Abstract
Objectives
To explore whether the predictive power of mid‐life ECG abnormalities and conventional cardiovascular risk factors for future stroke change over a 30‐year follow‐up period, and whether a repeated examination improves their predictive power.
Design and setting
Longitudinal population‐based study.
Participants
2322 men aged 50 years, with a follow‐up period of 30 years. 1221 subjects were re‐examined at age 70 years
Main outcome measure
Risk for fatal and non‐fatal stroke during three decades of follow‐up. Investigations included resting ECG and traditional cardiovascular risk factors.
Results
When measured at age 50 years, ST segment depression and T wave abnormalities, together with ECG‐left ventricular hypertrophy, were of importance only during the first 20 years, but regained importance when re‐measured at age 70 years. Blood pressure was a significant predictor for stroke over all three decades of follow‐up. In elderly people only, there is evidence that apolipoprotein A1 may protect from future stroke.
Conclusion
Mid‐life values for blood pressure and ECG abnormalities retain their predictive value over long follow‐up periods even though they improved in predictive power when re‐measured in elderly people. Despite lower prevalence, ECG abnormalities had greater impact at age 50 years than at age 70 years. By contrast, apolipoprotein A1 was protective for future stroke only at age 70 years.
Even though there is a trend towards decreasing mortality due to stroke in many countries, the number of patients with stroke is growing because of changes in the age structure of Western populations.1 The clinical syndrome of stroke consists of different pathologies, the two main types being ischaemic stroke, which accounts for 72–82%,2 and haemorrhagic stroke. Most ischaemic strokes are caused by thromboemboli arising from atheromatous disease. Non‐atheromatous causes of stroke include cardiac arrhythmias, cardiomyopathies or intracranial vessel disease. Clinical evidence of atherosclerosis in one vascular bed is probably a reflection of more widespread atheromatous disease.3 However, risk factor patterns for complications of atherosclerosis may differ in the various parts of the cardiovascular tree.4 Also, risk factors for cardiovascular disease (CVD) may differ between young and older subjects, and stroke, congestive heart failure, atrial fibrillation and chronic nephropathy may become more important in very old subjects.5
ECG abnormalities have been shown to be associated with increased long‐term risk of mortality due to coronary heart disease (CHD) and CVD,6,7,8 and the prognostic value of major ECG findings for CVD and CHD have been shown to be more powerful than well‐established risk factors.9,10 Even though ECG findings are not a direct cause of stroke, the presence of CHD could be a good predictor of stroke since the two diseases could have common causes.
The effect of follow‐up time and re‐measurement of the ECG and conventional risk factors on their predictive value for stroke is especially important to understand, since many subjects with traditional cardiovascular risk factors will have died of cardiac disease before reaching the age at which stroke commonly occurs.
Our hypothesis was that the impact of risk factors for stroke might change over time, more so for some variables than for others. Hence, we studied how the impact of risk factors for stroke changed over time, with special emphasis on ECG abnormalities, using data from the Uppsala Longitudinal Study of Adult Men (ULSAM), a cohort with 30 years of follow‐up. The subjects in ULSAM were examined at age 50 years and re‐examined 20 years later, allowing us to investigate how risk factors measured in mid‐life changed in predictive power during three decades of follow‐up, and to study the impact of cardiovascular risk factors in the same cohort with baseline at two different time points, at ages 50 and 70 years
Method
Study sample and design
All 50‐year‐old men, born between 1920 and 1924, in Uppsala, Sweden, were invited to participate in ULSAM, aimed at identifying risk factors for CVD.11 Of the invited subjects, 2322 (82%) participated in 1970–3. Twenty years later, between 1991 and 1995, eligible participants were invited for re‐examination at age 70 years. Of these, 1221 (73%) participated. Between the two surveys, 422 had died and 219 had moved. Official registry data were available, the censored date being 31 December 2001.
Information on the men born between 1920 and 1924, who had been hospitalised for cerebrovascular disease during the 10 years before the investigation, was collected from hospital records, and two participants who had had a cerebrovascular disease were excluded. Drugs were classified according to the current list of pharmaceutical specialities available in Sweden. Only daily long‐term medication was included.
Baseline investigations and laboratory methods
Investigations at age 50 years
These investigations have been described extensively elsewhere.11 Twelve‐lead resting ECGs were recorded and coded according to the Minnesota code.12,13 Abnormal Q/QS pattern was defined as a Q/QS wave with a certain magnitude according to the Minnesota codes 1.1–1.3, ST segment depression as 4.1–4.2, T wave abnormalities as 5.1–5.3 and atrial fibrillation as 8.3. High R‐amplitude codes 3.1/3.3, together with 4.1–4.2, were considered to indicate ECG‐left ventricular hypertrophy (ECG‐LVH). Concentrations of serum low‐density lipoprotein (LDL)‐cholesterol, high‐density lipoprotein (HDL)‐cholesterol and apolipoproteins A1 (apoA1) and B (apoB) were measured on a subset of serum samples that had been stored since sampling, initially in liquid nitrogen and later in freezers.
The overall incidence rate for stroke in subjects where serum samples were analysed for LDL‐cholesterol, HDL‐cholesterol and apoA1 and apoB compared with subjects where it was not, was 0.6 and 0.65 per 100 patient‐years, respectively. Diabetes mellitus was defined as fasting blood glucose ⩾6.1 mmol/l14 and/or pharmacological treatment for diabetes mellitus.
Smoking habits were collected through interviews. In middle‐aged men 51% were smokers. The percentage of ex‐smokers (those who had stopped smoking at least one month ago) was 23.2%, and 25.8% had never smoked.
Investigations at age 70 years
The cohort was re‐investigated at age 70 years, at which time all investigations performed at age 50 years, including standard resting ECG, were repeated. At age 70 years, the apoB/apoA1 ratio was only determined in a random sample of 551 subjects. Technical problems with one of the freezers led to a random loss of samples, accounting for the reduced amount of samples available for apolipoprotein analyses. At the age of 70 years glucose levels were analysed as fasting plasma glucose levels, so the criteria for diabetes mellitus were changed to take this into account. Diabetes mellitus was defined according to latest guidelines as fasting plasma glucose ⩾7 mmol/l14 and/or pharmacological treatment for diabetes mellitus.
End point and follow‐up
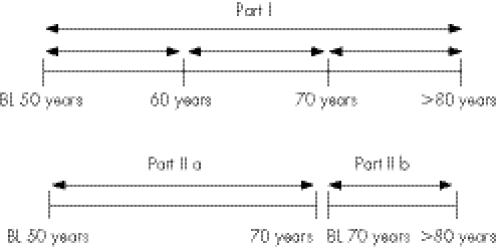
All men who were free from stroke at baseline were followed for the first occurrence of fatal or non‐fatal any‐cause stroke, using the Swedish cause‐of‐death registry and the hospital discharge registry data. Stroke was defined as International classification of disease, ninth revision codes 431 and 433–436 or 10th revision I61, I63–I66, thereby including both ischaemic (81%) and haemorrhagic stroke (19%). Transitory cerebral ischaemic attacks (58 cases) were included among ischaemic stroke. Separate analyses were also carried out for ischaemic stroke. The maximal follow‐up period was 31.7 years. The study was divided into two parts (fig 1). In part I of the study, the follow‐up period was divided into three consecutive periods, between ages 50 and 60, 60 and 70 and 70 and 80. Part II of the study was divided into two follow‐up periods. Part IIa, with a maximum follow‐up time of 23.8 years, covered the period from date of the first examination at age 50 years (n = 2322) to date of the first non‐fatal or fatal stroke event or second examination at age 70 years; and part IIb, with a maximum follow‐up time of 10.4 years, covered the period from baseline at age 70 years (n = 1221) until event date or censored date. Only men who were free from stroke at baseline at age 70 years were included in part IIb.
Figure 1 Study design. BL, baseline, outcome: fatal and non‐fatal any‐cause stroke/ischaemic stroke.
Statistical analyses
The statistical analyses were carried out using Stata V.8.0. Continuous variables were standardised to 1 SD, with the mean value being zero (mean = 0) and 1 SD being 1 (1SD = 1). Univariate Cox's proportional hazards regression analyses were used to determine the relationship of various risk factors and subsequent any‐cause stroke and ischaemic stroke in the different follow‐up periods. The proportional hazard assumption was checked for each of the variables for the whole follow‐up period (0–30 years) and for each of the follow‐up periods using the test for Schoenfeld's residuals. Also, to test for proportionality within each of the follow‐up periods, time‐dependent covariates were generated by creating interactions of the predictors and a function of survival time, and time‐dependant covariates were included in all multivariate models and tested for significance using a likelihood‐ratio test. Testing the time‐independent covariates is equivalent to testing for zero slope in a generalised linear regression of the scaled Schoenfeld's residuals on functions of time. All factors with p <0.15, in the different follow‐up periods in parts I and II, were evaluated for independence in a backward stepwise multiple model. In multivariate analyses, hazard ratios (HRs) were adjusted for age at entry and only variables with p<0.05 were allowed to stay in the models. To investigate whether the relationship between the independent risk factors and stroke outcomes was maintained if subjects with a history of myocardial infarction were excluded, multivariate analyses were repeated after excluding subjects with first myocardial infarction before the 50‐year examination or before first stroke events.
To further analyse the relative difference in impact of the specific ischaemic ECG abnormalities on stroke prognosis in young and elderly people, attributable fractions were estimated by dividing the incidence difference of stroke in subjects with and without the specific ECG abnormality by the incidence of stroke (Is) in subjects with the specific ECG abnormality:
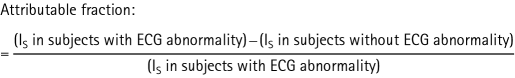 |
Results
During the total follow‐up period of up to 30 years, 1019 (43.9%) had died and 343 had either been hospitalised or died from any‐cause stroke, and of these, 221 were due to ischaemic stroke. Of the stroke events, 13% were fatal (n = 45). Baseline characteristics at ages 50 and 70 years are displayed in table 1. There was a significant difference for all the continuous cardiovascular risk factors between ages 50 and 70 years
Table 1 Baseline characteristics of cardiovascular risk factors at 50‐ and 70‐year‐surveys.
| Characteristics | Survey at age 50 (all subjects at age 50 included) | n | Survey at age 50 (only subjects attending both surveys included) | n | Survey at age 70 | n | p Value for t test (age 50 vs age 70 years)† |
|---|---|---|---|---|---|---|---|
| ECG items | |||||||
| Q/QS wave pattern (%) | 1.3 | 2322 | 0.9 | 1221 | 12.8 | 1139 | |
| ECG‐LVH (%) | 1.2 | 0.5 | 6.5 | ||||
| ST segment depression (%) | 2.3 | 1.2 | 14.3 | ||||
| T wave abnormal (%) | 5.9 | 3.8 | 16.0 | ||||
| AF (%) | 0.3 | 0.3 | 4.6 | ||||
| Conventional cardiovascular risk factors | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| BMI (kg/m2) | 25.0 (3.2) | 2322 | 24.9 (2.9) | 1221 | 26.3 (3.4) | 1215 | <0.001 |
| SBP supine (mm Hg) | 133.1 (18.1) | 2321 | 131.5 (16.7) | 1221 | 146.8 (18.5) | 1216 | <0.001 |
| DBP supine (mm Hg) | 83.7 (11.2) | 2321 | 82.7 (10.5) | 1221 | 83.8 (9.5) | 1216 | 0.007 |
| Fasting glucose (mmol/l)* | 5.5 (1.0) | 2314 | 5.0 (0.7) | 1217 | 5.8 (1.5) | 1219 | <0.001 |
| Fasting insulin (μU/ml) | 13.1 (7.8) | 1794 | 12.5 (7.0) | 985 | 13.0 (8.3) | 1206 | 0.01 |
| Serum triglycerides (mmol/l) | 1.9 (1.2) | 2322 | 1.8 (0.9) | 1221 | 1.4 (0.8) | 1220 | <0.001 |
| Serum total cholesterol (mmol/l) | 6.9 (1.3) | 2322 | 6.8 (1.3) | 1221 | 5.8 (1.0) | 1220 | <0.001 |
| HDL cholesterol (mmol/l) | 1.4 (0.4) | 1880 | 1.4 (0.4) | 988 | 1.3 (0.3) | 1218 | <0.001 |
| LDL cholesterol (mmol/l) | 5.3 (1.3) | 1880 | 5.2 (1.2) | 988 | 3.9 (0.9) | 1214 | <0.001 |
| ApoA1 (g/l) | 1.4 (0.3) | 1826 | 1.4 (0.2) | 965 | 1.3 (0.2) | 548 | <0.001 |
| ApoB (g/l) | 1.2 (0.3) | 1826 | 1.2 (0.3) | 965 | 1.03 (0.2) | 551 | <0.001 |
| ApoB/apoA1 ratio | 0.89 (0.26) | 1826 | 0.88 (0.24) | 965 | 0.82 (0.2) | 548 | <0.001 |
| Prevalence of smoking (%) | 51.1 | 2322 | 44.6 | 1221 | 20.8 | 1181 | |
| Diabetes mellitus (%) | 5.4 | 2315 | 4.2 | 1217 | 9.8 | 1219 |
AF, atrial fibrillation; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; ECG, electrocardiogram; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
*Data given are plasma concentrations (blood‐glucose concentrations at age 50 were multiplied by a conversion‐factor of 1.11 to be comparable with plasma, according to IFCC recommendation, Clinical Chemistry 2005 51:1573–6).
†p Value for one‐sample, two‐sided, t test for H0 = mean at survey age 50–mean at survey at age 70 years = 0
According to the test of Schoenfeld's residuals, proportional hazards assumption was fulfilled for each variable and follow‐up period. Also, the insertion of time‐dependent variables into the multivariate models for the different follow‐up periods did not affect the models significantly.
Part I: predictive value of mid‐life risk factors over three different follow‐up periods
The incidence rates for any‐cause stroke increased for the three different decades of follow‐up: 0.17, 0.62 and 1.3/100 person‐years at risk. Tables 2 and 3 show the results from the univariate analyses, for any‐cause stroke (including transient ischaemic attack (TIA) and haemorrhagic), and for ischaemic stroke. Analyses carried out with the outcome of ischaemic stroke showed a trend similar to any‐cause stroke.
Table 2 Baseline cardiovascular risk factors associated with future fatal and non‐fatal any‐cause stroke (including transient ischaemic attack and haemorrhagic stroke) over a total period of 30 years of follow‐up and divided into three different follow‐up periods.
| Follow‐up period | 0–30 (age period 50–80 years) | 0–9.9 (age period 50–60 years) | 10–19.9 (age period 60–70 years) | 20–30 (age period 70–80 years) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of cases | 343 | 40 | 122 | 181 | ||||
| PYAR | 57 003 | 23 317 | 19 719 | 13 966 | ||||
| Characteristics | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI†) | P Value | HR (95% CI) | p Value |
| ECG items | ||||||||
| Q/QS on ECG | 2.71 (1.21 to 6.08) | 0.016 | 4.66 (1.12 to 19.3) | 0.03 | 3.07 (0.98 to 9.67) | 0.06 | 1.33 (0.19 to 9.50) | 0.8 |
| ECG‐LVH | 5.68 (3.11 to 10.4) | <0.001 | 16.0 (6.25 to 40.8) | <0.001 | 5.39 (1.99 to 14.6) | 0.001 | 2.27 (0.56 to 9.14) | 0.3 |
| ST depression | 4.60 (2.89 to 7.31) | <0.001 | 11.3 (4.92 to 25.2) | <0.001 | 5.10 (2.49 to 10.5) | <0.001 | 2.04 (0.8 to 5.51) | 0.2 |
| T wave abnormality | 2.58 (1.82 to 3.66) | <0.001 | 4.33 (2.00 to 9.40) | <0.001 | 3.56 (2.16 to 5.87) | <0.001 | 1.36 (0.70 to 2.66) | 0.4 |
| Atrial fibrillation | 1.68 (0.24 to 11.98) | 0.6 | ||||||
| Conventional cardiovascular risk factors | ||||||||
| BMI | 1.16 (1.05 to 1.30) | 0.004 | 1.23 (0.92 to 1.64) | 0.2 | 1.18 (0.99 to 1.41) | 0.06 | 1.14 (0.98 to 1.32) | 0.1 |
| SBP supine | 1.39 (1.27 to 1.52) | <0.001 | 1.67 (1.34 to 2.07) | <0.001 | 1.51 (1.31 to 1.73) | <0.001 | 1.21 (1.05 to 1.40) | 0.008 |
| DBP supine | 1.34 (1.21 to 1.48) | <0.001 | 1.53 (1.17 to 1.99 | 0.002 | 1.41 (1.20 to 1.65) | <0.001 | 1.24 (1.07 to 1.43) | 0.004 |
| Fasting glucose | 1.11 (1.01 to 1.22) | 0.04 | 1.21 (1.02 to 1.44) | 0.03 | 1.13 (0.99,1.30) | 0.07 | 1.02 (1.86 to 1.22) | 0.8 |
| Fasting insulin | 1.16 (1.04 to 1.29) | 0.007 | 1.31 (1.05 to 1.62) | 0.014 | 1.17 (0.98 to 1.38) | 0.08 | 1.10 (0.93 to 1.30) | 0.3 |
| Triglycerides | 1.06 (0.94 to 1.19) | 0.3 | 1.11 (0.93 to 1.33 | 0.2 | 1.00 (0.81 to 1.24) | 0.98 | 1.07 (0.88 to 1.29) | 0.5 |
| Cholesterol | 1.03 (0.93 to 1.15) | 0.5 | 0.91 (0.66 to 1.25) | 0.5 | 1.08 (0.90 to 1.28) | 0.4 | 1.04 (0.89 to 1.20) | 0.6 |
| HDL | 0.99 (0.88 to 1.13 | 0.96 | 0.88 (0.60 to 1.27) | 0.5 | 1.01 (0.82 to 1.23) | 0.95 | 1.02 (0.86 to 1.21) | 0.9 |
| LDL | 1.03 (0.92 to 1.17) | 0.6 | 0.90 (0.63 to 1.28) | 0.6 | 1.09 (0.90 to 1.32) | 0.4 | 1.03 (0.87 to 1.22) | 0.7 |
| ApoA1 | 0.97 (0.86 to 1.10) | 0.8 | 0.79 (0.55 to 1.14) | 0.2 | 1.02 (0.83 to 1.24) | 0.9 | 0.79 (0.84 to 1.18) | 0.9 |
| ApoB | 1.03 (0.91 to 1.16) | 0.6 | 1.04 (0.74 to 1.45) | 0.8 | 1.00 (0.82 to 1.22) | 0.97 | 1.04 (0.88 to 1.23) | 0.6 |
| ApoB/ApoA1 | 1.05 (0.93 to 1.18) | 0.4 | 1.15 (0.84 to 1.59) | 0.4 | 1.05 (0.86,1.28) | 0.6 | 1.02 (0.86 to 1.22) | 0.8 |
| Smoking | 1.35 (1.10 to 1.68) | 0.005 | 2.96 (1.46 to 6.06) | 0.003 | 1.45 (1.01 to 2.08) | 0.04 | 1.11 (0.83 to 1.49) | 0.5 |
| DM | 1.69 (1.13 to 2.51) | 0.01 | 2.57 (1.00 to 6.56) | 0.05 | 1.93 (1.04 to 3.58) | 0.04 | 1.29 (0.68 to 2.45) | 0.4 |
ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVH, left ventricular hypertrophy; PYAR, patient years at risk; SBP, systolic blood pressure.
HRs with 95% CI derived from univariate Cox's proportional hazards regression were applied to variables standardised to 1 SD. Due to the low number of ECGs with atrial fibrillation at age 50 (n = 7), it was only possible to determine HRs for atrial fibrillation for the total follow‐up period and for the first 10 years of follow‐up
Table 3 Baseline cardiovascular risk factors associated with future fatal and non‐fatal ischaemic stroke (excluding transient ischaemic attack and haemorrhagic stroke) over a total period of 30 years of follow‐up and divided into three different follow‐up periods.
| Follow‐up period | 0–30 (age period 50–80 years) | 0–9.9 (age period 50–60 years) | 10–19.9 (age period 60–70 years) | 20–30 (age period 70– 80 years) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of cases | 221 | 16 | 75 | 130 | ||||
| PYAR | 54 873 | 22 174 | 18 933 | 13 766 | ||||
| Characteristics | HR (95% CI) | P Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| ECG items | ||||||||
| Q/QS on ECG | 3.67 (1.51 to 8.93) | 0.004 | 5.85 (0.77 to 44.3) | 0.09 | 4.83 (1.52 to 15. 4) | 0.008 | 1.92 (0.27 to 13.8) | 0.5 |
| ECG‐LVH | 7.64 (3.92 to 14.90) | <0.001 | 39.3 (12.7 to 122) | <0.001 | 6.74 (2.12 to 12.4) | 0.001 | 3.18 (0.79 to 12.9) | 0.1 |
| ST depression | 6.29 (3.78 to 10.47) | <0.001 | 32.5 (11.8 to 89.5) | <0.001 | 6.26 (2.72 to 14.4) | <0.001 | 2.82 (1.04 to 7.62) | 0.04 |
| T wave abnormality | 3.02 (1.99 to 4.58) | <0.001 | 10.7 (3.89 to 29.5) | <0.001 | 4.38 (2.41 to 7.97) | <0.001 | 1.28 (0.57 to 2.91) | 0.6 |
| Atrial fibrillation | 2.71 (0.38 to 19.31) | 0.3 | 25.7 (3.39 to 194) | 0.002 | ||||
| Conventional cardiovascular risk factors | ||||||||
| BMI | 1.26 (1.11 to 1.43) | <0.001 | 1.28 (0.81 to 2.00) | 0.3 | 1.33 (1.08 to 1.64) | 0.008 | 1.21 (1.02 to 1.44) | 0.03 |
| SBP supine | 1.47 (1.31 to 1.64) | <0.001 | 2.04 (1.52 to 2.74) | <0.001 | 1.62 (1.36 to 1.91) | <0.001 | 1.26 (1.07 to 1.48) | 0.005 |
| DBP supine | 1.43 (1.27 to 1.62) | <0.001 | 2.06 (1.41 to 2.74) | <0.001 | 1.51 (1.23 to 1.84) | <0.001 | 1.29 (1.09 to 1.53) | 0.003 |
| Fasting glucose | 1.18 (1.07 to 1.32) | 0.002 | 1.39 (1.16 to 1.66) | <0.001 | 1.14(0.95 to 1.36) | 0.2 | 1.15 (0.97 to 1.36) | 0.1 |
| Fasting insulin | 1.22 (1.09 to 1.37) | 0.001 | 1.46 (1.16 to 1.83) | 0.001 | 1.20 (0.99 to 1.45) | 0.06 | 1.17 (0.98 to 1.40) | 0.08 |
| Triglycerides | 1.09 (0.95 to 1.24) | 0.2 | 1.12 (0.86 to 1.46) | 0.4 | 1.05 (0.83 to 1.33) | 0.7 | 1.10 (0.89 to 1.37) | 0.4 |
| Cholesterol | 1.01 (0.89 to 1.16) | 0.9 | 0.69 (0.40 to 1.18) | 0.2 | 1.08 (0.86 to 1.35) | 0.5 | 1.02 (0.86 to 1.22) | 0.8 |
| HDL | 0.95 (0.81 to 1.11) | 0.5 | 0.80 (0.45 to 1.44) | 0.5 | 0.92 (0.69 to 1.22) | 0.6 | 0.99 (0.80 to 1.21) | 0.9 |
| LDL | 0.99 (0.85 to 1.15) | 0.9 | 0.66 (0.37 to 1.16) | 0.2 | 1.11 (0.86 to 1.42) | 0.4 | 0.98 (0.81 to 1.20) | 0.9 |
| ApoA1 | 0.97 (0.83 to 1.13) | 0.7 | 0.71 (0.39 to 1.31) | 0.3 | 0.97 (0.74 to 1.26) | 0.8 | 0.99 (0.81 to 1.22) | 0.96 |
| ApoB | 1.01 (0.87 to 1.18) | 0.9 | 0.88 (0.50 to 1.54) | 0.7 | 0.99 (0.77 to 1.29) | 0.96 | 1.03 (0.85 to 1.26) | 0.7 |
| ApoB/ApoA1 | 1.04 (0.89 to 1.21) | 0.6 | 1.13 (0.67 to 1.90) | 0.6 | 1.07 (0.83 to 1.38) | 0.6 | 1.01 (0.83 to 1.24) | 0.9 |
| Smoking | 1.24 (0.95 to 1.61) | 0.1 | 4.37 (1.25 to 15.3) | 0.02 | 1.17 (0.75 to 1.84) | 0.5 | 1.12 (0.79 to 1.58) | 0.5 |
| DM | 2.39 (1.55 to 3.69) | <0.001 | 5.85 (1.89 to 18.1) | 0.002 | 2.56 (1.28 to 5.14) | 0.008 | 1.86 (0.97 to 3.55) | 0.06 |
ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HDL, high density lipoprotein; LDL, low‐density lipoprotein; LVH, left ventricular hypertrophy; PYAR, patient years at risk; SBP, systolic blood pressure.
HRs with 95% CI derived from univariate Cox's proportional hazards regression were applied to variables standardised to 1 SD. Owing to the low number of ECGs with atrial fibrillation at age 50 years (n = 7), it was only possible to determine HRs for atrial fibrillation for the total follow‐up period and for the first 10 years of follow‐up.
When any‐cause stroke was considered, the ECG abnormalities (except atrial fibrillation), smoking and diabetes mellitus were significant predictors during the first two decades of follow‐up, whereas blood pressure was a significant predictor for all three decades in the univariate analyses (table 2). A similar pattern was seen when ischaemic stroke was analysed as the end point (table 3).
In stepwise multivariate analyses with any‐cause stroke included, systolic blood pressure (SBP) was an independent predictor during all follow‐up periods, even though the impact of SBP measured in middle age decreased with time. The presence of ST segment depression, T wave abnormality and smoking were significant independent predictors during the first two decades, whereas the only variable that still had a predictive value for events occurring between the ages of 70 and 80 years was SBP (table 4). In a similar multiple regression model including only ischaemic stroke cases, a similar picture was seen, but in that case, atrial fibrillation and diabetes mellitus were significant predictors during the first decade of follow‐up (table 5).
Table 4 Independent predictors for fatal and non‐fatal any‐cause stroke (including transient ischaemic attack and haemorrhagic stroke) over a period of 30 years of follow‐up divided into different follow‐up periods.
| Follow‐up period | 0–30 (age period 50–80 years) | 0–9.9 (age period 50–60 years) | 10–19.9 (age period 60–70 years) | 20–30 (age period 70–80 years) | ||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| SBP supine | 1.38 (1.26 to 1.51) | <0.001 | 1.50 (1.19 to 1.89) | 0.001 | 1.47 (1.27 to 1.70) | <0.001 | 1.21 (1.05 to 1.40) | 0.008 |
| ST segment depression | 3.36 (2.08 to 5.42) | <0.001 | 5.88 (2.30 to 15.0) | <0.001 | ||||
| T wave abnormality | 2.62 (1.55 to 4.42) | <0.001 | ||||||
| Smoking | 1.51 (1.21 to 1.87) | <0.001 | 3.22 (1.57 to 6.62) | 0.001 | 1.61 (1.12 to 2.30) | 0.01 | ||
SBP, systolic blood pressure.
HRs with 95% CIs from Cox's proportional hazards regression were applied to variables standardised to 1 SD. Stepwise multiple regression analysis were performed for each decade of follow‐up and only variables retained in the models are presented (p<0.05).
Table 5 Independent predictors for fatal and non‐fatal ischaemic stroke (excluding transient ischaemic attack and haemorrhagic stroke) over a period of 30 years of follow‐up divided into different follow‐up periods.
| Follow‐up period | 0–30 (age period 50–80 years) | 0–9.9 (age period 50–60 years) | 10–19.9 (age period 60–70 years) | 20–30 (age period 70–80 years) | ||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | P Value |
| SBP supine | 1.37 (1.22 to 1.55) | <0.001 | 1.59 (1.13 to 2.24) | 0.008 | 1.53 (1.28 to 1.83) | <0.001 | 1.26 (1.07 to 1.48) | 0.006 |
| BMI | 1.15 (1.00 to 1.31) | 0.04 | ||||||
| ST segment depression | 4.35 (2.57 to 7.39) | <0.001 | 12.82 (3.73 to 43.5) | <0.001 | ||||
| T wave abnormality | 3.18 (1.71 to 5.92) | <0.001 | ||||||
| Atrial fibrillation | 15.07 (1.77 to 128) | 0.01 | ||||||
| Diabetes mellitus | 1.72 (1.02 to 2.48) | 0.042 | 3.30 (1.01 to 10.8) | 0.05 | ||||
| Smoking | 1.44 (1.10 to 1.88) | 0.008 | 5.68 (1.56 to 20.7) | 0.008 | ||||
BMI, body mass index; SBP, systolic blood pressure.
HRs with 95% CIs from Cox's proportional hazards regression were applied to variables standardised to 1 SD. Stepwise multiple regression analysis were performed for each decade of follow‐up and only variables retained in the models are presented (p<0.05).
Parts IIa and IIb: comparison between risk factors in midlife and at age 70 years
For part IIa, with a follow‐up period from baseline at age 50 to 70 years, the incidence rate of any‐cause stroke was 0.38/100 person‐years, whereas for part IIb with a follow‐up period from baseline at age 70 years to the censor date, it was threefold higher: 1.22/per 100 person‐years.
Tables 6 and 7 show the results from the univariate analyses for any‐cause stroke and for ischaemic stroke.
Table 6 Hazard ratio for baseline characteristics at ages 50 and 70 years for fatal and non‐fatal any‐cause stroke (including transient ischaemic attack and haemorrhagic stroke) over different follow‐up periods.
| Follow‐up period | Part IIa (n = 2320) | Part IIb (n = 1148) | ||||
|---|---|---|---|---|---|---|
| No. of cases | 172 | 106 | ||||
| PYAR | 44 206 | 8667 | ||||
| Characteristics | HR (95% CI) | P Value | AF (%) | HR (95% CI) | p Value | AF (%) |
| ECG items | ||||||
| Q/QS on ECG | 3.51 (1.44 to 8.55) | 0.006 | 66 | 1.50 (0.88 to 2.57) | 0.1 | 33 |
| ECG‐LVH | 8.41 (4.29 to 16.5) | <0.001 | 86 | 2.75 (1.53 to 4.94) | 0.001 | 52 |
| ST depression | 6.61 (3.88 to 11.2) | <0.001 | 82 | 2.12 (1.32 to 3.42) | 0.002 | 46 |
| T wave abnormal | 3.66 (2.41 to 5.55) | <0.001 | 70 | 2.17 (1.38 to 3.43) | 0.001 | 47 |
| Atrial fibrillation | 2.93 (0.41 to 20.9) | 0.3 | 59 | 4.48 (2.49 to 8.06) | <0.001 | 59 |
| Conventional cardiovascular risk factors | ||||||
| BMI | 1.24 (1.08 to 1.43) | 0.003 | 1.09 (0.90 to 1.32) | 0.4 | ||
| SBP supine | 1.55 (1.38 to 1.74) | <0.001 | 1.47 (1.23 to 1.77) | <0.001 | ||
| DBP supine | 1.43 (1.26 to 1.64) | <0.001 | 1.27 (1.05 to 1.55) | 0.01 | ||
| Fasting glucose | 1.16 (1.04 to 1.29) | 0.009 | 1.13 (0.96 to 1.32) | 0.2 | ||
| Fasting insulin | 1.20 (1.05 to 1.38) | 0.006 | 1.10 (0.98 to 1.23) | 0.1 | ||
| Triglycerides | 1.06 (0.92 to 1.22) | 0.4 | 0.89 (0.72 to 1.10) | 0.3 | ||
| Cholesterol | 1.03 (0.88 to 1.20) | 0.7 | 1.07 (0.88 to 1.30) | 0.5 | ||
| HDL cholesterol | 0.97 (0.81 to 1.15) | 0.7 | 0.87 (0.71 to 1.06) | 0.2 | ||
| LDL cholesterol | 1.02 (0.86 to 1.20) | 0.8 | 1.18 (0.98 to 1.43) | 0.08 | ||
| ApoA1 | 0.92 (0.78 to 1.10) | 0.4 | 0.69 (0.52 to 0.91) | 0.009 | ||
| ApoB | 0.99 (0.84 to 1.17) | 0.9 | 1.11 (0.86 to 1.44) | 0.4 | ||
| ApoB/ApoA1 | 1.08 (0.92 to 1.27) | 0.4 | 1.32 (1.02 to 1.70) | 0.03 | ||
| Smoking | 1.59 (1.17 to 2.15) | 0.003 | 1.27 (0.81 to 2.00) | 0.3 | ||
| Diabetes mellitus | 1.99 (1.19 to 3.34) | 0.009 | 1.55 (0.88 to 2.72) | 0.13 | ||
AF, attributable fraction (calculated only for ECG abnormalities); ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; ECG, electrocardiogram; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVH, left ventricular hypertrophy; PYAR, patient years at risk; SBP, systolic blood pressure.
HRs with 95% CIs from Cox's proportional hazards regression were applied to variables standardised to 1 SD.
Subjects with a pacemaker at age 70 years were excluded from further analyses in part IIb.
Table 7 Hazard ratio for baseline characteristics at age 50 and age 70 years for fatal and non‐fatal ischaemic stroke (excluding transient ischaemic attack and haemorrhagic stroke) over different follow‐up periods.
| Follow‐up period | Part IIa (n = 2320) | Part IIb (n = 1148) | ||||
|---|---|---|---|---|---|---|
| No. of cases | 98 | 81 | ||||
| PYAR | 42 24 321 | 8573 | ||||
| Characteristics | HR (95% CI) | p Value | AF (%) | HR (95% CI) | p Value | AF (%) |
| ECG items | ||||||
| Q/QS on ECG | 4.94 (1.81 to 13.5) | 0.002 | 75 | 1.45 (0.78 to 2.69) | 0.2 | 27 |
| ECG‐LVH | 12.5 (5.8 to 27.0) | <0.001 | 90 | 3.37 (1.82 to 6.27) | <0.001 | 53 |
| ST depression | 9.97 (5.44 to 18.3) | <0.001 | 88 | 2.31 (1.36 to 3.93) | 0.002 | 46 |
| T wave abnormal | 5.14 (3.11 to 8.50) | <0.001 | 78 | 2.15 (1.28 to 3.62) | 0.004 | 44 |
| Atrial fibrillation | 5.10 (0.71 to 36.6) | 0.1 | 76 | 5.07 (2.67 to 9.63) | <0.001 | 59 |
| Conventional cardiovascular risk factors | ||||||
| BMI | 1.36 (1.14 to 1.64) | 0.001 | 1.04 (0.98 to 1.11) | 0.2 | ||
| SBP supine | 1.69 (1.47 to 1.95) | <0.001 | 1.02 (1.01 to 1.03) | 0.001 | ||
| DBP supine | 1.57 (1.32 to 1.87) | <0.001 | 1.02 (0.99 to 1.05) | 0.06 | ||
| Fasting glucose | 1.21 (1.06 to 1.38) | 0.004 | 1.13 (1.01 to 1.27) | 0.03 | ||
| Fasting insulin | 1.27 (1.09 to 1.47) | 0.002 | 1.02 (1.00 to 1.03) | 0.03 | ||
| Triglycerides | 1.09 (0.92 to 1.29) | 0.3 | 0.87 (0.63 to 1.19) | 0.4 | ||
| Cholesterol | 0.99 (0.81 to 1.22) | 0.9 | 1.14 (0.91 to 1.42) | 0.3 | ||
| HDL cholesterol | 0.88 (0.69 to 1.13) | 0.99 | 0.70 (0.36 to 1.36) | 0.3 | ||
| LDL cholesterol | 0.97 (0.77 to 1.22) | 0.2 | 1.29 (1.00 to 1.64) | 0.04 | ||
| ApoA1 | 0.87 (0.69 to 1.11) | 0.3 | 0.21 (0.05 to 0.85) | 0.03 | ||
| ApoB | 0.95 (0.76 to 1.20) | 0.7 | 1.67 (0.43 to 6.53) | 0.5 | ||
| ApoB/ApoA1 | 1.09 (0.87 to 1.36) | 0.4 | 3.62 (0.93 to 14.1) | 0.06 | ||
| Smoking | 1.33 (0.89 to 1.98) | 0.2 | 1.36 (0.82 to 2.27) | 0.2 | ||
| Diabetes mellitus | 2.91 (1.62 to 5.22) | <0.001 | 2.75 (1.32 to 5.74) | 0.007 | ||
AF, Attributable fraction (calculated only for ECG abnormalities); ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; ECG, electrocardiogram; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVH, left ventricular hypertrophy; PYAR, patient years at risk; SBP, systolic blood pressure.
HRs with 95% CIs from Cox's proportional hazards regression were applied to variables standardised to 1 SD.
Subjects with pacemaker at age 70 years were excluded from further analyses in part IIb.
In the univariate analyses for any‐cause stroke, ECG‐LVH, ST segment depression and T wave abnormalities were significant predictors for stroke when evaluated both at age 50 and at age 70 years (tables 6 and 7). On the contrary, atrial fibrillation was a significant predictor at age 70 years only. According to the attributable fraction, the impact of ECG abnormalities for future stroke was higher at age 50 than 70 years.
Blood pressure was a significant predictor for any‐cause stroke both at age 50 and at 70 years, whereas fasting glucose and insulin, smoking and diabetes mellitus were predictors at age 50 years only. On the other hand, apoA1 was a protective factor at age 70 years only. A similar trend was seen when only cases with ischaemic stroke were analysed (table 7), but for ischaemic stroke, fasting insulin and glucose as well as diabetes mellitus were significant predictors at both ages 50 and 70 years
In stepwise multiple regression analyses with any‐cause stroke, ST depression, T wave abnormalities, SBP and smoking were independent predictors of stroke when measured at age 50 years, while atrial fibrillation and LDL‐cholesterol were independent predictors at age 70 years. ApoA1 protected against stroke in elderly people. Similar data were obtained when only cases with ischaemic stroke were analysed, with the exception that diabetes mellitus was an independent predictor at age 50 years (table 8). Also, similar trends, with similar point estimates and overlapping confidence intervals, were obtained if subjects with a history of myocardial infarction before the first stroke event were excluded from the multivariate analyses. A total of 16% of subjects with any‐cause stroke and 19% with ischaemic stroke had a non‐fatal myocardial infarction before the first stroke event (data not shown).
Table 8 Independent predictors for fatal and non‐fatal any‐cause stroke and ischaemic stroke, in multivariate analyses, at ages 50 and 70 years over different follow‐up periods.
| Characteristics | Any‐cause stroke Part IIa Age 50–70 | Any‐cause stroke Part IIb Age 70 years to censored data | Ischaemic stroke Part IIa Age 50–age 70 years | Ischaemic stroke Part IIb Age 70 years to censored data | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| ST depression | 2.45 (1.20 to 5.00) | 0.014 | 2.91 (1.27 to 6.67) | 0.01 | ||||
| T wave abnormality | 1.82 (1.04 to 3.19) | 0.035 | 2.27 (1.16 to 4.46) | 0.02 | ||||
| SBP | 1.48 (1.31 to 1.67) | <0.001 | 1.53 (1.31 to 1.79) | <0.001 | ||||
| Smoking | 1.77 (1.30 to 2.40) | <0.001 | 1.59 (1.06 to 2.39) | 0.03 | ||||
| Diabetes mellitus | 1.92 (1.04 to 3.56) | 0.04 | ||||||
| Atrial fibrillation | 8.95 (4.39 to 18.26) | <0.001 | 10.11 (4.53 to 22.6) | <0.001 | ||||
| LDL | 1.39 (1.05 to 1.84) | 0.02 | 1.47 (1.07 to 2.02) | 0.02 | ||||
| ApoA1 | 0.59 (0.43 to 0.82) | 0.002 | 0.58 (0.40 to 0.84) | 0.004 | ||||
ApoA1, apolipoprotein A1; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
HRs with 95% CIs from Cox's proportional hazards regression were applied to variables standardised to 1 SD and adjusted for age at entry.
Discussion
This study shows that the effect of traditional risk factors in middle‐aged men change over prolonged follow‐up periods and when re‐measured at old age. Furthermore, the study also points out that ECG changes, such as ST segment depression and T wave abnormalities, are also important risk factors for stroke.
Consistent with another population‐based study carried out in Swedish men aged 47–55 years in 1970–3,15 high blood pressure, smoking and diabetes mellitus were associated with future stroke in middle‐aged men, whereas high cholesterol levels were not. In our cohort, atrial fibrillation, a well‐known risk factor for stroke,15,16 was found to be of importance only in elderly people, probably owing to low prevalence in mid‐life subjects (n = 7). SBP was an important risk factor for any‐cause stroke and for ischaemic stroke over three decades of follow‐up when measured at age 50 years. Although being of major importance during the first decades of follow‐up when evaluated at age 50 years, ST segment depression, T wave abnormalities and ECG‐LVH regained significance when re‐measured at age 70 years. Despite lower prevalence, ECG abnormalities had greater impact on risk at age 50 years than at age 70 years. In elderly people only, LDL‐cholesterol was an independent risk factor for future stroke, whereas apoA1 seemed to protect against future stroke.
The strength of our study is that the subjects have been followed for >30 years with repeated investigations. An obvious limitation of our study is the lack of women. There was no validation of the individual stroke cases. However, in previous studies, stroke, defined by combining data from the Cause of Death Registry and Hospital Discharge Registry in Sweden, has been shown to be an efficient, validated alternative to revised hospital discharge notes and death certificates.17 The association between risk factors and any‐cause stroke will be substantially influenced by ischaemic stroke, the dominant stroke subtype in our study population, so it was not surprising that the same results were obtained whether any‐cause stroke cases were analysed or when only those with ischaemic stroke were used in the models. The only main difference was a more pronounced impact of glucose and insulin when only ischaemic stroke cases were considered.
Differences in risk factor patterns for stroke between studies may depend on discrepancies in classification of stroke, dominant stroke subtype in the study population and different follow‐up time or differences in baseline age.18 Since stroke usually occurs later in life than CHD some of the participants in the cohort studies will have died from CHD before reaching the age when stroke occurs, thereby reducing the impact of some traditional cardiovascular risk factors on future development of stroke. This could explain why risk factors such as smoking, diabetes mellitus and high body mass index (BMI) were significant predictors in mid‐life but not at the age of 70 years. Consistent with other studies,19,20,21,22 we found smoking to be associated with stroke, but only in middle‐aged men. This finding could be explained not only by premature death but also by a decrease in smoking with age. The data should not be interpreted that smoking is not a risk factor in old age. Diabetes mellitus was associated with stroke in middle‐aged men only; however, considering the similar point estimates and overlapping confidence intervals it is not possible to exclude the significance in elderly people. Similarly, as found in another study,23 BMI in middle‐aged men was associated with stroke.
The roles of serum cholesterol and lipoprotein fractions as risk factors for stroke are far less consistent and more controversial18,19,24,25,26 than those for myocardial infarction. The Prospective Studies Collaboration, involving >45 prospective observational cohorts and 450 000 individuals, was not able to demonstrate an association between stroke and cholesterol levels even though they recognise that the lack of an overall relationship might conceal a positive association with ischaemic stroke together with a negative association with haemorrhagic stroke.25 Also, analysis of the EUROSTROKE Project could not disclose an association of total cholesterol with fatal, non‐fatal, haemorrhagic or ischaemic stroke26 even though, in men, an increase in HDL‐cholesterol was associated with a non‐significant reduced risk of stroke in all centres. In our study, none of the lipid variables were associated with stroke in mid‐life. However, LDL‐cholesterol was an independent predictor in multivariate analysis in elderly people. Also, in our study, the apoB/apoA1 ratio, with apoA1 driving the significance of the ratio, was found to be a significant predictor for stroke in elderly people. Recent studies have shown that the apoB/apoA1 ratio, which indicates the balance between atherogenic apoB‐containing particles and atheroprotective apoA1‐containing particles, may be a better predictor for CVD than the more traditional markers for dyslipidaemia.27,28,29,30 Qureshi et al31 were unable to demonstrate any statistically significant association between either apoA1 or apoB and stroke.31 However, their baseline investigations were performed at a younger age. A case–control study carried out in a Chinese population found that apoA1 decreased the risk of stroke. In that study, 57% of the patients were older than 70 years,32 further supporting our finding of an important protective role of apoA1 in elderly people. Our study also supports the recent findings by Walldius et al,28 where the apoB/apoA1 ratio was shown to be associated with stroke.
ST segment depression and T wave abnormalities were seen both in LVH and myocardial ischaemia.33 In a study with up to 14 years of follow‐up, in apparently healthy individuals with essential hypertension, LVH diagnosed by ECG or echocardiography was shown to confer an excess risk for stroke and TIA, independently of other individual risk factors.34 The association been ECG‐LVH and stroke in the EUROSTROKE project has been found to be more pronounced in smokers.35 However, it needs to be emphasised that the causal inter‐relationship between ECG‐LVH and specific stroke types is complex and different mechanisms may be involved.
The EUROSTROKE Project, even though the results indicated that ECG‐LVH was a predictor for stroke,35 did not consider ECG characteristics necessary in screening for stroke in the general population, since ECG abnormalities did not contribute to the prediction of stroke according to their model of risk scores.36 Our findings suggest that a standard resting ECG could play an important role in identifying patients at increased risk for stroke and that even minor ECG abnormalities may be of importance when assessing the individual patient's global burden of risk. ECG abnormalities together with low apoA1 levels could help identify elderly patients at increased risk for stroke and thereby contribute to reduction of suffering and disability if effective preventive measures are instituted.
In conclusion, mid‐life values for blood pressure and ECG abnormalities retain their predictive value over long follow‐up periods, even though they improved in predictive power when re‐measured in elderly people. Despite lower prevalence, ECG abnormalities had greater impact at age 50 years than at age 70 years. By contrast, there was evidence that ApoA1 may protect from future stroke in elderly people.
What this paper adds
During a follow‐up period of over 30 years, systolic blood pressure retained its predictive value for stroke.
ECG ischaemic abnormalities were of importance only during the first 20 years, but regained importance when re‐measured at age 70 years.
In the elderly people only, apolipoprotein A1 protected against future stroke.
Policy implications
Our findings suggest that a standard resting ECG could play an important role in assessing the patients' global burden of risk for stroke.
ECG abnormalities, together with low apolipoprotein A1 levels, could help to identify elderly patients at increased risk for stroke.
Abbreviations
ApoA1 - apolipoprotein A1
apoB apolipoprotein B - CHD, coronary heart disease
CVD - cardiovascular disease
ECG‐LVH - ECG‐left ventricular hypertrophy
HDL - high‐density lipoprotein
LDL - low‐density lipoprotein
SBP - systolic blood pressure
TIA - transient ischaemic attack
ULSAM - Uppasala Longitudinal Study of Adult Men
Footnotes
Funding: This work was funded by the Medical Faculty at Uppsala University, Royal Society of Science, Swedish Council for Planning and Co‐ordination of Research, Swedish National Association against Heart and Lung Disease and the Swedish Medical Research Council. None of the funders had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Competing interests: BZ, BW and JS have nothing to declare. During the time the study was conducted, CSM, JH and LL were also employed by AstraZeneca, Research and Development, Sweden but AstraZeneca has not had any role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Contributions: All authors participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript. CSM performed the statistical analyses and JH provided statistical expertise.
Ethical approval: All subjects have given informed consent and the ethics committee of the Faculty of Medicine, Uppsala University, has approved the Uppsala Longitudinal Study of Adult Men.
References
- 1.Sarti C, Rastenyte D, Cepaitis Z.et al International trends in mortality from stroke, 1968 to 1994. Stroke 2000311588–1601. [DOI] [PubMed] [Google Scholar]
- 2.Sudlow C L, Warlow C P. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 199728491–499. [DOI] [PubMed] [Google Scholar]
- 3.Guillot F. Atherothrombosis as a marker for disseminated atherosclerosis and a predictor of further ischaemic events. A review. Eur Heart J 19991A14–A26. [Google Scholar]
- 4.Lithell H. Pathogenesis and prevalence of atherosclerosis in hypertensive patients. Am J Hypertens 199472S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Arboix A, Miguel M, Ciscar E.et al Cardiovascular risk factors in patients aged 85 or older with ischaemic stroke. Clin Neurol Neurosurg 20051717. [DOI] [PubMed] [Google Scholar]
- 6.Greenland P, Xie X, Liu K.et al Impact of minor electrocardiographic ST‐segment and/or T‐wave abnormalities on cardiovascular mortality during long‐term follow‐up. Am J Cardiol 2003911068–1074. [DOI] [PubMed] [Google Scholar]
- 7.Kannel W, Anderson K, McGee D.et al Nonspecific electrocardiographic abnormality as a predictor of coronary heart disease: the Framingham Study. Am Heart J 1987113370–376. [DOI] [PubMed] [Google Scholar]
- 8.Daviglus M L, Liao Y, Greenland P.et al Association of nonspecific minor ST‐T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. JAMA 1999281530–536. [DOI] [PubMed] [Google Scholar]
- 9.De Bacquer D, GDB. Kornitzer M.et al Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart 199880570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bacquer D, De Backer G, Kornitzer M.et al Prognostic value of ischemic electrocardiographic findings for cardiovascular mortality in men and women. J Am Coll Cardiol 199832680–685. [DOI] [PubMed] [Google Scholar]
- 11.Hedstrand H. A study of middle‐aged men with particular reference to risk factors for cardiovascular disease. Ups J Med Sci 197519(Suppl)1–61. [PubMed] [Google Scholar]
- 12.Blackburn H, Keys A, Simonson E.et al The electrocardiogram in population studies. A classification system. Circulation 1960211160–1175. [DOI] [PubMed] [Google Scholar]
- 13.Prineas R, Crow R, Blackburn H.The Minnesota code manual of electrocardiographic findings, standards and procedures for measuring and classification. Bristol: John Wright, 1982
- 14.Alberti K G, Zimmet P Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 199815539–553. [DOI] [PubMed] [Google Scholar]
- 15.Harmsen P, Rosengren A, Tsipogianni A.et al Risk factors for stroke in middle‐aged men in Goteborg, Sweden. Stroke 199021223–229. [DOI] [PubMed] [Google Scholar]
- 16.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 19871471561–1564. [PubMed] [Google Scholar]
- 17.Merlo J, Lindblad U, Pessah‐Rasmussen H.et al Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol 200016235–243. [DOI] [PubMed] [Google Scholar]
- 18.Simons L A, Simons J, Friedlander Y.et al Cholesterol and other lipids predict coronary heart disease and ischaemic stroke in the elderly, but only in those below 70 years. Atherosclerosis 2001159201–208. [DOI] [PubMed] [Google Scholar]
- 19.Wolf P A, Belanger A J, D'Agostino R B. Management of risk factors. Neurol Clin 199210177–191. [PubMed] [Google Scholar]
- 20.Shinton R, Beevers G. Meta‐analysis of relation between cigarette smoking and stroke. BMJ 1989298789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whisnant J P. Modeling of risk factors for ischaemic stroke. The Willis Lecture. Stroke 1997281840–1844. [DOI] [PubMed] [Google Scholar]
- 22.Welin L, Eriksson H, Larsson B.et al Hyperinsulinaemia is not a major coronary risk factor in elderly men. The study of men born in 1913. Diabetologia 199235766–770. [DOI] [PubMed] [Google Scholar]
- 23.Jood K, Jern C, Wilhelmsen L.et al Body mass index in mid‐life is associated with a first stroke in men: a prospective population study over 28 years. Stroke 2004352764–2769. [DOI] [PubMed] [Google Scholar]
- 24.Propective Studies Collaboration. Welin L, Svardsudd K, Wilhelmsen L.et al Analysis of risk factors for stroke in a cohort of men born in 1913. N Engl J Med 1987317521–526. [DOI] [PubMed] [Google Scholar]
- 25.Prospective studies collaboration Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Lancet 19953461647–1653. [PubMed] [Google Scholar]
- 26.Bots M L, Elwood P C, Nikitin Y.et al Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 20025619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A‐I: risk indicators of coronary heart disease and targets for lipid‐modifying therapy. J Intern Med 2004255188–205. [DOI] [PubMed] [Google Scholar]
- 28.Walldius G, Aastveit A H, Jungner I. Stroke mortality and the apoB/apoA‐I ratio: results of the AMORIS prospective study. J Intern Med 2006259259–266. [DOI] [PubMed] [Google Scholar]
- 29.Walldius G, Jungner I, Holme I.et al High apolipoprotein B, low apolipoprotein A‐I, and improvement in the prediction of fatal myocardial infarction (AMORIS Study): a prospective study. Lancet 20013582026–2033. [DOI] [PubMed] [Google Scholar]
- 30.Avogaro P, Bon G B, Cazzolato G.et al Are apolipoproteins better discriminators than lipids for atherosclerosis? Lancet 19791901–903. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi A I, Giles W H, Croft J B.et al Apolipoproteins A‐1 and B and the likelihood of non‐fatal stroke and myocardial infarction — data from The Third National Health and Nutrition Examination Survey. Med Sci Monit 20028CR311–CR316. [PubMed] [Google Scholar]
- 32.Woo J, Ho S C, Lau J.et al Cardiovascular symptoms, electrocardiographic abnormalities, and associated risk factors in an elderly Chinese population. Int J Cardiol 199342249–255. [DOI] [PubMed] [Google Scholar]
- 33.Ashley E A, Raxwal V K, Froelicher V F. The prevalence and prognostic significance of electrocardiographic abnormalities. Curr Probl Cardiol 2000251–72. [DOI] [PubMed] [Google Scholar]
- 34.Verdecchia P, Porcellati C, Reboldi G.et al Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 20011042039–2044. [DOI] [PubMed] [Google Scholar]
- 35.Bots M L, Nikitin Y, Salonen J T.et al Left ventricular hypertrophy and risk of fatal and non‐fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe, J Epidemiol Community Health 2002568–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moons K G, Bots M L, Salonen J T.et al Prediction of stroke in the general population in Europe (EUROSTROKE): Is there a role for fibrinogen and electrocardiography? J Epidemiol Community Health 20025630–36. [DOI] [PMC free article] [PubMed] [Google Scholar]