Abstract
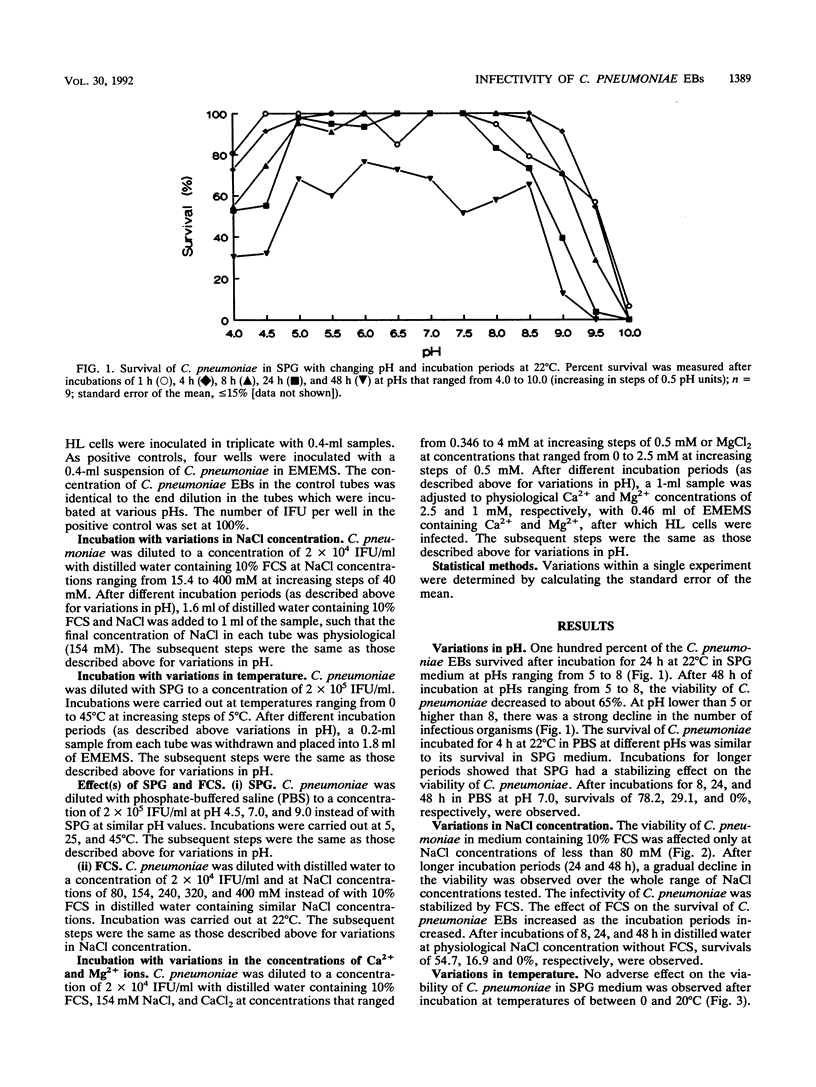
The influence of variations in the pH, NaCl concentration, temperature, and concentrations of calcium and magnesium ions on the survival of Chlamydia pneumoniae elementary bodies (EBs) outside the host cells was investigated. The survival was determined after various incubation periods by counting the inclusion-forming units after C. pneumoniae was cultured for 72 h on monolayers of HL cells. The normal physiological conditions were restored prior to infecting the HL cells with C. pneumoniae. Declines in the infectivities of C. pneumoniae EBs were observed at pH values of lower than 5 and higher than 8 or at NaCl concentrations of less than 80 mM. The viability of C. pneumoniae EBs in SPG medium decreased as the temperature and/or incubation period increased. Incubation temperatures of up to 20 degrees C and incubation periods of up to 48 h did not affect the viability of C. pneumoniae. One hundred percent of the C. pneumoniae EBs were infective after 1 h of incubation at 35 degrees C, whereas 90, 50, and 40% survived after incubations of 8, 24, and 48 h, respectively. The viability of C. pneumoniae was unaffected within the investigated range of Ca2+ and Mg2+ ion concentrations in the medium. The presence of 10% fetal calf serum in the incubation medium had a stabilizing effect on the viability of C. pneumoniae. This effect became more pronounced as the incubation period increased.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cles L. D., Stamm W. E. Use of HL cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1990 May;28(5):938–940. doi: 10.1128/jcm.28.5.938-940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Kuo C. C., Campbell L. A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989 Mar;8(3):191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- Hyman C. L., Augenbraun M. H., Roblin P. M., Schachter J., Hammerschlag M. R. Asymptomatic respiratory tract infection with Chlamydia pneumoniae TWAR. J Clin Microbiol. 1991 Sep;29(9):2082–2083. doi: 10.1128/jcm.29.9.2082-2083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemola M., Saikku P., Visakorpi R., Wang S. P., Grayston J. T. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988 Feb;157(2):230–236. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Sep;162(3):755–758. doi: 10.1093/infdis/162.3.755. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988 May;26(5):812–815. doi: 10.1128/jcm.26.5.812-815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Grayston J. T., Wang S. P., Kuo C. C. Pneumonia associated with the TWAR strain of Chlamydia. Ann Intern Med. 1987 Apr;106(4):507–511. doi: 10.7326/0003-4819-106-4-507. [DOI] [PubMed] [Google Scholar]
- Narita T., Wyrick P. B., Manire G. P. Effect of alkali on the structure of cell envelopes of Chlamydia psittaci elementary bodies. J Bacteriol. 1976 Jan;125(1):300–307. doi: 10.1128/jb.125.1.300-307.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K. Epidemiological and clinical aspects on infections due to Chlamydia pneumoniae (strain TWAR). Scand J Infect Dis Suppl. 1990;69:63–67. [PubMed] [Google Scholar]
- Seelig J. Interaction of phospholipids with Ca2+ ions. On the role of the phospholipid head groups. Cell Biol Int Rep. 1990 Apr;14(4):353–360. doi: 10.1016/0309-1651(90)91204-h. [DOI] [PubMed] [Google Scholar]
- Thom D. H., Grayston J. T., Wang S. P., Kuo C. C., Altman J. Chlamydia pneumoniae strain TWAR, Mycoplasma pneumoniae, and viral infections in acute respiratory disease in a university student health clinic population. Am J Epidemiol. 1990 Aug;132(2):248–256. doi: 10.1093/oxfordjournals.aje.a115654. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Nakada H., Sakurai N., Kuo C. C., Wang S. P., Grayston J. T. Transmission of Chlamydia pneumoniae in young children in a Japanese family. J Infect Dis. 1990 Dec;162(6):1390–1392. doi: 10.1093/infdis/162.6.1390. [DOI] [PubMed] [Google Scholar]


