Abstract
Background
Many studies have shown a consistent association between ambient air pollution and an increase in death due to cardiovascular causes. An increase in blood pressure is a common risk factor for a variety of cardiovascular diseases. However, the association between air pollution and blood pressure has not been evaluated extensively.
Methods
In this cross‐sectional study, we measured blood pressure in 10 459 subjects who had a health examination from 2001 to 2003, and calculated individual's exposure to ambient levels of air pollutants. To evaluate the relationship between exposure to air pollutants and blood pressure with respect to season, we performed a multiple regression analysis, separately, according to season, controlling for individual characteristics and meteorological variables.
Results
In the warm‐weather season (July–September), particulate air pollutant of <10 μm (PM10) and nitrogen dioxide (NO2) concentrations were significantly associated with measures of blood pressure. During cold weather (October–December), blood pressure was significantly associated with sulphur dioxide (SO2) and ozone (O3) concentrations. The significant association between PM10 or NO2 and blood pressure disappeared during the cold‐weather season.
Conclusion
We found a seasonal variation for the association between ambient air‐pollutant concentrations and blood pressure.
In the past decade, many studies have reported an association between air pollution and daily deaths resulting from either respiratory or cardiovascular causes.1 In particular, exposure to fine particulate air pollution was found to be associated with increased cardiopulmonary deaths.2,3 Although the mechanisms responsible for this association remain unclear, it is commonly explained by alteration in the autonomic nervous system, change in blood coagulability or inflammation. As a result of the observations that exposure to fine particulate air pollutants (<2.5 μm in diameter, PM2.5) is associated with an altered autonomic balance, it might be reasonable to expect that air pollution exposure can affect blood pressure by stimulating sympathetic activation.4,5,6
In the population‐based study by Ibald‐Mulli et al,7 an increase in systolic blood pressure was associated with exposure to total suspended particulates and sulphur dioxide (SO2).7 However, de Paula Santos et al8 have reported that particulate matter (<10 μm in diameter, PM10) and nitrogen dioxide (NO2) concentrations had no significant effect on blood pressure, but concentrations of SO2 were associated with both systolic and diastolic blood pressure. Many other studies have been performed with a variety of subjects or study designs, but the results on the association between air‐pollutant concentrations and blood pressure have been inconsistent.9,10,11,12
It has been shown that the chemical characteristics of the particulate air pollutants change throughout the year.13 Therefore, exposure to particulate air pollutants can affect the short‐term health effects of air pollution differently in different seasons. On the other hand, gaseous air pollutants do not change in quality according to season, possibly giving a robust effect on blood pressure regardless of season.
Therefore, we conducted this study to investigate the effects of particulate and gaseous air pollutants on measures of blood pressure and evaluated the seasonal change of the effects of air pollution.
Methods
Study participants, weather and air pollution data
The subjects were participants at Inha University Hospital (Incheon, South Korea) health examination, from 2001 to 2003. Because of the possible influence of pre‐existing circulatory disease, those who had a medical history of hypertension or other cardiovascular disease were excluded from the study, and all the evaluated individuals were residents of the city. In all, 10 459 (6715 males and 3744 females) individuals participated in the study during three warm‐weather seasons (July–September 2001–2003) and three cold‐weather seasons (October–December 2001–2003). The information obtained included age, sex, smoking habits and alcohol consumption. In addition, weight and height were measured at the time of the examination. Blood pressure measurements were recorded by nurses from both arms in a sitting position after at least a 5 min resting period using a fully automated electronic device (BP‐203RVII, Colin Corporation, Tokyo, Japan) and the mean values from both measurements were recorded. The blood pressure measurements and other health examinations were performed in the morning from 9:00 to 12:00. Automated instruments for measuring blood pressure are known to reduce observer error, ensuring that recorded data are accurate.14 We also collected data on serum total cholesterol and fasting blood sugar.
The city of Incheon is the third largest city in Korea and located on the western side of the country, with the sea to the west and metropolitan Seoul to the east. It has a four‐season climate and low‐level temperature inversions are common during the winter months. Data on air pollutants (PM10, NO2, SO2 and O3) were provided by the National Institute of Environmental Research (Korea). We calculated the hourly mean of PM10, NO2 and SO2 by averaging the data from nine monitoring stations and then computed their 24 h averages. The 8 h average for O3 between 9:00 and 17:00 was calculated from the data obtained from the monitoring stations because we thought daytime exposure to O3 is more relevant to health effects than 24 h averages. Hourly mean temperature, humidity and barometric pressure data were obtained from the Incheon Meteorological Administration. Our study was approved by the Institutional Review Board of Inha University Hospital and written informed consent was obtained from all participants.
Statistical analysis
The associations of personal characteristics and blood pressure were assessed using the t test and analysis of variance. To evaluate the relationship between exposure to air pollutants and blood pressure with respect to season, we performed a regression analysis separately according to season controlling for age, sex, body mass index, total cholesterol, fasting blood sugar, smoking habits and alcohol consumption in addition to meteorological variables such as temperature, relative humidity and barometric pressure. Air pollutants may affect blood pressure with a time lag and the appropriate average time for exposure may exceed 24 h. Therefore, exposures from the same day to the 3 days before (lag0, lag1, lag2 and lag3) were examined. The 24 h averages of weather variables subject to corresponding lag days were also used in the statistical models. All tests of statistical significance were two‐sided and p<0.01 was considered significant. Statistical analyses were conducted using the SAS V.8.02.
Results
The subjects included 6715 males and 3744 females with a mean (SD) age of 43.08 (12.3) years, mean (SD) height 165.3 (8.7) cm and mean (SD) weight 64.4 (11.1) kg (table 1). Age, sex, height, weight, cholesterol, glucose, smoking and alcohol consumption were found to be significantly associated with blood pressure (p<0.01).
Table 1 Characteristics of the study population and the relationship with blood pressure (n = 10 459).
| Variables | n (%) | Blood pressure (mean (SD)) (mm Hg) | |||
|---|---|---|---|---|---|
| Systolic BP | p Value | Diastolic BP | p Value | ||
| Gender | |||||
| Male | 6715 (64.2) | 127.2 (15.8) | <0.001 | 77.6 (11.1) | <0.001 |
| Female | 3744 (35.8) | 119.1 (16.6) | 72.4 (10.7) | ||
| Age (years) | |||||
| ⩾43 | 5258 (50.3) | 126.1 (17.7) | <0.001 | 77.5 (11.5) | <0.001 |
| <43 | 5201 (49.7) | 122.4 (15) | 74.0 (10.6) | ||
| Height (cm) | |||||
| ⩾166 | 5070 (48.5) | 126.4 (15.2) | <0.001 | 77.0 (10.8) | <0.001 |
| <166 | 5389 (51.5) | 122.3 (17.4) | 74.5 (11.4) | ||
| Weight (kg) | |||||
| ⩾64 | 5309 (50.8) | 127.4 (15.6) | <0.001 | 78.0 (11) | <0.001 |
| <64 | 5150 (49.2) | 121.1 (16.9) | 73.4 (11) | ||
| Body mass index (kg/m2) | |||||
| ⩾23.4 | 5219 (49.9) | 127.3 (16.2) | <0.001 | 78.0 (11) | <0.001 |
| <23.4 | 5240 (50.1) | 121.3 (16.3) | 73.5 (10.9) | ||
| Cholesterol (mg/dl) | |||||
| ⩾185 | 5267 (50.4) | 126.2 (16.8) | <0.001 | 77.4 (11.3) | <0.001 |
| <185 | 5192 (49.6) | 122.3 (16) | 74.0 (10.9) | ||
| Glucose (mg/dl) | |||||
| ⩾94 | 5377 (51.4) | 127.6 (6.7) | <0.001 | 77.9 (11.3) | <0.001 |
| <94 | 5082 (48.6) | 120.8 (15.6) | 73.4 (0.7) | ||
| Smoking | |||||
| Never smoker | 5518 (52.8) | 122.3 (17) | <0.001 | 74.5 (11.3) | <0.001 |
| Former smoker | 1041 (9.9) | 127.9 (16.4) | 78.3 (11.1) | ||
| Current smoker | 3900 (37.3) | 126.0 (15.5) | 76.8 (10.9) | ||
| Alcohol consumption | |||||
| Non‐drinker | 4590 (43.9) | 121.9 (16.8) | <0.001 | 74.2 (10.9) | <0.001 |
| 2–3 times/month | 2247 (21.5) | 124.0 (15.6) | 75.3 (10.8) | ||
| 1–2 times/week | 2513 (24) | 126.7 (16) | 77.3 (11.3) | ||
| Almost everyday | 842 (8) | 129.0 (16.7) | 79.7 (11.6) | ||
| Everyday | 267 (2.6) | 129.6 (16.4) | 79.7 (11) | ||
BP, blood pressure.
Table 2 shows the air‐pollutant concentrations and weather conditions for each season. The 24 h average (SD) concentrations of PM10, NO2 and SO2 were 42.1 (20.9) μg/m3, 22.5 (7.1) ppb and 5.1 (1.4) ppb for the warm‐weather season (July–September), and 53.5 (29.3) μg/m3, 29.2 (11.8) ppb and 6.9 (2.3) ppb for the cold‐weather season (October–December), respectively. The 8 h average (SD) concentrations of O3 were 26.6 (11.8) and 17.5 (7.3) ppb, respectively.
Table 2 Distribution of air pollution concentrations and meteorological measures.
| Pollutants | Mean | SD | Minimum | 25% | Median | 75% | Maximum |
|---|---|---|---|---|---|---|---|
| Warm‐weather season (July–September) | |||||||
| PM10 (μg/m3) | 42.1 | 20.9 | 15.4 | 26.7 | 36.7 | 52.2 | 136.7 |
| NO2 (ppb) | 22.5 | 7.1 | 8.4 | 17.2 | 22.3 | 26.9 | 49.3 |
| SO2 (ppb) | 5.1 | 1.4 | 2.5 | 4.0 | 5.0 | 5.9 | 11.3 |
| O3 (ppb) | 26.6 | 11.8 | 5.2 | 17.4 | 26.9 | 34.8 | 62.4 |
| Weather | |||||||
| Temperature (°C) | 23.8 | 2.5 | 16.8 | 22.0 | 23.9 | 25.4 | 30.9 |
| Humidity (%) | 77.3 | 10.9 | 41.7 | 70.0 | 79.5 | 85.5 | 95.8 |
| Air pressure (hPa) | 1009.7 | 5.3 | 990.8 | 1006.4 | 1010.0 | 1013.0 | 1025.6 |
| Cold‐weather season (October–December) | |||||||
| PM10 (μg/m3) | 53.5 | 29.3 | 11.9 | 34.3 | 45.7 | 64.5 | 209.6 |
| NO2 (ppb) | 29.2 | 11.8 | 9.9 | 19.9 | 27.2 | 34.7 | 74.0 |
| SO2 (ppb) | 6.9 | 2.3 | 2.9 | 5.3 | 6.5 | 8.1 | 15.1 |
| O3 (ppb) | 17.5 | 7.3 | 3.8 | 10.9 | 17.4 | 22.9 | 33.9 |
| Weather | |||||||
| Temperature (°C) | 8.0 | 6.9 | −5.2 | 2.4 | 7.7 | 14.1 | 20.3 |
| Humidity (%) | 62.0 | 12.6 | 33.7 | 53.2 | 61.1 | 71.5 | 92.4 |
| Air pressure (hPa) | 1022.1 | 5.5 | 1006.0 | 1018.4 | 1021.9 | 1025.3 | 1035.9 |
PM10, particulate air pollutant of <10 μm in diameter.
In the warm‐weather season, PM10 concentrations were significantly associated with systolic blood pressure of lag0–lag2 days and diastolic blood pressure of lag0 and lag1 days (p<0.01). Considering the distribution of lag effects of PM10 exposure in the adjusted model, we found that the 1‐day‐lag model was the most appropriate for evaluating the air pollution effect on blood pressure. Concentrations of NO2 were also significantly associated with blood pressure of lag1 day. However, SO2 and O3 concentrations were not significantly associated with blood pressure of lag1 day in the adjusted model (p<0.01; table 3). Outdoor temperature was significantly associated with blood pressure in the warm‐weather season.
Table 3 Effects of air pollutants on the systolic and diastolic blood pressure of participants in the warm‐weather season (July–September).
| Observations (n) | Regression coefficient (p value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before adjusted | After adjusted | ||||||||
| Present | Lag1 day | Lag2 day | Lag3 day | Present | Lag1 day | Lag2 day | Lag3 day | ||
| PM10 | |||||||||
| sBP | 6095 | 0.0798 (<0.001) | 0.0791 (<0.001) | 0.0514 (<0.001) | 0.0355 (<0.001) | 0.0651 (<0.001) | 0.0702 (<0.001) | 0.0387 (<0.001) | 0.0233 (0.023) |
| dBP | 6095 | 0.0240 (0.001) | 0.0210 (<0.001) | 0.0073 (0.151) | 0.0010 (0.848) | 0.0175 (0.004) | 0.0195 (0.001) | 0.0083 (0.101) | 0.0056 (0.29) |
| NO2 | |||||||||
| sBP | 6095 | 0.1453 (<0.001) | 0.1605 (<0.001) | 0.0263 (0.331) | −0.0163 (0.55) | 0.1131 (0.002) | 0.1858 (<0.001) | −0.0188 (0.534) | −0.0431 (0.147) |
| dBP | 6095 | 0.0157 (0.444) | 0.0157 (0.426) | −0.0368 (0.047) | −0.0618 (0.001) | 0.0113 (0.645) | 0.0544 (0.016) | −0.0201 (0.331) | −0.0261 (0.197) |
| SO2 | |||||||||
| sBP | 6095 | 0.0875 (0.518) | 0.4526 (0.001) | 0.0835 (0.512) | −0.1341 (0.327) | −0.2503 (0.066) | 0.2443 (0.114) | −0.2182 (0.089) | −0.3718 (0.007) |
| dBP | 6095 | 0.2764 (0.003) | 0.3290 (0.001) | 0.0448 (0.607) | 0.0113 (0.904) | 0.0571 (0.539) | 0.2022 (0.022) | 0.0179 (0.838) | 0.0731 (0.434) |
| O3 | |||||||||
| sBP | 6095 | −0.0239 (0.199) | −0.0126 (0.468) | −0.0006 (0.971) | −0.0006 (0.973) | −0.0338 (0.067) | 0.0381 (0.058) | 0.0357 (0.039) | 0.0002 (0.989) |
| dBP | 6095 | 0.0011 (0.932) | −0.0117 (0.325) | −0.0163 (0.157) | −0.0073 (0.512) | 0.0045 (0.723) | 0.0068 (0.618) | 0.0110 (0.35) | 0.0093 (0.395) |
dBP, diastolic blood pressure; PM10, particulate air pollutant at <10 μm diameter; sBP, systolic blood pressure.
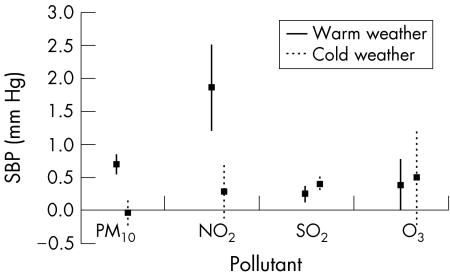
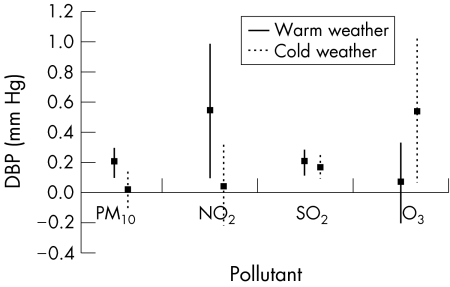
For the cold‐weather season, the pollutants were associated with blood pressure differently. Systolic blood pressure was significantly associated with concentrations of SO2 1 day before. In addition, systolic blood pressure of lag0 and lag2 was significantly associated with O3 concentrations (table 4). However, the significant association between PM10 or NO2 concentrations and blood pressure disappeared during the cold‐weather season. When we evaluated the interactive effect between season and air pollutants, we found that PM10, NO2 and O3 were significantly interactive with season for the effect on blood pressure whereas there was no significant interaction between SO2 and season. Figures 1 and 2 show the pollutant effect on blood pressure of lag1 day by an increase of 10 μg/m3 or ppb for PM10, NO2 and O3 and an increase of 1 ppb for SO2 according to season. Outdoor temperature was not significantly associated with blood pressure in the cold‐weather season.
Table 4 Effect of air pollutants on the systolic and diastolic blood pressure in the cold‐weather season (October–December).
| Observations (n) | Regression coefficient (p value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before adjusted | After adjusted | ||||||||
| Present | Lag1 day | Lag2 day | Lag3 day | Present | Lag1 day | Lag2 day | Lag3 day | ||
| PM10 | |||||||||
| sBP | 4364 | −0.0190 (0.028) | −0.0110 (0.265) | −0.0240 (0.008) | −0.0147 (0.083) | −0.0114 (0.178) | −0.0032 (0.749) | −0.0169 (0.06) | −0.0173 (0.04) |
| dBP | 4364 | −0.0171 (0.003) | −0.0093 (0.159) | −0.0133 (0.027) | −0.0126 (0.025) | −0.0103 (0.068) | 0.0007 (0.917) | −0.0041 (0.494) | −0.0125 (0.025) |
| NO2 | |||||||||
| sBP | 4364 | 0.0034 (0.863) | 0.0073 (0.723) | −0.0429 (0.043) | −0.0167 (0.417) | 0.0256 (0.181) | 0.0275 (0.195) | −0.0273 (0.223) | −0.0118 (0.579) |
| dBP | 4364 | −0.0244 (0.062) | −0.0226 (0.1) | −0.0230 (0.105) | −0.0277 (0.044) | −0.0072 (0.573) | 0.0035 (0.802) | 0.0114 (0.445) | −0.0198 (0.16) |
| SO2 | |||||||||
| sBP | 4364 | −0.1290 (0.289) | 0.1602 (0.19) | −0.1677 (0.196) | −0.1650 (0.218) | −0.0074 (0.951) | 0.3897 (0.001) | 0.0670 (0.57) | 0.0842 (0.515) |
| dBP | 4364 | −0.1653 (0.042) | 0.0207 (0.8) | 0.0058 (0.947) | −0.1106 (0.216) | −0.1217 (0.126) | 0.1596 (0.043) | 0.1596 (0.051) | 0.0025 (0.977) |
| O3 | |||||||||
| sBP | 4364 | 0.1345 (<0.001) | 0.0685 (0.058) | 0.1497 (<0.001) | 0.0705 (0.034) | 0.1170 (0.001) | 0.0490 (0.177) | 0.1413 (<0.001) | 0.0157 (0.654) |
| dBP | 4364 | 0.0316 (0.148) | 0.0382 (0.113) | 0.0313 (0.158) | 0.0150 (0.498) | 0.0397 (0.066) | 0.0532 (0.027) | 0.0247 (0.288) | 0.0132 (0.569) |
dBP, diastolic blood pressure; PM10, particulate air pollutant <10 μm in diameter; sBP, systolic blood pressure.
Figure 1 Systolic blood pressure (SBP) change by the increase of 10 μg/m3 or ppb for particulate air pollutant of <10 μm in diameter (PM10), NO2 and O3 and an increase of 1 ppb for SO2 1 day before, according to season. Solid and dotted lines indicate 95% CI.
Figure 2 Diastolic blood pressure change (DBP) by the increase of 10 μg/m3 or ppb for particulate air pollutant of <10 μm in diameter (PM10), NO2 and O3 and an increase of 1 ppb for SO2 1 day before according to season. Solid and dotted lines indicate 95% CI.
Discussion
We found that air pollution was associated with blood pressure. In the warm‐weather season, PM10 and NO2 concentrations were significantly associated with measures of blood pressure. During cold weather, blood pressure was significantly associated with SO2 and O3. Particularly, PM10 concentrations were consistently associated with blood pressure of lag0–lag2 days in the warm‐weather season. Therefore, it might be postulated that the PM10 is the pollutant most responsible for the persistent effect on blood pressure in the warm weather. Exposure to an increase of 100 μg/m3 of PM10 is predicted to increase 7.0 mm Hg systolic and 2.0 mm Hg diastolic blood pressure of lag1 day.
We found that NO2 concentrations were significantly associated with systolic blood pressure of lag0–lag1 days during the warm‐weather seasons. Even though there is not enough evidence to support this association such as increased resting heart rate or cardiovascular disease risk in response to elevated NO2 concentrations, other reported findings also suggest that NO2 may have a significant role in elevating blood pressure.15,16 In the case of SO2, an observed effect on increase in blood pressure was noted in our study and the same result was reported in the study by de Paula Santos et al.8 Ibald‐Mulli et al7 also found a positive association between SO2 concentration and systolic blood pressure. However, there have been studies on animal exposure to SO2, showing the opposite effect the rats exposed to SO2 had decreased blood pressure in a dose‐dependent manner.17,18 The explanation for these contradictory results between the animal model and human population is unclear. However, the exposure concentrations of SO2 in the animal experiments were 10–50 ppm, which is not comparable to the ambient concentrations of SO2.
We found a significant association between O3 and blood pressure in the cold‐weather season. Ozone is a highly reactive gas, but cellular responses to O3 may not be the result of direct reaction of O3 with cell‐surface components. They are mediated through a cascade of secondary, free‐radical‐derived ozonation products.19 Thus, it is possible that O3 could promote systemic oxidative stress through activation of lung macrophages or alveolar cells by interaction with the cell membranes.20
There is little information available on the underlying biological mechanisms that might explain the change of blood pressure in association with air pollutants. A study on the acute effect of inhaled urban air‐pollution particles in the rat showed increased plasma levels of endothelin‐1, which is thought to have an active role in the maintenance of basal systemic vascular tone.21 In the animal model, injection of endothelin‐1 leads to dose‐related increases in sympathetic nerve activity, therefore alteration in the central endothelin‐1 system could result in blood pressure elevation.22 However, these studies have been performed in animal models and therefore, the mechanisms require confirmation in the human population.
In a controlled experimental design, Urch et al6 found evidence that air pollution actually has a causal role in elevating blood pressure. In a study on healthy adults, Brook et al23 showed that inhalation of PM2.5 and O3 causes acute arterial vasoconstriction. A potential biological mechanism for vasoconstriction was suggested to include a reflex increase in the sympathetic nervous system activity. Oxidative stress and subsequent systemic inflammation caused by air pollution is also suggested as a possible mechanism for many cardiovascular diseases including hypertension.24 PM2.5 inhalation has been shown to induce systemic inflammation, which is possibly related to the free radical activity of components in particulate matter.25 Cytokines have been reported to be involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants.26
In this study, PM10‐induced increases in blood pressure were observed in the warm‐weather season whereas no significant effect was found in the cold‐weather season. The seasonal variation may be explained by the fact that particles from different seasons have different toxicity profiles due to the different composition, concentrations and dimensions of the particles.27 We also found that gaseous air pollutants affected blood pressure differently according to the season. In particular, NO2 showed the same pattern of seasonal change with PM10 for the effect, indicating that NO2 possibly represents toxic chemicals shared with particulate pollutants. SO2 and O3 showed a relatively smaller change of effect between seasons, even though their effects on blood pressure were more apparent in the cold weather.
Our results are subject to the following limitations. First, we used environmental monitoring data for the exposure of each individual to air pollution. Therefore, measurement errors resulting from differences between actual exposure and ambient levels cannot be avoided. However, this error is more likely to cause a bias towards the null and underestimate the pollution effects. Second, there is also a possibility of error in the blood pressure measurement because we used the data measured once from both arms by an automated electronic device. The possible misclassification of blood pressure could cause an underestimation of the pollutant effect if the measurement error occurred non‐differentially. Third, the cross‐sectional design of this study makes causal inference limited. We can only infer that an individual sampled on a higher‐pollution day tends to have a higher blood pressure than an individual sampled on a lower‐pollution day.
Despite these limitations, this study has several strengths. We controlled many potential confounding factors such as body mass index, total cholesterol, fasting glucose, smoking and alcohol consumption in the statistical model. In addition, our findings were based on blood pressure measurements for a large number of subjects. Therefore, our findings strongly support the evidence that air pollution is associated with an increase in blood pressure. Even though a relatively small increase in blood pressure was found during the daily change in level of air pollution, this increase in blood pressure could increase the risk of cardiovascular diseases and strokes in susceptible individuals. In addition, the mean increase in blood pressure could indicate that a susceptible subgroup might actually experience a much greater effect because susceptibility to air pollution differs within the population.28
In conclusion, our study showed the association between ambient air‐pollutant concentrations and blood pressure. PM10 and NO2 concentrations were significantly associated with blood pressure in the warm season whereas SO2 and O3 were more likely associated with blood pressure in the cold season. As we increase our understanding that the exposure to air pollution leads to elevated blood pressure, which causes an increase in risk for a cardiovascular event or stroke, it will be necessary to keep air pollution level as low as possible for prevention of blood pressure‐related diseases.
What is already known about the topic
The study results on the association between air pollution concentrations and blood pressure have been inconsistent
Exposure to air pollution can affect short‐term health effects differently in different seasons
What this paper adds
This study shows the association between ambient air‐pollutant concentrations and blood pressure
The effect of air pollution on blood pressure varies between warm‐weather and cold‐weather seasons
PM10 and NO2 concentrations were significantly associated with blood pressure in the warm season whereas SO2 and O3 were more likely associated with blood pressure in the cold season
Policy implications
Increase the understanding of the effect of air pollution on health risk and its impact on vulnerable persons to high blood pressure
Necessary actions to keep air pollution level as low as possible for prevention of circulatory diseases and other pollution‐related diseases
Target pollutant‐oriented strategies to reduce emission of hazardous pollutants, particularly particulate pollutants, in accordance with seasonal characteristics
Abbreviations
PM2.5 - particulate air pollutant of <2.5 μm diameter
PM10 - particulate air pollutant of <10 μm diameter
Footnotes
Funding: This study was supported by Ecotechnopia 21 Project of Ministry of Environment, South Korea.
Competing interests: None declared.
References
- 1.Zmirou D, Schwartz J, Saez M.et al Time‐series analysis of air pollution and cause‐specific mortality. Epidemiology 19989495–503. [PubMed] [Google Scholar]
- 2.Pope C A, 3rd, Burnett R T, Thun M J.et al Lung cancer, cardiopulmonary mortality, and long‐term exposure to fine particulate air pollution. JAMA 20022871132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samet J M, Dominici F, Curriero F C.et al Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 20003431742–1749. [DOI] [PubMed] [Google Scholar]
- 4.Devlin R B, Ghio A J, Kehrl H.et al Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl 20034076s–80s. [DOI] [PubMed] [Google Scholar]
- 5.Magari S R, Hauser R, Schwartz J.et al Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001104986–991. [DOI] [PubMed] [Google Scholar]
- 6.Urch B, Silverman F, Corey P.et al Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 20051131052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibald‐Mulli A, Stieber J, Wichmann H E.et al Effects of air pollution on blood pressure: a population‐based approach. Am J Public Health 200191571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Paula Santos U, Braga A L, Giorgi D M.et al Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur Heart J 200426193–200. [DOI] [PubMed] [Google Scholar]
- 9.Linn W S, Gong H, Jr, Clark K W.et al Day‐to‐day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc 199949108–115. [DOI] [PubMed] [Google Scholar]
- 10.Brauer M, Ebelt S T, Fisher T V.et al Exposure of chronic obstructive pulmonary disease patients to particles: respiratory and cardiovascular health effects. J Expo Anal Environ Epidemiol 200111490–500. [DOI] [PubMed] [Google Scholar]
- 11.Zanobetti A, Canner M J, Stone P H.et al Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation 20041102184–2189. [DOI] [PubMed] [Google Scholar]
- 12.Ibald‐Mulli A, Timonen K L, Peters A.et al Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect 2004112369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoletti L, De Berardis B, Diociaiuti M. Physico‐chemical characterisation of the inhalable particulate matter (PM10) in an urban area: an analysis of the seasonal trend. Sci Total Environ 2002292265–275. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien E, Waeber B, Parati G.et al Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ 2001322531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruidavets J B, Cassadou S, Cournot M.et al Increased resting heart rate with pollutants in a population based study. J Epidemiol Commun Health 200559685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grazuleviciene R, Maroziene L, Dulskiene V.et al Exposure to urban nitrogen dioxide pollution and the risk of myocardial infarction. Scand J Work Environ Health 200430293–298. [DOI] [PubMed] [Google Scholar]
- 17.Drew R T, Kutzman R S, Costa D L.et al Effects of sulfur dioxide and ozone on hypertension sensitive and resistant rats. Fundam Appl Toxicol 19833298–302. [DOI] [PubMed] [Google Scholar]
- 18.Meng Z Q, Geng H, Bai J.et al Blood pressure of rats lowered by sulfur dioxide and its derivatives. Inhalation Toxicol 200315951–959. [DOI] [PubMed] [Google Scholar]
- 19.Kelly F J. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 200360612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson K, Stone V, Borm P J.et al Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol Med 2003341369–1382. [DOI] [PubMed] [Google Scholar]
- 21.Bouthillier L, Vincent R, Goegan P.et al Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin‐1. Am J Pathol 19981531873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Sasaki S, Moriguchi J.et al Central effects of endothelin and its antagonists on sympathetic and cardiovascular regulation in SHR‐SP. J Cardiovasc Pharmacol 199933876–882. [DOI] [PubMed] [Google Scholar]
- 23.Brook R D, Brook J R, Urch B.et al Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 20021051534–1536. [DOI] [PubMed] [Google Scholar]
- 24.Oparil S, Zaman M A, Calhoun D A. Pathogenesis of hypertension. Ann Intern Med 2003139761–776. [DOI] [PubMed] [Google Scholar]
- 25.Dagher Z, Garcon G, Gosset P.et al Pro‐inflammatory effects of Dunkerque city air pollution particulate matter 2.5 in human epithelial lung cells (L132) in culture. J Appl Toxicol 200525166–175. [DOI] [PubMed] [Google Scholar]
- 26.Ishii H, Fujii T, Hogg J C.et al Adenoviral E1A modulates inflammatory mediator expression by lung epithelial cells exposed to PM10. Am J Physiol Lung Cell Mol Physiol 2003284L290–L297. [DOI] [PubMed] [Google Scholar]
- 27.Becker S, Dailey L A, Soukup J M.et al Seasonal variations in air pollution particle‐induced inflammatory mediator release and oxidative stress. Environ Health Perspect 20051131032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward D J, Ayres J G. Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med 200461e13. [DOI] [PMC free article] [PubMed] [Google Scholar]