Abstract
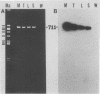
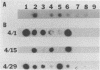
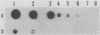
Carrier cattle infected with Babesia bovis are difficult to detect because of the low numbers of parasites that occur in peripheral blood. However, diagnosis of low-level infections with the parasite is important for evaluating the efficacies of vaccines and in transmission and epidemiological studies. We used the polymerase chain reaction (PCR) to amplify a portion of the apocytochrome b gene from the parasite and tested the ability of this method to detect carrier cattle. The target sequence is associated with a 7.4-kb DNA element in undigested B. bovis genomic DNA (as shown previously), and the amplified product was detected by Southern and dot blot hybridization. The assay was specific for B. bovis, since no amplification was detected with Babesia bigemina, Trypanosoma brucei, Anaplasma marginale, or leukocyte DNA. The target sequence was amplified in DNA from B. bovis Mexico, Texas, and Australia S and L strains, demonstrating the applicability of the method to strains from different geographic regions. The sensitivity of the method ranged from 1 to 10 infected erythrocytes extracted from 0.5 ml of blood. This sensitivity was about 1,000 times greater than that from the use of unamplified parasite DNA. By the PCR method, six B. bovis carrier cattle were detected 86% of the time (range, 66 to 100%) when they were tested 11 times, while with microscopic examination of thick blood smears, the same carrier cattle were detected only 36% of the time (range, 17 to 66%). The method provides a useful diagnostic tool for detecting B. bovis carrier cattle, and the sensitivity is significantly improved over that of current methods. The results also suggest that characteristics of the apocytchrome b gene may make this a valuable target DNA for PCR-based detection of other hemoparasites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldritt S. M., Joseph J. T., Wirth D. F. Sequence identification of cytochrome b in Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3614–3620. doi: 10.1128/mcb.9.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio R. E., Potgieter F. T., Nel N. A column purification procedure for the removal of leucocytes from parasite-infected bovine blood. Onderstepoort J Vet Res. 1986 Sep;53(3):179–180. [PubMed] [Google Scholar]
- Curnow J. A. Studies on antigenic changes and strain differences in Babesia argentina infections. Aust Vet J. 1973 Jun;49(6):279–283. doi: 10.1111/j.1751-0813.1973.tb06806.x. [DOI] [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Jasmer D. P., Reduker D. W., Goff W. L., Stiller D., McGuire T. C. DNA probes distinguish geographical isolates and identify a novel DNA molecule of Babesia bovis. J Parasitol. 1990 Dec;76(6):834–841. [PubMed] [Google Scholar]
- Joseph J. T., Aldritt S. M., Unnasch T., Puijalon O., Wirth D. F. Characterization of a conserved extrachromosomal element isolated from the avian malarial parasite Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3621–3629. doi: 10.1128/mcb.9.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther D. G., Cox H. U., Nelson W. O. Comparisons of serotests with calf inoculations for detection of carriers in anaplasmosis- vaccinated cattle. Am J Vet Res. 1980 Dec;41(12):2085–2086. [PubMed] [Google Scholar]
- Mahoney D. F. Bovine babesiasis: a study of factors concerned in transmission. Ann Trop Med Parasitol. 1969 Mar;63(1):1–14. doi: 10.1080/00034983.1969.11686595. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Ketterer P. J. Babesia argentina: the infectivity and immunogenicity of irradiated blood parasites for splenectomized calves. Int J Parasitol. 1973 Mar;3(2):209–217. doi: 10.1016/0020-7519(73)90026-x. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Mirre G. B. Bovine babesiasis: the persistence of immunity to Babesia argentina and B. bigemina in calves (Bos taurus) after naturally acquired infection. Ann Trop Med Parasitol. 1973 Jun;67(2):197–203. doi: 10.1080/00034983.1973.11686877. [DOI] [PubMed] [Google Scholar]
- McLaughlin G. L., Edlind T. D., Ihler G. M. Detection of Babesia bovis using DNA hybridization. J Protozool. 1986 Feb;33(1):125–128. doi: 10.1111/j.1550-7408.1986.tb05571.x. [DOI] [PubMed] [Google Scholar]
- Megson A., Inman G. J., Hunt P. D., Baylis H. A., Hall R. The gene for apocytochrome B of Theileria annulata resides on a small linear extrachromosomal element. Mol Biochem Parasitol. 1991 Sep;48(1):113–115. doi: 10.1016/0166-6851(91)90171-2. [DOI] [PubMed] [Google Scholar]
- Reduker D. W., Jasmer D. P., Goff W. L., Perryman L. E., Davis W. C., McGuire T. C. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol Biochem Parasitol. 1989 Jul;35(3):239–247. doi: 10.1016/0166-6851(89)90210-7. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C. E., Palmer G. H., Jasmer D. P., Hines S. A., Perryman L. E., McElwain T. F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol Biochem Parasitol. 1991 May;46(1):45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Akella R., Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989 Jun 15;35(2):97–107. doi: 10.1016/0166-6851(89)90112-6. [DOI] [PubMed] [Google Scholar]
- Wright I. G. Immunodiagnosis of and immunoprophylaxis against the haemoparasites Babesia sp. and Anaplasma sp. in domestic animals. Rev Sci Tech. 1990 Jun;9(2):345–356. doi: 10.20506/rst.9.2.508. [DOI] [PubMed] [Google Scholar]