Abstract
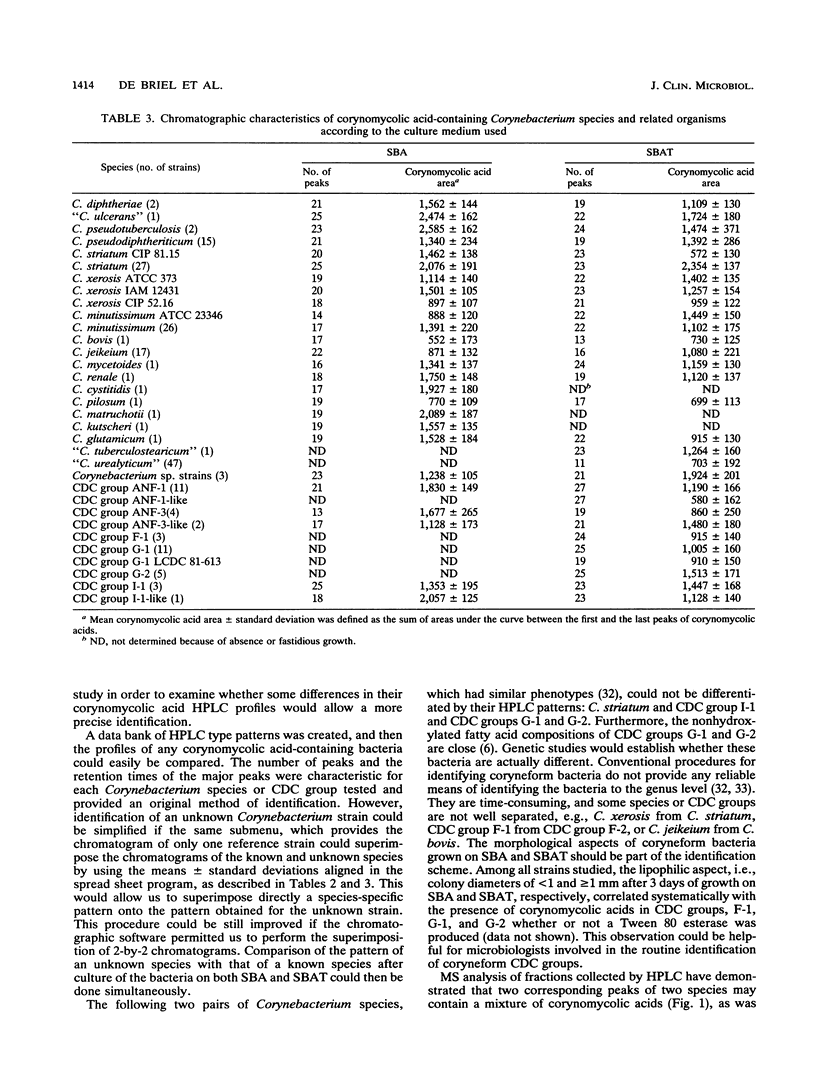
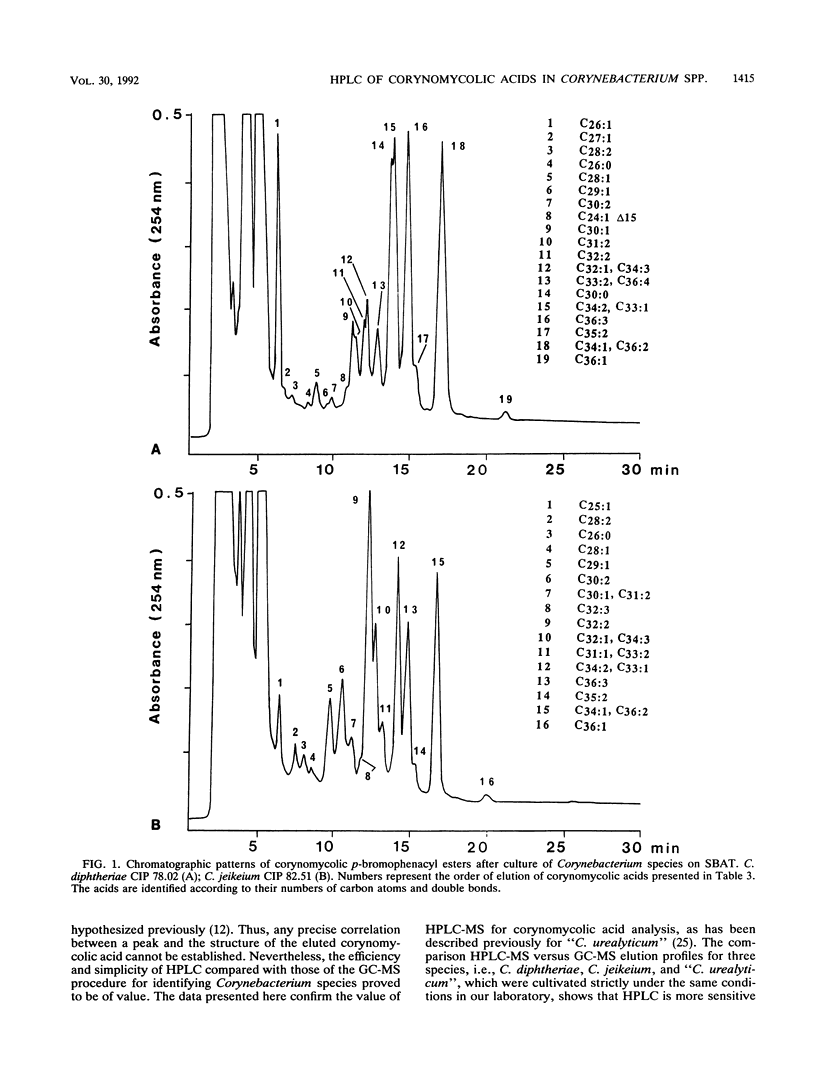
A high-performance liquid chromatography (HPLC) study of 307 strains of Corynebacterium species and related taxa revealed that strains classified as "Corynebacterium aquaticum"; "Corynebacterium asperum"; and Centers for Disease Control (CDC) groups 1, 2, A-3, A-4, A-5, B-1, B-3, E, F-2, and I-2 as well as some unidentified coryneforms do not contain any corynomycolic acids; therefore, they should not be included in the genus Corynebacterium. Such an HPLC method of identification permitted the correct assignment to the genus Rhodococcus of two unpigmented strains of coryneform bacteria whose mycolic acid profiles were comparable to those of Rhodococcus equi. Bacteria belonging to CDC groups ANF-1, ANF-3, F-1, G-1, G-2, and I-1, as well as some other Corynebacterium sp. strains, yielded corynomycolic acid HPLC patterns related to those of Corynebacterium species. Either similarities or differences were observed in the corynomycolic acid profiles of Corynebacterium species tested after culture on sheep blood agar and/or sheep blood agar supplemented with Tween 80, which demonstrated that identification at the species or group level is possible. However, Corynebacterium striatum and CDC group I-1 bacteria as well as CDC group G-1 and group G-2 bacteria had indistinguishable HPLC patterns. Conversely, some variations were observed within some species as Corynebacterium xerosis, C. striatum, and Corynebacterium minutissimum. The evaluation procedure of this HPLC method by mass spectrometry analysis of isolated eluted peaks revealed that analytical reverse-phase HPLC alone does not provide any structural information, since isomers with identical polarities coeluted as a single peak. Nevertheless, HPLC is a rapid and reliable method for identification of corynomycolic acid-containing bacteria in the clinical microbiological laboratory.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alashamaony L., Goodfellow M., Minnikin D. E. Free mycolic acids as criteria in the classification of Nocardia and the 'rhodochrous' complex. J Gen Microbiol. 1976 Jan;92(1):188–199. doi: 10.1099/00221287-92-1-188. [DOI] [PubMed] [Google Scholar]
- Alshamoany L., Goodfellow M., Minnikin D. E., Bowden G. H., Hardie J. M. Fatty and mycolic acid composition of Bacterionema matruchotii and related organisms. J Gen Microbiol. 1977 Jan;98(1):205–213. doi: 10.1099/00221287-98-1-205. [DOI] [PubMed] [Google Scholar]
- Athalye M., Noble W. C., Mallet A. I., Minnikin D. E. Gas chromatography-mass spectrometry of mycolic acids as a tool in the identification of medically important coryneform bacteria. J Gen Microbiol. 1984 Mar;130(3):513–519. doi: 10.1099/00221287-130-3-513. [DOI] [PubMed] [Google Scholar]
- Barnass S., Holland K., Tabaqchali S. Vancomycin-resistant Corynebacterium species causing prosthetic valve endocarditis successfully treated with imipenem and ciprofloxacin. J Infect. 1991 Mar;22(2):161–169. doi: 10.1016/0163-4453(91)91591-k. [DOI] [PubMed] [Google Scholar]
- Bernard K. A., Bellefeuille M., Ewan E. P. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991 Jan;29(1):83–89. doi: 10.1128/jcm.29.1.83-89.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Lanéelle M. A., Asselineau J., Barksdale L. Description of Corynebacterium tuberculostearicum sp. nov., a leprosy-derived Corynebacterium. Ann Microbiol (Paris) 1984 Nov-Dec;135B(3):251–267. doi: 10.1016/s0769-2609(84)80093-9. [DOI] [PubMed] [Google Scholar]
- Butler W. R., Ahearn D. G., Kilburn J. O. High-performance liquid chromatography of mycolic acids as a tool in the identification of Corynebacterium, Nocardia, Rhodococcus, and Mycobacterium species. J Clin Microbiol. 1986 Jan;23(1):182–185. doi: 10.1128/jcm.23.1.182-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. R., Jost K. C., Jr, Kilburn J. O. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991 Nov;29(11):2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. R., Kilburn J. O. High-performance liquid chromatography patterns of mycolic acids as criteria for identification of Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium smegmatis. J Clin Microbiol. 1990 Sep;28(9):2094–2098. doi: 10.1128/jcm.28.9.2094-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. R., Kilburn J. O. Identification of major slowly growing pathogenic mycobacteria and Mycobacterium gordonae by high-performance liquid chromatography of their mycolic acids. J Clin Microbiol. 1988 Jan;26(1):50–53. doi: 10.1128/jcm.26.1.50-53.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. R., Kilburn J. O., Kubica G. P. High-performance liquid chromatography analysis of mycolic acids as an aid in laboratory identification of Rhodococcus and Nocardia species. J Clin Microbiol. 1987 Nov;25(11):2126–2131. doi: 10.1128/jcm.25.11.2126-2131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier J., Pommier M. T., Cremieux A., Michel G. Influence of Tween 80 on the mycolic acid composition of three cutaneous corynebacteria. J Gen Microbiol. 1988 Sep;134(9):2457–2461. doi: 10.1099/00221287-134-9-2457. [DOI] [PubMed] [Google Scholar]
- Chomarat M., Breton P., Dubost J. Osteomyelitis due to Corynebacterium group D2. Eur J Clin Microbiol Infect Dis. 1991 Jan;10(1):43–43. doi: 10.1007/BF01967098. [DOI] [PubMed] [Google Scholar]
- Chou S. P., Kasatiya S., Irvine N. Cellular fatty acid composition of Oerskovia species, CDC Coryneform groups A-3, A-4, A-5, Corynebacterium aquaticum, Listeria denitrificans and Brevibacterium acetylicum. Antonie Van Leeuwenhoek. 1990 Aug;58(2):115–119. doi: 10.1007/BF00422727. [DOI] [PubMed] [Google Scholar]
- Cocito C., Delville J. Biological, chemical, immunological and staining properties of bacteria isolated from tissues of leprosy patients. Eur J Epidemiol. 1985 Sep;1(3):202–231. doi: 10.1007/BF00234095. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Goodfellow M., Minnikin D. E. A survey of the structures of mycolic acids in Corynebacterium and related taxa. J Gen Microbiol. 1982 Jan;128(1):129–149. doi: 10.1099/00221287-128-1-129. [DOI] [PubMed] [Google Scholar]
- Corina D. L., Sesardic D. Profile analysis of total mycolic acids from skin corynebacteria and from named Corynebacterium strains by gas-liquid chromatography and gas-liquid chromatography/mass spectrometry. J Gen Microbiol. 1980 Jan;116(1):61–68. doi: 10.1099/00221287-116-1-61. [DOI] [PubMed] [Google Scholar]
- Couderc F., De Briel D., Demont N., Gilard V., Promé J. C. Mass spectrometry as a tool for identifying group D2 corynebacteria by their fatty acid profiles. J Gen Microbiol. 1991 Aug;137(8):1903–1909. doi: 10.1099/00221287-137-8-1903. [DOI] [PubMed] [Google Scholar]
- Coyle M. B., Lipsky B. A. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev. 1990 Jul;3(3):227–246. doi: 10.1128/cmr.3.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
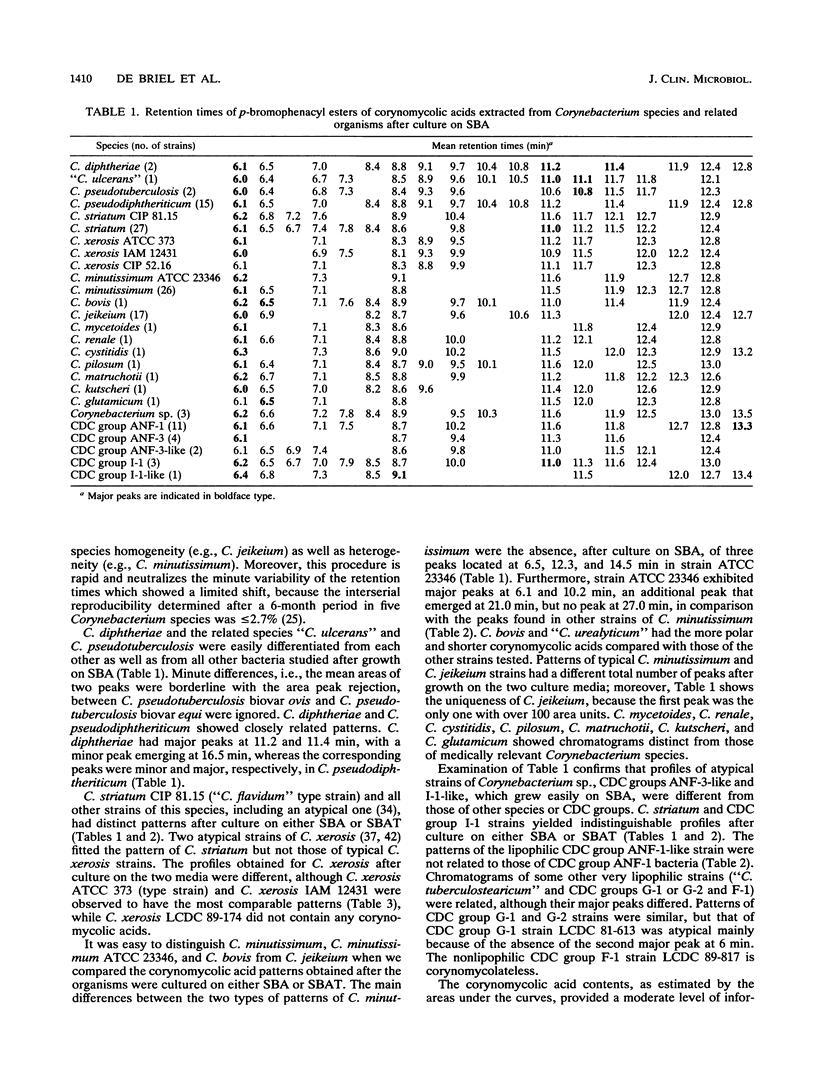
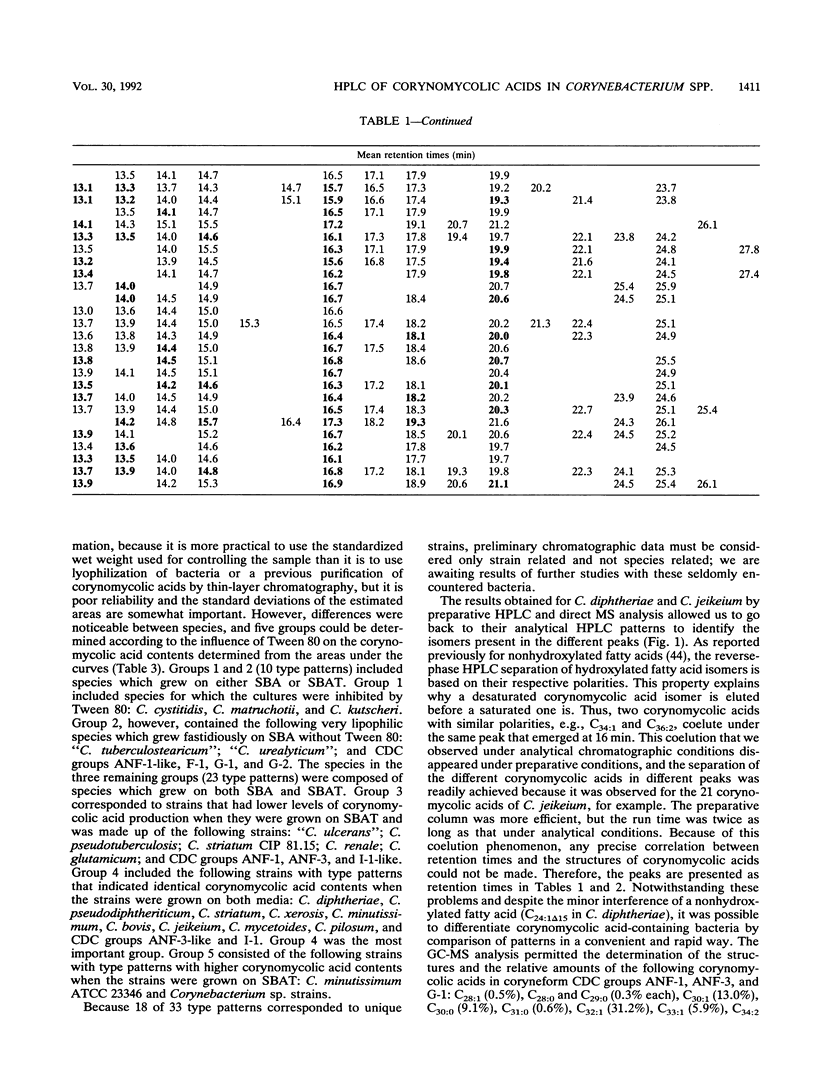
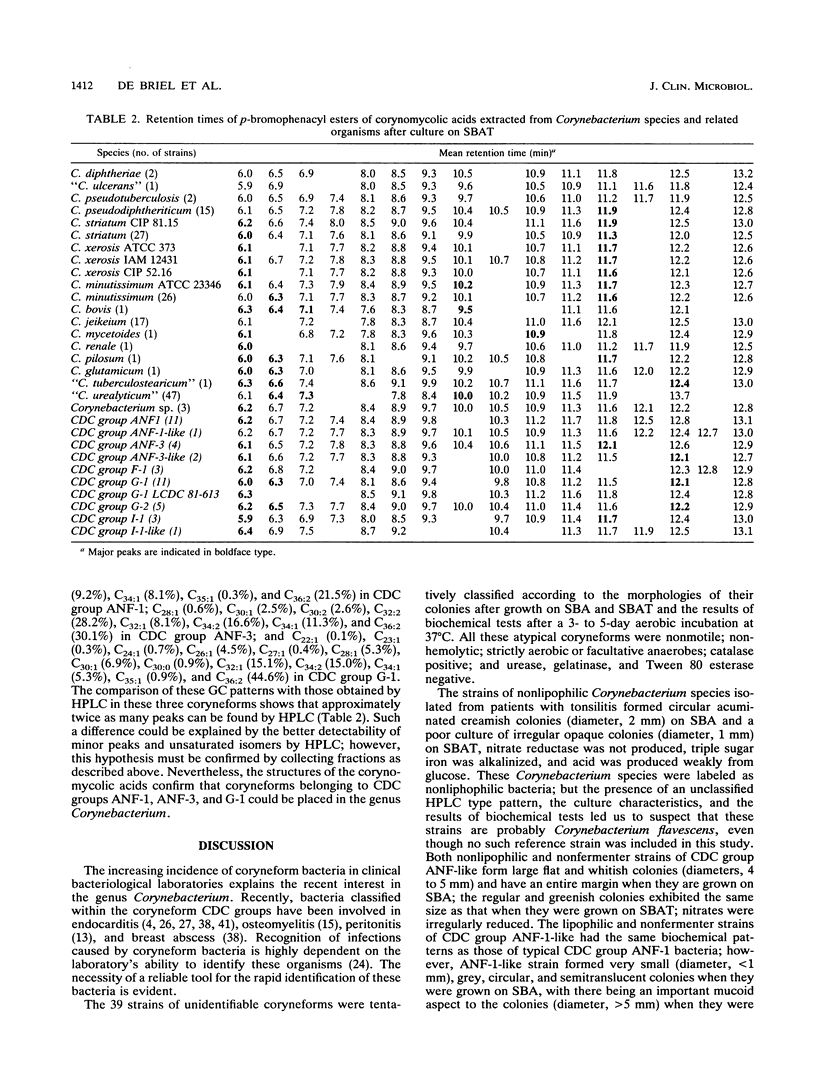
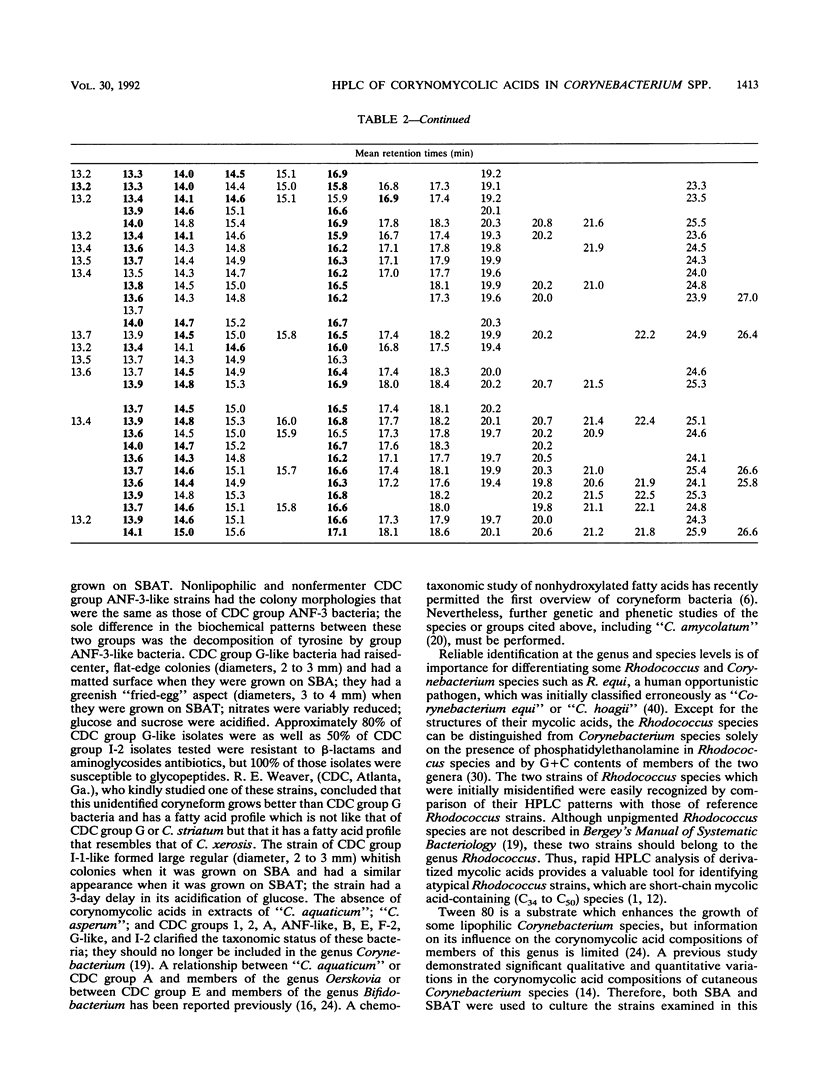
- De Briel D., Couderc F., Riegel P., Gallion C., Langs J. C., Jehl F. Contribution of high-performance liquid chromatography to the identification of some Corynebacterium species by comparison of their corynomycolic acid patterns. Res Microbiol. 1992 Feb;143(2):191–198. doi: 10.1016/0923-2508(92)90008-c. [DOI] [PubMed] [Google Scholar]
- De Briel D., Langs J. C., Rougeron G., Chabot P., Le Faou A. Multiresistant corynebacteria in bacteriuria: a comparative study of the role of Corynebacterium group D2 and Corynebacterium jeikeium. J Hosp Infect. 1991 Jan;17(1):35–43. doi: 10.1016/0195-6701(91)90075-j. [DOI] [PubMed] [Google Scholar]
- Ena J., Berenguer J., Peláez T., Bouza E. Endocarditis caused by Corynebacterium group D2. J Infect. 1991 Jan;22(1):95–96. doi: 10.1016/0163-4453(91)91150-v. [DOI] [PubMed] [Google Scholar]
- Freney J., Duperron M. T., Courtier C., Hansen W., Allard F., Boeufgras J. M., Monget D., Fleurette J. Evaluation of API Coryne in comparison with conventional methods for identifying coryneform bacteria. J Clin Microbiol. 1991 Jan;29(1):38–41. doi: 10.1128/jcm.29.1.38-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly C., Sandra P., Verzele M., Cocito C. Analysis of mycolic acids from a group of corynebacteria by capillary gas chromatography and mass spectrometry. Eur J Biochem. 1982 Jun 15;125(1):83–94. doi: 10.1111/j.1432-1033.1982.tb06654.x. [DOI] [PubMed] [Google Scholar]
- Herrera-Alcaraz E. A., Valero-Guillén P. L., Martín-Luengo F., Soriano F. Taxonomic implications of the chemical analysis of the D2 group of corynebacteria. FEMS Microbiol Lett. 1990 Nov;60(3):341–344. doi: 10.1016/0378-1097(90)90328-n. [DOI] [PubMed] [Google Scholar]
- Markowitz S. M., Coudron P. E. Native valve endocarditis caused by an organism resembling Corynebacterium striatum. J Clin Microbiol. 1990 Jan;28(1):8–10. doi: 10.1128/jcm.28.1.8-10.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Schaal K. P., von Graevenitz A., von Moos L., Woolcock J. B., Wüst J., Yassin A. F. Characterization of Rhodococcus equi-like bacterium isolated from a wound infection in a noncompromised host. J Clin Microbiol. 1988 Apr;26(4):618–620. doi: 10.1128/jcm.26.4.618-620.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Soto A., Soriano F., Valero-Guillén P. Classification of coryneform bacteria associated with human urinary tract infection (group D2) as Corynebacterium urealyticum sp. nov. Int J Syst Bacteriol. 1992 Jan;42(1):178–181. doi: 10.1099/00207713-42-1-178. [DOI] [PubMed] [Google Scholar]
- Prescott J. F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991 Jan;4(1):20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn A. G., Comaish J. S., Pedler S. J. Septic arthritis and endocarditis due to group G-2 coryneform organism. Lancet. 1991 Jul 6;338(8758):62–63. doi: 10.1016/0140-6736(91)90062-t. [DOI] [PubMed] [Google Scholar]
- Røder B. L., Frimodt-Møller N. Corynebacterium xerosis as a cause of community-acquired endocarditis. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):233–234. doi: 10.1007/BF01963847. [DOI] [PubMed] [Google Scholar]



