study provides empirical data relating to these assumptions. The programme does not have a significant effect on routine health service utilisation over 6 months, but it is associated with improvements in outcomes and health‐related quality of life at no increased cost, and is likely to be cost effective.
The programme may be a useful addition to current provision for long‐term conditions.
Acknowledgements
We thank Fran Morris, Claire Chandler and Sophie Jerrim for their hard work in the administration of the trial; the patients who kindly agreed to participate and complete the written assessments; the staff within the Expert Patients Programme and the Primary Care Trusts who helped recruit patients; and Ayesha Dost and Geoff Royston at the Strategy Directorate of the Department of Health. NPCRDC is a department of the University of Manchester but is funded by the Department of Health. The views expressed in this paper are the views of the authors and not the funding body.
Abbreviations
CDSMP - chronic disease self management programme
EPP - Expert Patients Programme
NHS - National Health Service
REPORT - Research into Expert Patients—Outcomes in a Randomised Trial
QALY - quality‐adjusted life year
Footnotes
Funding: The REPORT study was funded by the Department of Health. The funding body was not involved in the design, analysis and reporting of the REPORT trial beyond commissioning reviews of the protocol, and checking the accuracy of certain facts and material included in the final draft.
Competing interests: None.
References
- 1.Murray C, Lopez A.The global burden of disease: a comprehensive assessment of mortality and disability from disease, injuries and risk factors in 1990. Boston: Harvard School of Public Health on behalf of the World Bank, 1996
- 2.Epping‐Jordan J, Pruitt S, Bengoa R.et al Improving the quality of health care for chronic conditions. Qual Saf Health Care 200413299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weingarten S, Henning J, Badamgarav E.et al Interventions used in disease management programmes for patients with chronic illness—which ones work? Meta‐analysis of published reports. BMJ 2002325925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner E, Austin B, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 199674511–543. [PubMed] [Google Scholar]
- 5.Department of Health Self care—a real choice: self care support—a practical option. London: Department of Health, 2005
- 6.Department of Health The expert patient: a new approach to chronic disease management for the 21st century. London: Department of Health, 2001
- 7.Barlow J, Turner A, Wright C. A randomized controlled study of the Arthritis Self‐Management Programme in the UK. Health Educ Res Theory Pract 200015665–680. [DOI] [PubMed] [Google Scholar]
- 8.Lewin B, Robertson I, Cay E.et al Effects of self‐help post‐myocardial infarction rehabilitation on psychological adjustment and use of health services. Lancet 19923391036–1040. [DOI] [PubMed] [Google Scholar]
- 9.Barlow J, Wright C, Sheasby J.et al Self‐management approaches for people with chronic conditions: a review. Pat Educ Couns 200248177–187. [DOI] [PubMed] [Google Scholar]
- 10.Newman S, Steed L, Mulligan K. Self‐management interventions for chronic illness. Lancet 20043641523–1537. [DOI] [PubMed] [Google Scholar]
- 11.Lorig K, Sobel D, Stewart A.et al Evidence suggesting that chronic disease self‐management can improve health status while reducing hospitalisation: a randomized trial. Med Care 1999375–14. [DOI] [PubMed] [Google Scholar]
- 12.Wagner E, Groves T. Care for chronic diseases. BMJ 2002325913–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorig K, Ritter P, Gonzalez V. Hispanic chronic disease self‐management programme. Nurs Res 200352361–369. [DOI] [PubMed] [Google Scholar]
- 14.Fu D, Fu H, McGowan P.et al Implementation and quantitative evaluation of chronic disease self‐management programme in Shanghai, China: randomized controlled trial. Bull World Health Organ 200381174–182. [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths C, Mitlib J, Azad A.et al Randomised controlled trial of a lay‐led self‐management programme for Bangladeshi patients with chronic disease. Br J Gen Pract 200555831–837. [PMC free article] [PubMed] [Google Scholar]
- 16.Wanless D.Securing our future health: taking a long term view. London: HM Treasury, 2002
- 17.Gravelle H, Richardson G, Weatherly H.et alAn assessment of the quality of economic evaluations of self management. York: University of York, Centre for Health Economics, 2003
- 18.Bury M, Newbould J, Taylor D.A rapid review of the current state of knowledge regarding lay led self‐management of chronic illness: evidence review. London: National Institute for Health and Clinical Excellence, 2005
- 19.Cooper J, Thompson J.Stepping stones to success: an implementation, training and support framework for lay led self‐management programmes. London: NHS, 2005
- 20.Medical Research Council A framework for development and evaluation of RCTs for complex interventions to improve health. London: Medical Research Council, 2000
- 21.Bandura A.Self‐efficacy: the exercise of control. New York: WH Freeman and Company, 1997
- 22.Lorig K, Seleznick M, Lubeck D.et al The beneficial outcomes of the arthritis self‐management course are not adequately explained by behaviour change. Arthritis Rheum 19893291–95. [DOI] [PubMed] [Google Scholar]
- 23.Lorig K, Stewart A, Ritter P.et alOutcome measures for health education and other health care interventions. Thousand Oaks: Sage, 1996
- 24.Kind P. The EuroQoL instrument: an index of health‐related quality of life. In: Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott‐Raven, 1996
- 25.Bower P, Kennedy A, Reeves D.et al Recruitment to a trial of self care skills training in long term health conditions: analysis of the impact of patient attitudes and preferences. Contemp Clin Trials 20062749–56. [DOI] [PubMed] [Google Scholar]
- 26.King M, Nazareth I, Lampe F.et al Impact of participant and physician intervention preferences on randomized trials: a systematic review. JAMA 20052931089–1099. [DOI] [PubMed] [Google Scholar]
- 27.Committee for Proprietary Medicinal Products Points to consider on adjustment for baseline covariates. London: The European Agency for the Evaluation of Medicinal Products, 2003
- 28.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol 200154343–349. [DOI] [PubMed] [Google Scholar]
- 29.Assman S, Pocock S, Enos L.et al Subgroup analysis and other misuses of baseline data in clinical trials. Lancet 20003551064–1069. [DOI] [PubMed] [Google Scholar]
- 30.Van Hout B, Al M, Gordon G.et al Costs, effects, and C/E ratios alongside a clinical trial. Health Econ 19943309–313. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy A, Gately C, Rogers A.Process evaluation of the EPP—Report II: examination of the implementation of the Expert Patients Programme within the structures and locality contexts of the NHS in England. Manchester: University of Manchester, National Primary Care Research and Development Centre, 2005
- 32.National Institute of Health and Clinical Excellence Guide to the methods of technology appraisal. London: NICE, 2004 [PubMed]
- 33.Schulz K, Chalmers I, Hayes R.et al Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995273408–412. [DOI] [PubMed] [Google Scholar]
- 34.Charlton B. The future of clinical research: from megatrials towards methodological rigour and representative sampling. J Eval Clin Pract 19962159–169. [DOI] [PubMed] [Google Scholar]
- 35.National Centre for Social Research and University College London Health Survey for England, 2003 [computer file]. Colchester, Essex. UK Data Archive [distributor] 2005
- 36.Dost A.EPP Internal monitoring national level analysis (unpublished report). London: Strategy Directorate, Department of Health, 2005
- 37.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ 20003201530–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]