Abstract
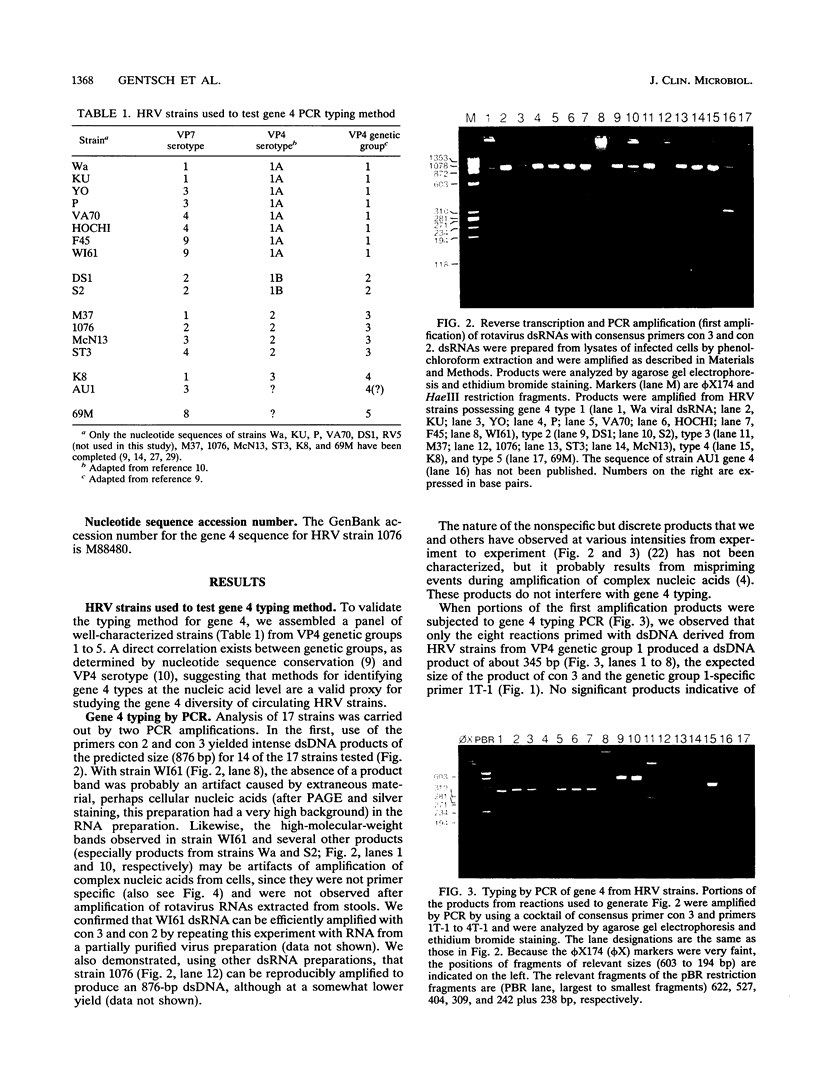
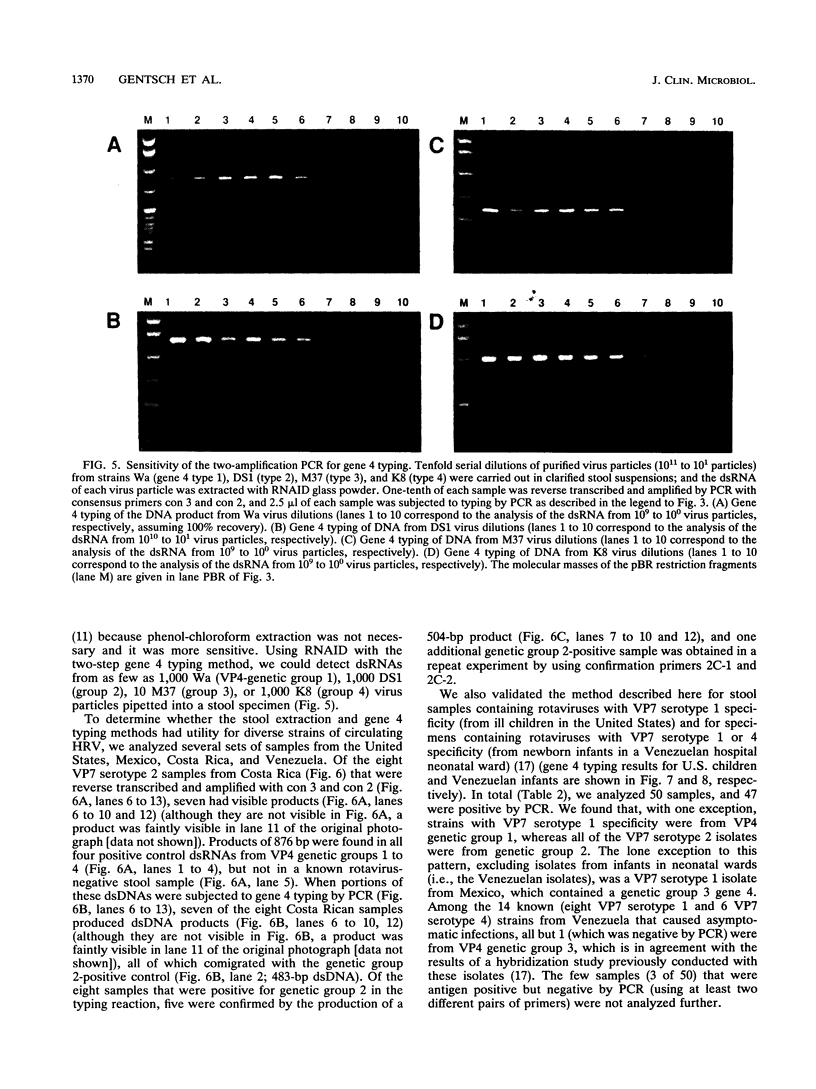
Five genetically distinct human rotavirus (HRV) gene 4 groups have been described on the basis of comparative nucleotide sequencing and the predicted amino acid sequences, and at least four of them represent distinct VP4 antigenic types. To identify each gene 4 type and investigate its distribution in HRV isolates from patients with diarrhea, we developed a polymerase chain reaction (PCR) typing method using sequence information available for four genetically distinct gene 4 types. Rotavirus double-stranded RNAs (dsRNAs) isolated from stool samples were first reverse transcribed and amplified by PCR by using two oligonucleotide primers that correspond to regions that are highly conserved among all known HRV gene 4 types. The 876-bp dsDNA products were then reamplified by PCR in the presence of a cocktail containing one conserved plus-sense primer and four type-specific minus-sense primers (selected from the hypervariable region of gene 4), resulting in products of 345, 483, 267, and 391 bp corresponding to gene 4 types 1, 2, 3, and 4, respectively. This method reliably identified the gene 4 types of 16 well-characterized HRV isolates. Our results were independently confirmed for all 16 strains by reverse transcription and PCR amplification of HRV dsRNA in the presence of alternate type-specific primer pairs. For direct gene 4 typing of HRV in stool samples, we developed a method to extract rotavirus dsRNA from stool specimens by using glass powder. Our results suggest that gene 4 typing will be useful in providing more a complete characterization of HRV strains of epidemiologic or vaccine-related interest.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Fenyö E. M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990 Jul;28(7):1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Sears J., Schael I. P., White L., Garcia D., Lanata C., Kapikian A. Z. Identification of human rotavirus serotype by hybridization to polymerase chain reaction-generated probes derived from a hyperdivergent region of the gene encoding outer capsid protein VP7. J Virol. 1990 Aug;64(8):4021–4024. doi: 10.1128/jvi.64.8.4021-4024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Green K., Nishikawa K., Taniguchi K., Jones R., Kapikian A. Z., Chanock R. M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988 Aug;62(8):2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A. Z., Chanock R. M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Allen J. R., Glass R. I., Fang Z. Y., Bremont M., Cohen J., McCrae M. A., Saif L. J., Sinarachatanant P., Caul E. O. Detection of group B and C rotaviruses by polymerase chain reaction. J Clin Microbiol. 1991 Mar;29(3):519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larralde G., Flores J. Identification of gene 4 alleles among human rotaviruses by polymerase chain reaction-derived probes. Virology. 1990 Nov;179(1):469–473. doi: 10.1016/0042-6822(90)90317-k. [DOI] [PubMed] [Google Scholar]
- Larralde G., Li B. G., Kapikian A. Z., Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991 Jun;65(6):3213–3218. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron C. W., Lew J., Glass R. I., Weber J. M., Ruiz-Palacios G. M. Annual rotavirus epidemic patterns in North America. Results of a 5-year retrospective survey of 88 centers in Canada, Mexico, and the United States. Rotavirus Study Group. JAMA. 1990 Aug 22;264(8):983–988. doi: 10.1001/jama.264.8.983. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl K. A., Gould A. R. Detection and characterisation of bluetongue virus using the polymerase chain reaction. Virus Res. 1991 Sep;21(1):19–34. doi: 10.1016/0168-1702(91)90069-8. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Akatani K., Ikegami N. Identification of rotavirus genogroups by RNA-RNA hybridization. Mol Cell Probes. 1989 Sep;3(3):251–261. doi: 10.1016/0890-8508(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T. Molecular evidence for naturally occurring single VP7 gene substitution reassortant between human rotaviruses belonging to two different genogroups. Arch Virol. 1991;119(1-2):67–81. doi: 10.1007/BF01314324. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. G., Gouvea V. S., Fialho A. M. A comparison of simian rotavirus SA11 preparations maintained in different laboratories. Mem Inst Oswaldo Cruz. 1986 Oct-Dec;81(4):389–393. doi: 10.1590/s0074-02761986000400005. [DOI] [PubMed] [Google Scholar]
- Qian Y., Green K. Y. Human rotavirus strain 69M has a unique VP4 as determined by amino acid sequence analysis. Virology. 1991 May;182(1):407–412. doi: 10.1016/0042-6822(91)90691-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Nishikawa K., Urasawa T., Urasawa S., Midthun K., Kapikian A. Z., Gorziglia M. Complete nucleotide sequence of the gene encoding VP4 of a human rotavirus (strain K8) which has unique VP4 neutralization epitopes. J Virol. 1989 Sep;63(9):4101–4106. doi: 10.1128/jvi.63.9.4101-4106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Woods P. A., Gentsch J., Gouvea V., Mata L., Santosham M., Bai Z. S., Urasawa S., Glass R. I. Distribution of serotypes of human rotavirus in different populations. J Clin Microbiol. 1992 Apr;30(4):781–785. doi: 10.1128/jcm.30.4.781-785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M. A. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J Virol Methods. 1990 Jan;27(1):29–37. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]
- Yamada O., Matsumoto T., Nakashima M., Hagari S., Kamahora T., Ueyama H., Kishi Y., Uemura H., Kurimura T. A new method for extracting DNA or RNA for polymerase chain reaction. J Virol Methods. 1990 Feb;27(2):203–209. doi: 10.1016/0166-0934(90)90136-4. [DOI] [PubMed] [Google Scholar]