Abstract
Background
Rapid fluid delivery from ingested beverages is the goal of oral rehydration solutions (ORS) and sports drinks.
Objective
The aim of the present study was to investigate the effects of increasing carbohydrate and sodium content upon fluid delivery using a deuterium oxide (D2O) tracer.
Design
Twenty healthy male subjects were divided into two groups of 10, the first group was a carbohydrate group (CHO) and the second a sodium group (Na). The CHO group ingested four different drinks with a stepped increase of 3% glucose from 0% to 9% while sodium concentration was 20 mmol/L. The Na group ingested four drinks with a stepped increase of 20 mmol/L from 0 mmol/L to 60 mmol/l while glucose concentration was 6%. All beverages contained 3 g of D2O. Subjects remained seated for two hours after ingestion of the experimental beverage, with blood taken every 5 min in the first hour and every 10 min in the second hour.
Results
Including 3% glucose in the beverage led to a significantly greater AUC 60 min (19640 ± 1252 δ‰ vs. VSMOW.60 min) than all trials. No carbohydrate (18381 ± 1198 δ‰ vs. VSMOW.60 min) had a greater AUC 60 min than a 6% (16088 ± 1359 δ‰ vs. VSMOW.60 min) and 9% beverage (13134 ± 1115 δ‰ vs. VSMOW.60 min); the 6% beverage had a significantly greater AUC 60 min than the 9% beverage. There was no difference in fluid delivery between the different sodium beverages.
Conclusion
In conclusion the present study showed that when carbohydrate concentration in an ingested beverage was increased above 6% fluid delivery was compromised. However, increasing the amount of sodium (0–60 mmol/L) in a 6% glucose beverage did not lead to increases in fluid delivery.
Introduction
One of the main aims of oral rehydration solutions (ORS) and sports drinks is to make the ingested fluid available to for use within the body as quickly as possible. Drinks designed for use in both ORS and sports nutrition contain a mixture of carbohydrate and electrolytes, with the main electrolyte being sodium.
Carbohydrates in ORS mainly come in the form of glucose, although they may also contain sucrose, maltodextrins or fructose. Increasing the amount of carbohydrate in an ingested beverage leads to a decrease in fluid delivery [1]. The increase in osmolality due to high carbohydrate concentrations leads to a net movement of water into the intestinal lumen causing a loss in the body water pool and may increase the effects of dehydration [2]. Previously it has been shown that a 6% CHO electrolyte solution leads to greater fluid delivery than a 15% glucose solution [3], but there was no difference shown in fluid delivery when a 6%, 8% and 10% glucose and fructose solution was compared [4].
Previous research has suggested that addition of sodium to ingested beverages will lead to increased fluid delivery [5] and a reduced plasma volume change during exercise indicating greater availability of fluid [6]. More recent research has suggested that sodium content may not be as important a factor as carbohydrate [7]. Indeed, increasing the sodium content of a 6% carbohydrate solution did not show any differences in intestinal water absorption [8]. However, this investigation employed the triple lumen technique which only measures a small section of the small intestine and does not take into account gastric emptying. Therefore, the triple lumen technique may not be representative of fluid availability to the whole body from an ingested beverage.
Deuterium oxide dilution is a relatively non invasive measure of fluid delivery. While it does not give a quantitative value of the amount of fluid absorbed as long as the amount and concentration of the tracer are kept the same the method can provide a measure of relative differences between ingested beverages [9]. Inclusion of D2O in an ingested beverage gives an integrative measure of gastric emptying and intestinal fluid absorption leading to fluid delivery. Studies which have compared different beverages using D2O [3,10] have found temporal D2O responses which would be expected [11].
The aim of the present study was to investigate the effects of increasing carbohydrate and sodium content upon fluid delivery using a deuterated water tracer.
Methods
Twenty healthy male subjects took part in the study. The study was approved by the Ethics Committee of the School of Sport and Exercise Sciences at the University of Birmingham. Subjects completed a General Health Questionnaire and provided written consent to take part in the study.
All experimental trials took place after an overnight fast. Each subject undertook four trials each at least 7 days apart. The subjects were divided into two groups of 10, the first group was a carbohydrate group (CHO) (Age: 20 ± 1 y, Body Mass: 81.2 ± 7.5 kg) and the second a sodium group (Na) (Age: 21 ± 2 y, Body Mass: 83.6 ± 9 kg). The experimental beverages given to the CHO group were:
G0: Water + 20 mmol/L sodium
G3: 3% Glucose + 20 mmol/L sodium
G6: 6% Glucose + 20 mmol/L sodium
G9: 9% Glucose + 20 mmol/L sodium
The Na group ingested the following beverages:
Na0: 6% Glucose
Na20: 6% Glucose + 20 mmol/L sodium
Na40: 6% Glucose + 40 mmol/L sodium
Na60: 6% Glucose + 60 mmol/L sodium
Glucose was obtained from Cerestar (Manchester, UK) and sodium from Sigma-Aldrich (Gillingham, UK). All trials were conducted in a randomised order.
Subjects arrived in the laboratory between 7 and 9 am. On arrival in the laboratory subjects were asked to empty their bladder before nude body mass was recorded (Champ II, Ohaus UK, Leicester, UK). Thereafter a Teflon catheter (Quickcath, Baxter, Norfolk, UK) was inserted into the antecubital vein for blood sampling.
Subjects remained seated for 20 min before a resting blood and saliva sample was taken. A 550 ml bolus of the experimental beverage was given containing 3.00 g of deuterated water (99.9% atom % deuterium oxide, Sigma Aldrich, St Louis USA), this was then followed by a further 50 ml of the experimental beverage which was used to swill out the mouth and ensure all deuterium oxide was ingested.
A 5 ml blood sample was taken every five minutes following ingestion of the experimental beverage for the first hour and every 10 min for the second hour. Therefore, subjects remained at rest for a total of 120 min. At all time point's blood was stored in tubes containing 0.054 ml K3EDTA (Becton Dickinson, Plymouth, UK). All EDTA tubes were centrifuged at 3200 g for 10 min and the plasma stored in a glass vial which was stored at -70°C.
Plasma D2O enrichment were analysed using the GasBench II (Thermo Electron, Bremen, Germany) – isotope ratio mass spectrometry (Finnigan, Delta XP, Bremen, Germany). Briefly, 200 μl of the aqueous sample was transferred into a vacutainer (Labco, High Wycombe, England). A platinum catalyst (Thermo Electron, Bremen, Germany) was added and the vacutainer flushed with a 2% H2 in Helium for 5 min gas followed by a 40 min equilibration period then followed where the hydrogen isotopes in the aqueous solution exchanged with hydrogen ions in the headspace. A sample of the headspace gas was then injected into an Isotope Ratio Mass Spectrometer (IRMS) (Thermo Electron, Bremen, Germany). The average of the last 9 measurements was used as the average for the sample. The isotopic enrichment is expressed as δ‰ against the international water standard Vienna Standard Mean Ocean Water (VSMOW). The CV of this measurement is 0.27%.
Non linear regression was employed to calculate the half time (T1/2), time to plateau (TTP) and plateau enrichment (PE) of the enrichment curve (GraphPad Prism, GraphPad software, San Diego, US). The non linear regression equation used was:
| Y = Ymax.(1-e(-K.t)) |
Where Y = plasma D2O enrichment, K = a constant, and t = time.
In both experiments plasma deuterium oxide enrichment were analysed using a two way (time and treatment) ANOVA for repeated measures. Area under the curve for the first 60 min (AUC 60 min), T1/2, time to plateau and plateau enrichment were analysed using a repeated measures ANOVA. All post hoc tests were Tukeys HSD. Significance was set at p < 0.05. All data analysis was conducted using SPSS version 12.
Results
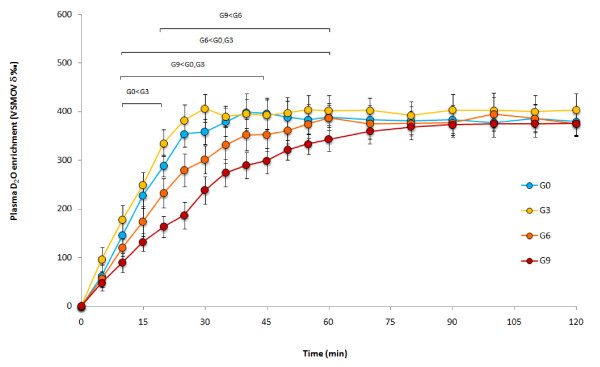
The glucose group showed a quick rise in plasma deuterium oxide enrichment before reaching a plateau. While not significantly different to each other there was a trend for the time to plateau to increase with increasing carbohydrate concentration (GO = 34 ± 7 min, G3 = 35 ± 10 min, G6 = 43 ± 13 min, G9 = 51 ± 15 min), all reached a plateau enrichment of similar value (G0 = 434 ± 113 δ‰ vs. VSMOW, G3 = 458 ± 96 δ‰ vs. VSMOW, G6 = 398 ± 106 δ‰ vs. VSMOW, G9 = 360 ± 55 δ‰ vs. VSMOW).
The glucose group showed a significant effect of trial (F3,27 = 37.250, P < 0.001), time (F18,162 = 88.973, P < 0.001) and an interaction between trial and time (F54,486 = 5.386, P < 0.001). Including 3% glucose in the beverage led to significantly greater plasma deuterium enrichment than having no carbohydrate between 20 and 30 min after ingestion. When carbohydrate content was increased to 6% there was lower plasma D2O enrichment than the 3% and 0% carbohydrate beverages between 10 and 45 min after ingestion. Further increasing the carbohydrate content to 9% led to lower plasma deuterium enrichment than a 3% and 0% beverage between 10 and 60 min after ingestion, and also had lower plasma deuterium enrichment than a 6% beverage between 20 and 60 min after ingestion (Figure 1).
Figure 1.
D2O enrichment over time after ingesting 4 different glucose beverages. Statistical differences (P < 0.05) are indicated.
There was a significant effect of trial for T1/2 (F3,27 = 17.528, P < 0.001). Trials G0 (11 ± 2 min) and G3 (9 ± 1 min) had the quickest T1/2 but were not different from each other and trial G6 (15 ± 2 min) was quicker than G9 (24 ± 3 min) (Table 1). There was a significant effect of trial for AUC 60 min (F3,27 = 65.861, P < 0.001). Trial G3 showed the greatest AUC 60 min (19640 ± 1252 δ‰ vs. VSMOW.60 min), with G0 (18381 ± 1198 δ‰ vs. VSMOW.60 min) having a greater AUC 60 min than G6 (16088 ± 1359 δ‰ vs. VSMOW.60 min) and G9 (13134 ± 1115 δ‰ vs. VSMOW.60 min), G6 had a significantly greater AUC 60 min than G9 (Table 1).
Table 1.
D2O enrichment characteristics with 4 different glucose beverages
| G0 | G3 | G6 | G9 | |
| T 1/2 (min) | 11 ± 2bc | 9 ± 1bc | 15 ± 2c | 24 ± 3 |
| AUC (δ‰ vs. VSMOW.60 min) | 18381 ± 1198bc | 19640 ± 1252abc | 16088 ± 1359c | 13134 ± 1115 |
| PE (δ‰ vs. VSMOW) | 434 ± 113 | 458 ± 96 | 398 ± 106 | 360 ± 55 |
| TTP (min) | 34 ± 7 | 35 ± 10 | 43 ± 13 | 51 ± 15 |
Half time (t1/2), area under the curve for first 60 min (AUC), plateau enrichment (PE) and Time to Plateau (TTP) with no glucose (G0), 3% glucose (G3), 6% glucose (G6) and 9% glucose (G9). All drinks contained 20 mmol/L sodium. Significant differences are indicated with a, b and c. a indicates different from G0, b indicates different from G6, c indicates different from G9. (P < 0.05)
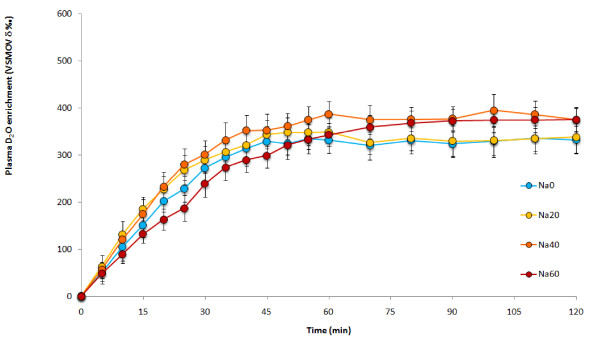
The sodium group also had an initial increase in plasma deuterium oxide enrichment before reaching a plateau (Figure 2). While not significantly different from each other there was a trend for a decrease in time to plateau with increasing sodium content (Na0 = 23 ± 3 min, Na20 = 19 ± 3 min, Na40 = 18 ± 2 min, Na60 = 16 ± 3 min), all had similar plateau enrichments (Na0 = 352 ± 17 δ‰ vs. VSMOW, Na20 = 356 ± 14 δ‰ vs. VSMOW, Na40 = 348 ± 15 δ‰ vs. VSMOW, Na60 = 354 ± 18 δ‰ vs. VSMOW) (Table 2).
Figure 2.
D2O enrichment over time after ingesting 4 different sodium beverages. There were no statistical differences.
Table 2.
D2O enrichment characteristics with 4 different sodium beverages
| Na0 | Na20 | Na40 | Na60 | |
| T 1/2 (min) | 16 ± 2 min | 13 ± 2 min | 12 ± 1 min | 11 ± 2 |
| AUC (δ‰ vs. VSMOW.60 min) | 13843 ± 785 | 15054 ± 674 | 15162 ± 467 | 15810 ± 719 |
| PE (δ‰ vs. VSMOW) | 352 ± 17 | 356 ± 14 | 348 ± 15 | 354 ± 18 |
| TTP (min) | 23 ± 3 | 19 ± 3 | 18 ± 2 | 16 ± 3 |
Half time (t1/2), area under the curve for first 60 min (AUC), plateau enrichment (PE) and Time to Plateau (TTP) with no sodium (Na0), 20 mmol/L sodium (Na20), 40 mmol/L sodium (Na40) and 60 mmol/L sodium (Na60) in a 6% glucose solution. There were no statistical differences between trials.
The sodium group failed to show any differences between the trials for plasma D2O enrichment (F3,27 = 1.719, P = 0.187) (Figure 2). While Na60 had the quickest T1/2 (11 ± 2 min) compared to Na40 (12 ± 1 min), Na20 (13 ± 2 min) and Na0 (16 ± 2 min), these were not significantly different from each other (F3,27 = 2.119, P = 0.121). Similarly Na60 had the greatest AUC 60 min (15810 ± 719 δ‰ vs. VSMOW.60 min) compared to Na40 (15162 ± 467 δ‰ vs. VSMOW.60 min), Na20 (15054 ± 674 δ‰ vs. VSMOW.60 min) and Na0 (13843 ± 785 δ‰ vs. VSMOW.60 min) but again were not significantly different from each other (Table 2).
Discussion
The present study investigated the effect of increasing the amounts of glucose and sodium in ingested beverages on fluid delivery. A deuterium oxide tracer was incorporated in the test beverages and plasma deuterium oxide enrichment was used as a measure of fluid delivery.
Increasing the glucose content of the beverage above 6% decreased fluid delivery compared to water, whereas sodium content in the range investigated did not affect fluid delivery.
The present study shows that increasing the carbohydrate content of a beverage above 6% can lead to a decrease in fluid delivery compared to water. While previously a 15% glucose solution [3] and a 20% carbohydrate solution [12] have been shown to cause a slowing of the accumulation of deuterium oxide in plasma following ingestion of the beverage, this is the first study which has showed that a 6% carbohydrate solution can slow the accumulation of D2O in plasma. Davis et al. [4] did not report any differences in the appearance of D2O in plasma when comparing 6%, 8% and 10% glucose and fructose beverages with water. A possible reason for the discrepancies between these studies is the inclusion of fructose in the beverages of Davis et al. [4]. Fructose is absorbed by GLUT5 in the intestinal cell membrane [13], whereas glucose is absorbed by SGLT1 [14]. The inclusion of these multiple transportable carbohydrates can lead to a reduction of the inhibiting effect of hyperosmolality on fluid absorption [15].
As the use of a D2O tracer is an integrated measure of gastric emptying and intestinal absorption either of these could be the site of the decreased fluid delivery seen with the ingestion of the 6% and 9% glucose beverages. Upon ingestion of the beverage it first enters the stomach. A 20% carbohydrate solution was shown to empty slower from the stomach than a 6% carbohydrate solution [12]. Concentrations of carbohydrate less than 10% have also been shown to slow gastric emptying, with a 5% glucose beverage having a slower gastric emptying when compared to water alone, and a 10% glucose beverage slowing gastric emptying even further [1]. One study has reported that carbohydrate concentrations less than 10% do not affect gastric emptying [16]. It is possible that the 6% and 9% carbohydrate beverages in the present study slowed gastric emptying although this is not clear.
Once the ingested beverage has been emptied from the stomach it enters the small intestine. The proximal section of the small intestine, the duodenum is the most water permeable section of the small intestine. Water is absorbed down an osmotic gradient in the duodenum, therefore when water is compared to a carbohydrate beverage water leads to greater fluid absorption in the duodenum as it causes a greater osmotic gradient [17]. The increased carbohydrate content in the 6% and 9% beverages may have decreased fluid absorption in the duodenum compared to water [15].
As the ingested beverage continues down the small intestine to the jejunum more solute is absorbed [17]. The absorption of glucose by SGLT1 in the small intestine is directly coupled with the absorption of 2 sodium molecules and approximately 300 water molecules [18]. Therefore fluid can be absorbed against a concentration gradient. This may explain why the 3% glucose beverage led to greater fluid delivery than the water beverage. The absorption of glucose in the jejunum will lead to increased fluid absorption, both in the transcellular route of SGLT1 and through paracellular pathways as SGLT1 has been shown to increase the permeability of the intestinal tight junctions [19].
The present study did not show any effect of increasing sodium concentration on fluid delivery. It has been shown that when 25 mmol/L sodium chloride solution was ingested that the decrease in plasma volume seen during exercise was lowered compared to water alone, suggesting that fluid delivery was increased [6]. The present study suggests that when carbohydrate is included in a beverage increasing the amount of sodium does not increase fluid absorption. Gisolfi et al. [8] investigated the absorption of fluid from a 6% glucose beverage containing either 0, 25 or 50 mmol/L and found that there was no difference in fluid absorption. Similarly, during exercise increasing the amount of sodium in carbohydrate beverages ingested during exercise did not lead to an increase in fluid absorption [20]. Therefore, the presence of 6% glucose in the beverages in the present study may have masked any effect that sodium has upon fluid delivery.
Despite no effect of sodium on fluid delivery it might still be useful to include sodium into carbohydrate electrolyte beverages. Including sodium in an ingested beverage leads to an increase in palatability [21]. Palatability is an important factor in increasing voluntary drinking [22], which may be useful in situations such as during prolonged exercise in hot conditions as the fluid intake may enable a lower core body temperature to be maintained when compared to no fluid and prevent a decrease in performance [23]. Sodium is also important for rehydration after a period of dehydration as sodium helps with fluid retention [24].
Although deuterium oxide tracers have been used extensively to measure total body water (TBW), the use of this tracer to measure fluid delivery has received relatively little attention in the literature. Studies have successfully employed a deuterium oxide tracer to compare fluid delivery between drinks [3,12,25,26]. The advantages of the technique are that it is relatively non invasive and provides an integrated measure of the effects of gastric emptying and intestinal absorption on fluid delivery. One suggested disadvantage to the technique is that it is not able to measure net fluid absorption [2]. The results of these studies should always be interpreted with caution. The data presented here may not reflect absorption per se. This would require an assumption that steady state conditions exist for extra and intra cellular fluid volumes; and this assumption is not always valid. This would have been a particular concern in the sodium trials in this study. If increasing beverage sodium commensurately expanded plasma volume, one could have increased water absorption from the gut without a parallel increase in plasma D2O enrichment. To a lesser extent, a similar concern applies to the glucose trials. As insulin-mediated glucose uptake will promptly move absorbed glucose into the intracellular space, it will be followed by water, again creating a non-steady state dynamic during the period that the data are collected. The exact impact of these fluid shifts is unknown and therefore results have to be interpreted with caution.
In conclusion the present study showed that when carbohydrate concentration in an ingested beverage was increased above 6% fluid delivery was compromised. Sodium content (0–60 mmol/L) in a 6% glucose beverage did not lead to increases in fluid delivery.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AJ was the principle investigator, managed the project and finalized the paper. KC performed the experimental trials and analyses and drafted the first manuscript. JC helped in the daily running of the trials in the laboratory. JC helped in the daily running of the trials in the laboratory. AB provided technical assistance with the deuterium oxide analysis. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This study was supported by a grant of GlaxoSmithKline Consumer Healthcare UK.
Contributor Information
Asker E Jeukendrup, Email: a.e.jeukendrup@bham.ac.uk.
Kevin Currell, Email: a.e.jeukendrup@bham.ac.uk.
Juliette Clarke, Email: a.e.jeukendrup@bham.ac.uk.
Johnny Cole, Email: a.e.jeukendrup@bham.ac.uk.
Andrew K Blannin, Email: a.k.blannin@bham.ac.uk.
References
- Maughan RJ, Leiper JB. Limitations to fluid replacement during exercise. Can J Appl Physiol. 1999;24:173–187. doi: 10.1139/h99-015. [DOI] [PubMed] [Google Scholar]
- Gisolfi CV, Summers RW, Schedl HP, Bleiler TL, Oppliger RA. Human intestinal water absorption: direct vs. indirect measurements. Am J Physiol. 1990;258:G216–222. doi: 10.1152/ajpgi.1990.258.2.G216. [DOI] [PubMed] [Google Scholar]
- Davis JM, Lamb DR, Burgess WA, Bartoli WP. Accumulation of deuterium oxide in body fluids after ingestion of D2O-labeled beverages. J Appl Physiol. 1987;63:2060–2066. doi: 10.1152/jappl.1987.63.5.2060. [DOI] [PubMed] [Google Scholar]
- Davis JM, Burgess WA, Slentz CA, Bartoli WP. Fluid avalibility and sports drinks differing in carbohydrate type and concentration. Am J Clin Nutr. 1990;51:1054–1057. doi: 10.1093/ajcn/51.6.1054. [DOI] [PubMed] [Google Scholar]
- Leiper JB, Maughan RJ. Experimental models for the investigation of water and solute transport in man. Implications for oral rehydration solutions. Drugs. 1988;36:65–79. doi: 10.2165/00003495-198800364-00010. [DOI] [PubMed] [Google Scholar]
- Barr SI, Costill DL, Fink WJ. Fluid replacement during prolonged exercise: effects of water, saline, or no fluid. Med Sci Sports Exerc. 1991:23. [PubMed] [Google Scholar]
- Schedl HP, Maughan RJ, Gisolfi CV. Intestinal absorption during rest and exercise: implications for formulating oral rehydration solution (ORS) Med Sci Sport Exerc. 1994;26:267–280. [PubMed] [Google Scholar]
- Gisolfi CV, Summers RD, Schedl HP, Bleiler TL. Effect of sodium concentration in a carbohydrate-electrolyte solution on intestinal absorption. Med Sci Sports Exerc. 1995;27:1414–1420. [PubMed] [Google Scholar]
- Lambert CP, Ball D, Leiper JB, Maughan RJ. The use of a deuterium tracer technique to follow the fate of fluids ingested by human subjects: effects of drink volume and tracer concentration and content. Exp Physiol. 1999;84:391–399. doi: 10.1017/S0958067099018266. [DOI] [PubMed] [Google Scholar]
- Murray R, Eddy DE, Murray TW, Seifert JG, Paul GL, Halaby GA. The effect of fluid and carbohydrate feedings during intermittent cycling ecxercise. Med Sci Sports Exerc. 1987;19:597–604. [PubMed] [Google Scholar]
- Jeukendrup AE, Moseley L. Multiple transportable carbohydrates enhance gastric emptying and fluid delivery. Scand J Med Sci Sports. 2008 doi: 10.1111/j.1600-0838.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- Murray R, Bartoli WP, Eddy DE, Horn MK. Gastric emptying and plasma deuterium accumulation following ingestion of water and two carbohydrate-electrolyte beverages. Int J Sport Nutr. 1997;7:144–153. doi: 10.1123/ijsn.7.2.144. [DOI] [PubMed] [Google Scholar]
- Semenza G, Kessler M, Hosang M, Weber J, Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984;779:343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Summers RW, Schedl HP, Flanagan SW, Chang R, Gisolfi CV. Effects of carbohydrate type and concentration and solution osmolality on water absorption. Med Sci Sports Exerc. 1995;27:1607–1615. [PubMed] [Google Scholar]
- Zachwieja JJ, Costill DL, Beard GC, Robergs RA, Pascoe DD, Anderson DE. The effects of a carbonated carbohydrate drink on gastric emptying, gastrointestinal distress, and exercise performance. Int J Sport Nutr. 1992;2:229–238. doi: 10.1123/ijsn.2.3.239. [DOI] [PubMed] [Google Scholar]
- Lambert GP, Chang RT, Xia T, Summers RW, Gisolfi CV. Absorption from different intestinal segments during exercise. J Appl Physiol. 1997;83:204–212. doi: 10.1152/jappl.1997.83.1.204. [DOI] [PubMed] [Google Scholar]
- Loo DD, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR. Show me the pathway! Regulation of paracellular permeability by Na(+)-glucose cotransport. Adv Drug Deliv Rev. 2000;41:265–281. doi: 10.1016/S0169-409X(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Gisolfi CV, Lambert GP, Summers RW. Intestinal fluid absorption during exercise: role of sport drink osmolality and [Na+] Med Sci Sports Exerc. 2001;33:907–915. doi: 10.1097/00005768-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Murray R. The effects of consuming carbohydrate-electrolyte beverages on gastric emptying and fluid absorption during and following exercise. Sports Med. 1987;4:322–351. doi: 10.2165/00007256-198704050-00002. [DOI] [PubMed] [Google Scholar]
- Minehan MR, Riley MD, Burke LM. Effect of flavor and awareness of kilojoule content of drinks on preference and fluid balance in team sports. Int J Sport Nutr Exerc Metab. 2002;12:81–92. doi: 10.1123/ijsnem.12.1.81. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Montain SJ, Latzka WA. Hydration effects on thermoregulation and performance in the heat. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:679–690. doi: 10.1016/S1095-6433(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ. Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Med Sci Sports Exerc. 1996;28:1260–1271. doi: 10.1097/00005768-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Koulmann N, Melin B, Jimenez C, Charpenet A, Savourey G, Bittel J. Effects of different carbohydrate-electrolyte beverages on the appearance of ingested deuterium in body fluids during moderate exercise by humans in the heat. Eur J Appl Physiol Occup Physiol. 1997;75:525–531. doi: 10.1007/s004210050199. [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Leiper JB, Vist GE. Gastric emptying and fluid availability after ingestion of glucose and soy protein hydrolysate solutions in man. Exp Physiol. 2004;89:101–108. doi: 10.1113/expphysiol.2003.002655. [DOI] [PubMed] [Google Scholar]