Abstract
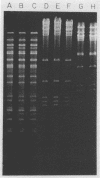
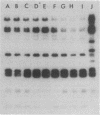
In 1990 an increased number of strains of Shigella boydii serotype 2 were isolated from different regions of Bulgaria. Strains were reported as sporadic, although they showed identical phenotypic characteristics, including resistance to ampicillin, carbenicillin, streptomycin, sulfonamide, tetracycline, ticarcillin, and trimethoprim. The objective of this study was to determine the genetic relatedness of the strains and the mechanism of their antimicrobial resistance. Plasmid fingerprinting showed an identical pattern for 23 of 25 of the selected strains. All 25 strains tested transferred their resistances en bloc to an Escherichia coli recipient. Transconjugants contained a 112-kb R plasmid which carried all the resistance genes, including that conferring type I dihydrofolate reductase-mediated trimethoprim resistance (MIC greater than 2,000 micrograms/ml). Riboprobe analysis showed identical restriction length fragment polymorphisms, suggesting a highly conserved genome. All findings indicate that strains of S. boydii serotype 2 isolated in 1990 from different regions of Bulgaria were highly related genetically and can be considered representatives of a single bacterial clone. The presence of an R plasmid and selection pressure because of the usage of antimicrobial agents, particularly trimethoprim, have likely facilitated the spread of the clone throughout the country.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bratoeva M. P., John J. F., Jr Dissemination of trimethoprim-resistant clones of Shigella sonnei in Bulgaria. J Infect Dis. 1989 Apr;159(4):648–653. doi: 10.1093/infdis/159.4.648. [DOI] [PubMed] [Google Scholar]
- Bratoeva M. P. Microbiological methods and plasmid pattern analysis in epidemiological control of Shigella sonnei infections. Microbiol Sci. 1986 Jul;3(7):216–219. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N., McLeod S., AuYong Y., Krishnan C. Increasing incidence of resistance among shigellae to trimethoprim. Lancet. 1991 Mar 9;337(8741):622–622. doi: 10.1016/0140-6736(91)91694-p. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Siitonen A., Jahkola M., Fling M., Sundström L., Huovinen P. Increase of trimethoprim resistance among Shigella species, 1975-1988: analysis of resistance mechanisms. J Infect Dis. 1990 Jun;161(6):1242–1248. doi: 10.1093/infdis/161.6.1242. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, Twitty J. A. Plasmids as epidemiologic markers in nosocomial gram-negative bacilli: experience at a university and review of the literature. Rev Infect Dis. 1986 Sep-Oct;8(5):693–704. doi: 10.1093/clinids/8.5.693. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Martinetti G., Altwegg M. rRNA gene restriction patterns and plasmid analysis as a tool for typing Salmonella enteritidis. Res Microbiol. 1990 Nov-Dec;141(9):1151–1162. doi: 10.1016/0923-2508(90)90088-8. [DOI] [PubMed] [Google Scholar]
- Nastasi A., Villafrate M. R., Mammina C., Pontello M., Ricci M. Molecular analysis of strains of Shigella boydii isolated in northern and southern Italy. Res Microbiol. 1990 Nov-Dec;141(9):1163–1172. doi: 10.1016/0923-2508(90)90089-9. [DOI] [PubMed] [Google Scholar]
- Prats G., Gurgui M., Mirelis B. Epidemiology of Shigella boydii serotype 2, a strain indigenous to Spain. Eur J Clin Microbiol. 1985 Aug;4(4):434–435. doi: 10.1007/BF02148707. [DOI] [PubMed] [Google Scholar]
- Rowe B., Gross R. J., Allen H. A. Shigella dysenteriae and Shigella boydii in England and Wales during 1972 and 1973. Br Med J. 1974 Dec 14;4(5945):641–642. doi: 10.1136/bmj.4.5945.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Parsonnet J., Greene K., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiologic techniques in analysis of epidemic and endemic Shigella dysenteriae type 1 strains. J Infect Dis. 1991 Feb;163(2):406–409. doi: 10.1093/infdis/163.2.406. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Trimethoprim resistance; epidemiology and molecular aspects. J Med Microbiol. 1990 Jan;31(1):1–19. doi: 10.1099/00222615-31-1-1. [DOI] [PubMed] [Google Scholar]